Wide–Narrow Row Planting Pattern Increases Root Lodging Resistance by Adjusting Root Architecture and Root Physiological Activity in Maize (Zea mays L.) in Northeast China
Abstract
1. Introduction
2. Materials and Methods
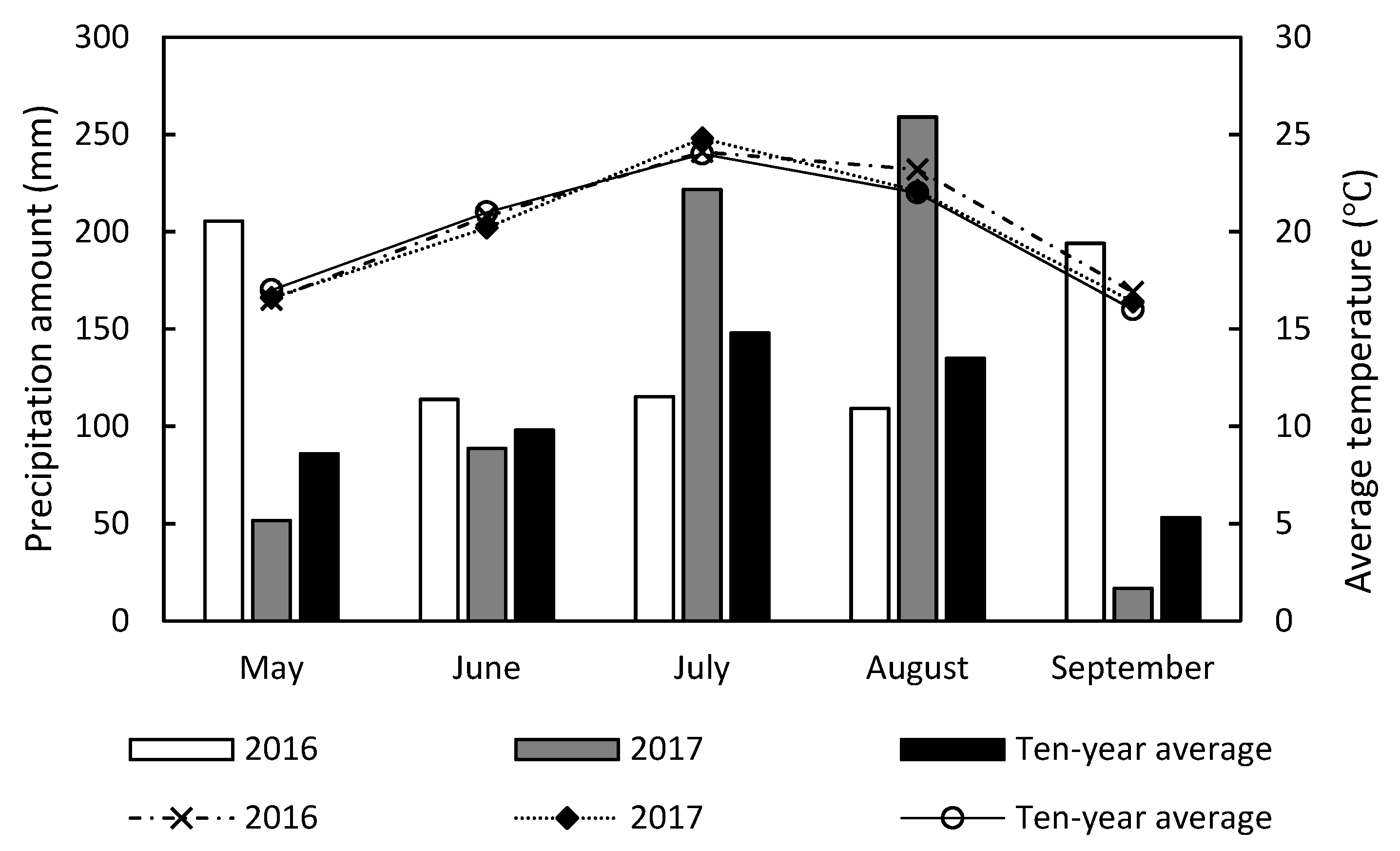
2.1. Materials and Cultivation of Plants
2.2. Number and Diameter of Nodal Roots
2.3. Root Length Density (RLD)
2.4. Root Bleeding Sap Intensity, Nitrate Nitrogen, Ammonium Nitrogen, Potassium, and Free Amino Acid Concentration of Root Bleeding Sap
2.5. Natural Root Lodging Rate and Root Failure Moment (Rfm)
2.6. Grain Yield
2.7. Statistical Analysis
3. Results
3.1. Number and Diameter
3.2. RLD
3.3. Root Bleeding Sap Intensity and Its Composition
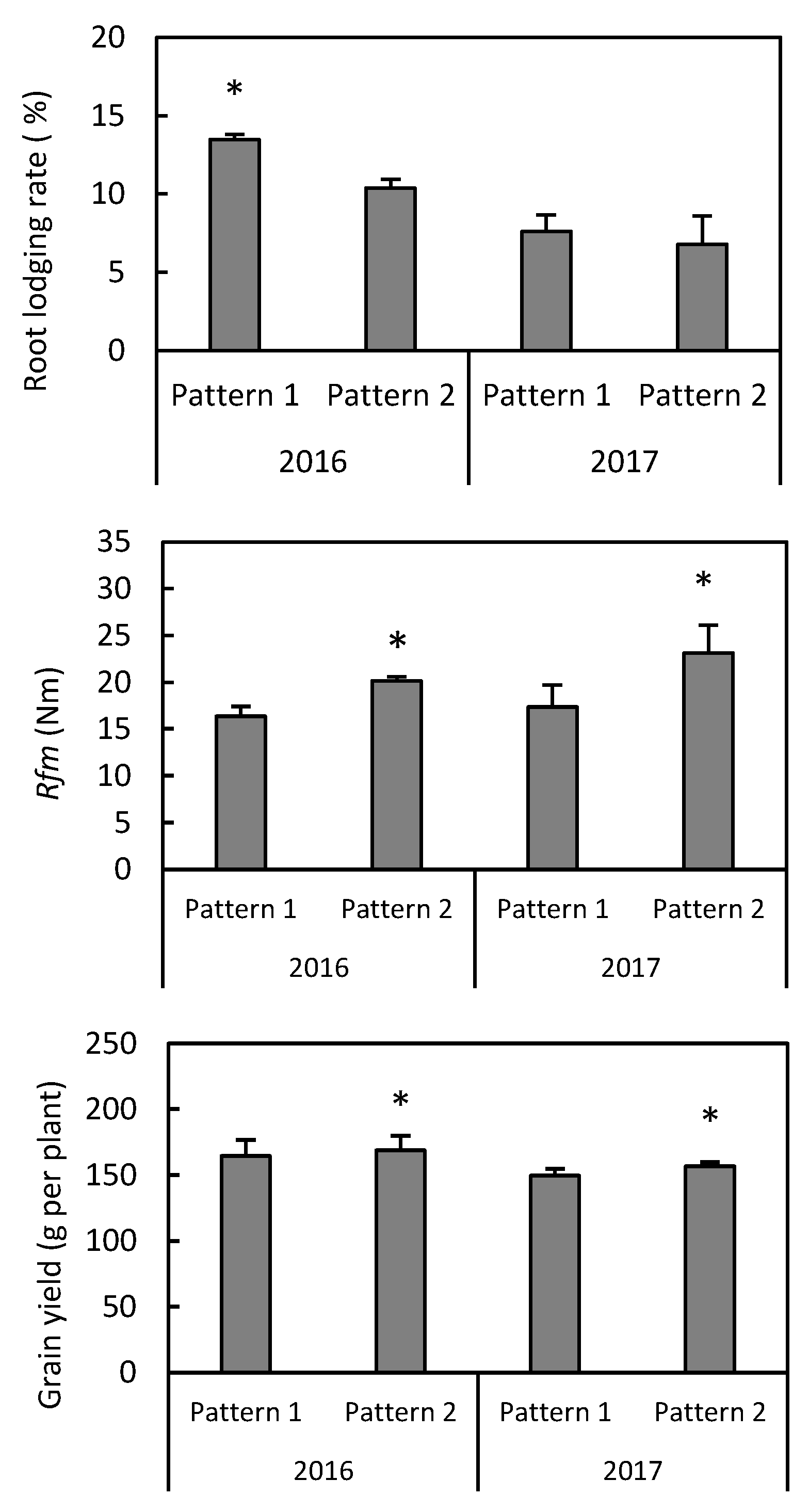
3.4. Grain Yield, Rfm, and Root Lodging Rate
3.5. Relationships between the Root Diameter and Root-Bleeding Intensity, the Root-Bleeding Intensity and Grain Yield, and the RLD and Root Lodging Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.H. The comparison about maize breeding ideas and technical level between China and American. Beijing Agric. 2007, 14, 13–16. [Google Scholar]
- Bian, D.; Jia, G.; Cai, L.; Ma, Z.; Eneji, A.E.; Cui, Y. Effects of tillage practices on root characteristics and root lodging resistance of maize. Field Crop. Res. 2016, 185, 89–96. [Google Scholar] [CrossRef]
- So, Y.S.; Adetimirin, V.O.; Kim, S.K. Observational study on the recovery from root lodging at flowering time and yield reduction in maize (Zea mays L.). Plant Bleeding Biotechnol. 2013, 1, 171–177. [Google Scholar] [CrossRef]
- Crook, M.J.; Ennos, A.R. The mechanics of root lodging in winter wheat Triticum aestivum L. J. Exp. Bot. 1993, 44, 1219–1224. [Google Scholar] [CrossRef]
- De Dorlodot, S.; Forster, B.; Pagès, L.; Price, A.; Tuberosa, R.; Draye, X. Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007, 12, 474–481. [Google Scholar] [CrossRef]
- Wu, K.; Zhou, F.; Zhou, S.; Zhang, X.; Wu, B. Enhancing root lodging resistance of maize with twin plants in wide-narrow rows: A case study. Plant Prod. Sci. 2020, 23, 286–296. [Google Scholar] [CrossRef]
- Liu, S.; Song, F.; Liu, F.; Zhu, X.; Xu, H. Effect of planting density on root lodging resistance and its relationship to nodal root growth characteristics in maize (Zea mays L.). J. Agric. Sci. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Berry, P.M.; Griffin, J.M.; Sylvester-Bradley, R.; Scott, R.K.; Spink, J.H.; Baker, C.J.; Clare, R.W. Controlling plant form through husbandry to minimize lodging in wheat. Field Crop. Res. 2000, 67, 59–81. [Google Scholar] [CrossRef]
- Sharratt, B.S.; McWilliams, D.A. Microclimatic and rooting characteristics of narrow-row versus conventional-row corn. Agron. J. 2005, 97, 1129–1135. [Google Scholar] [CrossRef]
- San-Oh, Y.; Sugiyama, T.; Yoshita, D.; Ookawa, T.; Hirasawa, T. The effect of planting pattern on the rate of photosynthesis and related processes during ripening in rice plants. Field Crop. Res. 2006, 96, 113–124. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Zhai, W.; Tong, Z.; Shen, T.; Li, Y. Controlled-release nitrogen fertilizer improved lodging resistance and potassium and silicon uptake of direct-seeded rice. Crop Sci. 2019, 59, 2733–2740. [Google Scholar] [CrossRef]
- Noguchi, A.; Kageyama, M.; Shinmachi, F.; Schmidhalter, U.; Hasegawa, I. Potential for using plant xylem sap to evaluate inorganic nutrient availability in soil: I. Influence of inorganic nutrients present in the rhizosphere on those in the xylem sap of Luffa cylindrica Roem. Soil Sci. Plant Nutr. 2005, 51, 333–341. [Google Scholar] [CrossRef]
- Guan, D.; Al-Kaisi, M.M.; Zhang, Y. 2Tillage practices affect biomass and grain yield through regulating root growth, root-bleeding sap and nutrients uptake in summer maize. Field Crop. Res. 2014, 157, 89–97. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Y.; Wang, Z.; Yang, J.; Zhang, J. Morphological and physiological traits of roots and their relationships with shoot growth in “super” rice. Field Crop. Res. 2009, 113, 31–40. [Google Scholar] [CrossRef]
- Cui, X.; Dong, Y.; Gi, P.; Wang, H.; Xu, K.; Zhang, Z. Relationship between root vigour, photosynthesis and biomass in soybean cultivars during 87 years of genetic improvement in the northern China. Photosynthetica 2016, 54, 81–86. [Google Scholar] [CrossRef]
- Kato, C.; Ohshima, N.; Kamada, H.; Satoh, S. Enhancement of the inhibitory activity for greening in xylem sap of squash root with waterlogging. Plant Physiol. Biochem. 2001, 39, 513–519. [Google Scholar] [CrossRef]
- Wang, H.; Xu, R.; Li, Y.; Yang, L.; Shi, W.; Liu, Y.; Chang, S.; Hou, F.; Jia, Q. Enhance root-bleeding sap flow and root lodging resistance of maize under a combination of nitrogen strategies and farming practices. Agric. Water Manag. 2019, 224, 105742. [Google Scholar] [CrossRef]
- Liang, X.; Guo, F.; Feng, Y.; Zhang, J.; Yang, S.; Meng, J.; Li, X.; Wan, S. Single-seed sowing increased pod yield at a reduced seeding rate by improving root physiological stage of Arachis hypogaea. J. Integr. Agric. 2020, 19, 1019–1032. [Google Scholar] [CrossRef]
- Tian, C.; Han, J.; Li, J.; Zhen, G.; Liu, Y.; Lu, Y.; Wang, Y.; Wang, Y. Effects of row direction and row spacing on maize leaf senescence. PLoS ONE 2019, 14, e0215330. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Song, F.; Qi, X.; Li, X.; Zhu, X. Responses of leaf architecture traits and yield in maize to different row orientation and planting patterns in northeast China. Rom. Agric. Res. 2017, 34, 243–254. [Google Scholar]
- Liu, T.; Song, S.; Zhu, X. Light interception and radiation use efficiency response to narrow-wide row planting patterns in maize. Aust. J. Crop Sci. 2012, 6, 506–513. [Google Scholar]
- Shao, H.; Xia, T.; Wu, D.; Chen, F.; Mi, G. Root growth and root system architecture of field-grown maize in response to high planting density. Plant Soil 2018, 430, 395–411. [Google Scholar] [CrossRef]
- Durieux, R.P.; Kamprath, E.J.; Jackson, W.A.; Moll, R.H. Root distribution of corn: The effect of nitrogen fertilization. Agron. J. 1994, 86, 958–962. [Google Scholar] [CrossRef]
- Böhm, W. Methods of Studying Root Systems. Ecological Studies; Springer: Berlin, Germany, 1979; Volume 33, pp. 20–25. [Google Scholar]
- He, Z.; Xu, B.; Liu, B.; Yao, B.; Wang, H.; Chen, Z.; Li, D.; Bai, Z.; Zhang, Z. Relationship between photosynthesis, bleeding-sap mass, and bleeding components in maize hybrids and corresponding parents in northern China. Photosynthetica 2019, 57, 698–704. [Google Scholar] [CrossRef]
- van Staden, J.F.; Taljaard, R.E. Determination of ammonia in water and industrial effluent streams with the indophenol blue method using sequential injection analysis. Anal. Chim. Acta 1997, 344, 281–289. [Google Scholar] [CrossRef]
- Pinthus, M.J. Lodging in wheat, barley and oats: The phenomenon, its causes, and preventive measures. Adv. Agron. 1974, 25, 209–263. [Google Scholar]
- Fouéré, A.; Pellerin, S.; Duparque, A. A portable electronic device for evaluating root lodging resistance in maize. Agron. J. 1995, 87, 1020–1024. [Google Scholar] [CrossRef]
- Yu, G.; Zhuang, J.; Nakayana, K.; Jin, Y. Root water uptake and profile soil water as affected by vertical root distribution. Plant Ecol. 2007, 189, 15–30. [Google Scholar] [CrossRef]
- Nakamoto, T. Effect of soil water content on the gravitropic behavior of nodal roots in maize. Plant Soil 1993, 152, 261–267. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Li, Q.; Ma, D.; Lu, H.; Feng, W.; Xie, Y.; Zhu, Y.; Guo, T. Effects of different irrigation and nitrogen regimes on root growth and its correlation with above-ground plant parts in high-yielding wheat under field conditions. Field Crop. Res. 2014, 165, 138–149. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, B.; Gao, J.; Sun, J. Effects of different management systems on root distribution of maize. Can. J. Plant Sci. 2015, 95, 21–28. [Google Scholar] [CrossRef]
- Widdicombe, W.D.; Thelen, K.D. Row width and plant density effects on corn grain production in the northern Corn Belt. Agron. J. 2002, 94, 1020–1023. [Google Scholar] [CrossRef]
- Pedersen, P.; Lauer, J.G. Corn and soybean responses to rotation sequence, row spacing, and tillage system. Agron. J. 2003, 95, 965–971. [Google Scholar] [CrossRef]
- Ansari, T.H.; Yamamoto, Y.; Yoshida, T.; Sakagami, K.; Miyazaki, A. Relation between bleeding rate during panicle formation stage and sink size in rice plant. Soil Sci. Plant Nutr. 2004, 50, 57–66. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Cai, Q.; Wang, Z.; Cao, J.; Yu, T.; Xie, T. Exogenous diethyl aminoethyl hexanoate ameliorates low temperature stress by improving nitrogen metabolism in maize seedlings. PLoS ONE 2020, 15, e0232294. [Google Scholar] [CrossRef] [PubMed]
- Husted, S.; Hebbern, C.A.; Mattsson, M.; Schjoerring, J.K. A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol. Plant 2000, 109, 167–179. [Google Scholar] [CrossRef]
- Tiwari, P.; Srivastava, D.; Chauhan, A.S.; Indoliya, Y.; Singh, P.K.; Tiwari, S.; Fatima, T.; Mishra, S.K.; Dwivedi, S.; Agarwal, L.; et al. Root system architecture, physiological analysis and dynamic transcriptomics unravel the drought-responsive traits in rice genotypes. Ecotox. Environ. Saf. 2020, 207, 111252. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Jian, S.; Li, X.; Wang, Y. Wide–Narrow Row Planting Pattern Increases Root Lodging Resistance by Adjusting Root Architecture and Root Physiological Activity in Maize (Zea mays L.) in Northeast China. Agriculture 2021, 11, 517. https://doi.org/10.3390/agriculture11060517
Liu S, Jian S, Li X, Wang Y. Wide–Narrow Row Planting Pattern Increases Root Lodging Resistance by Adjusting Root Architecture and Root Physiological Activity in Maize (Zea mays L.) in Northeast China. Agriculture. 2021; 11(6):517. https://doi.org/10.3390/agriculture11060517
Chicago/Turabian StyleLiu, Shengqun, Shulian Jian, Xiangnan Li, and Yang Wang. 2021. "Wide–Narrow Row Planting Pattern Increases Root Lodging Resistance by Adjusting Root Architecture and Root Physiological Activity in Maize (Zea mays L.) in Northeast China" Agriculture 11, no. 6: 517. https://doi.org/10.3390/agriculture11060517
APA StyleLiu, S., Jian, S., Li, X., & Wang, Y. (2021). Wide–Narrow Row Planting Pattern Increases Root Lodging Resistance by Adjusting Root Architecture and Root Physiological Activity in Maize (Zea mays L.) in Northeast China. Agriculture, 11(6), 517. https://doi.org/10.3390/agriculture11060517






