Impact of Ozonisation Time and Dose on Health Related and Microbiological Properties of Rapanui Tomatoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Treatmens
2.3. Titratable Acidity (TA) and Total Soluble Solids (TSS)
2.4. Total Phenolic Content (TPC)
2.5. Total Flavonoid Content (TFC)
2.6. Total Carotenoids and Lycopene Content
2.7. Vitamin C Content Analysis
2.8. Total Antioxidant Activity (TAA)
2.9. Colour Measurements
2.10. Firmness
2.11. Evaluation of Fruit Weight Losses during the Storage Time
2.12. Microbial Analysis (Yeasts and Moulds)
2.13. Statistical Analysis
3. Results and Discussion
3.1. Titratable Acidity (TA) and Total Soluble Solids (TSS)
3.2. Total Phenolic, Total Flavonoid, Lycopene, Carotenoids and Vitamin C Content Determination
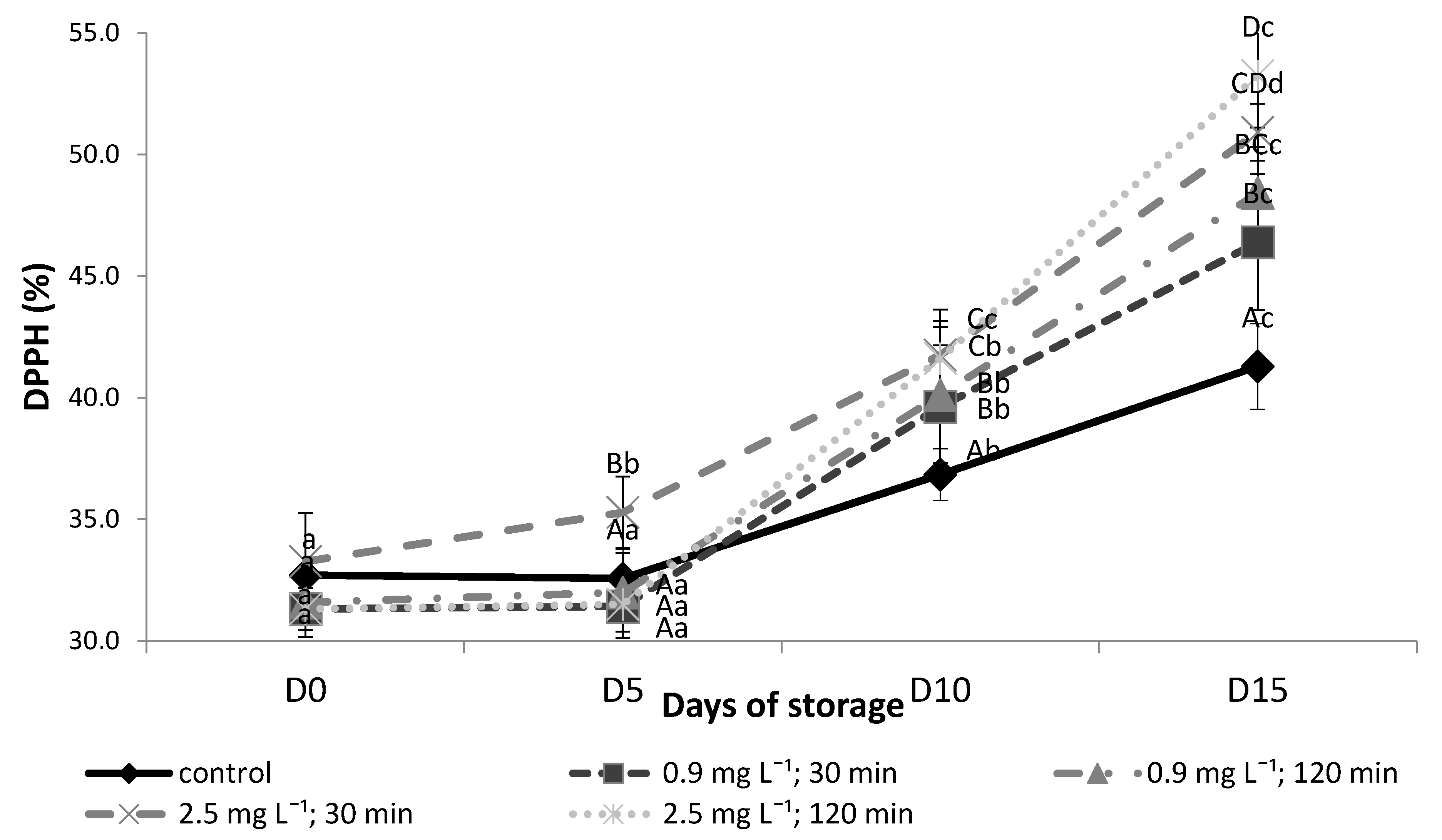
3.3. Total Antioxidant Activity (TAA)
3.4. Colour Measurements
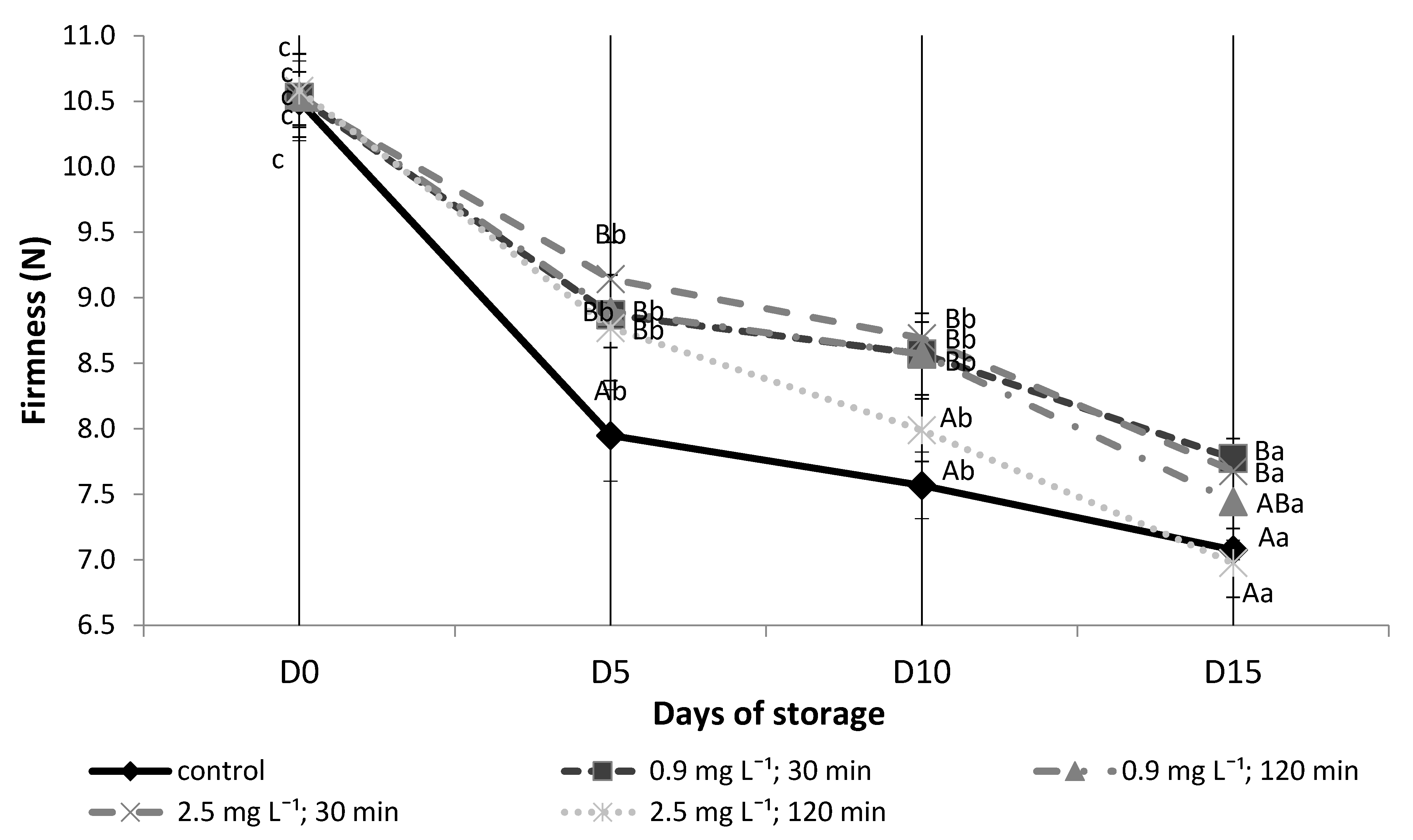
3.5. Firmness
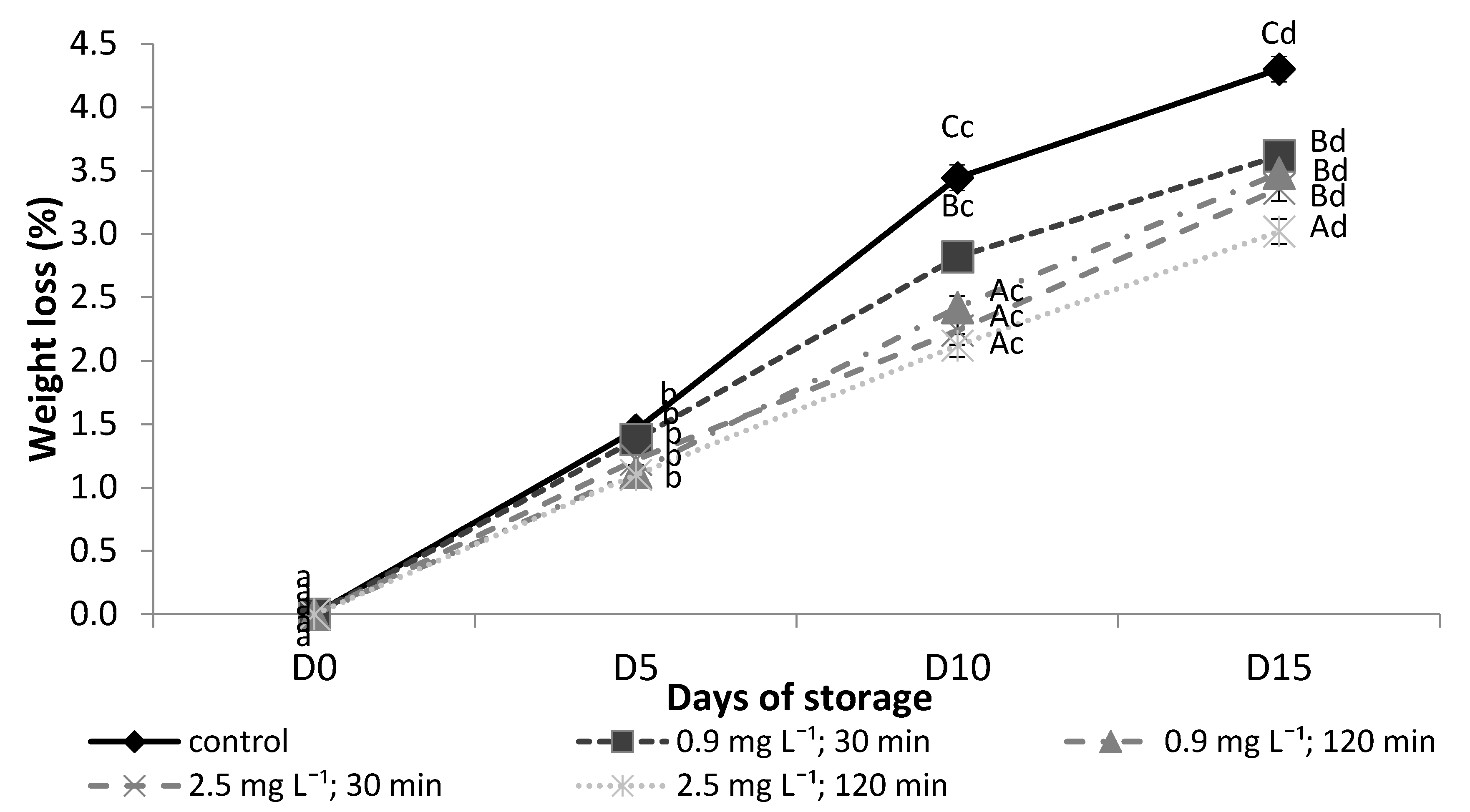
3.6. Assessment of Tomatoes Weight Losses during Storage
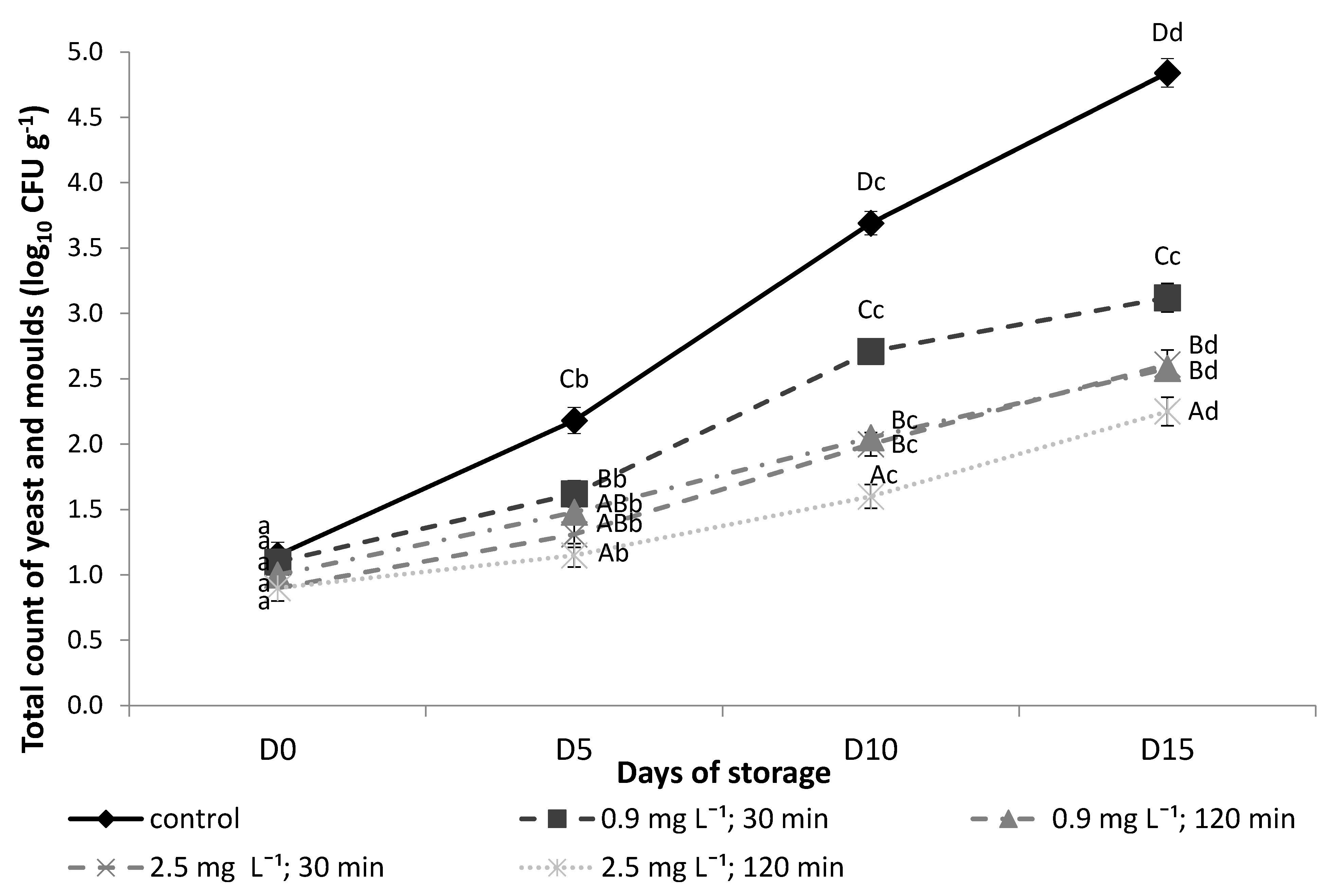
3.7. Microbiological Analysis (Yeasts and Moulds)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakir, S.; Capanoglu, E.; Hall, R.D.; de Vos, R.C.H. Variation in secondary metabolites in a unique set of tomato accessions collected in Turkey. Food Chem. 2020, 317, 126406. [Google Scholar] [CrossRef] [PubMed]
- Demiray, E.; Tulek, Y.; Yilmaz, Y. Degradation kinetics of lycopene, b-carotene and ascorbic acid in tomatoes during hot air drying. LWT-Food Sci. Technol. 2013, 50, 172–176. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Phytochemical composition and antioxidant activity of high-lycopene tomato (Solanum lycopersicum L.) cultivars grown in Southern Italy. Sci. Hortic. 2011, 127, 255–261. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, D.S.; Kozukue, N.; Kim, H.J.; Nishitani, Y.; Mizuno, M.; Levin, C.E.; Friedman, M. Protein, free amino acid, phenolic, β-carotene, and lycopene content, and antioxidative and cancer cell inhibitory effects of 12 greenhouse-grown commercial cherry tomato varieties. J. Food Compos. Anal. 2014, 34, 115–127. [Google Scholar] [CrossRef]
- Xu, S.; Sun, X.; Lu, H.; Yang, H.; Ruan, Q.; Huang, H.; Chen, M. Detecting and monitoring the flavor of tomato (Solanum lycopersicum) under the impact of postharvest handlings by physicochemical parameters and electronic nose. Sensors 2018, 18, 1847. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Rigano, M.M.; Calafiore, R.; Frusciante, L.; Barone, A. Enhancing the human-promoting effects of tomato fruit for biofortified food. Mediat. Inflamm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Tanambell, H.; Quek, S.Y.; Bishop, K.S. Screening of in vitro health benefits of tangerine tomatoes. Antioxidants 2019, 8, 230. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Cătoi, A.-F.; Vodnar, D.C. Screening of ten tomato varieties processing waste for bioactive components and their related antioxidant and antimicrobial activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colitz, G.A.; Willett, W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef]
- Paur, I.; Lilleby, W.; Bohn, S.K.; Hulander, E.; Klein, W.; Vlatkovic, L.; Axcrona, K.; Bolstad, N.; Bjøro, T.; Laake, P.; et al. Tomato based randomized controlled trial in prostate cancer patients: Effect on PSA. Clin. Nutr. 2017, 36, 672–679. [Google Scholar] [CrossRef]
- Giovannucci, E. Tomato base-products, lycopene and cancer. Review of epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W.C. A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002, 94, 391–398. [Google Scholar] [CrossRef]
- Ingallina, C.; Maccelli, A.; Spano, M.; Di Matteo, G.; Di Sotto, A.; Giusti, A.M.; Vinci, G.; Di Giacomo, S.; Rapa, M.; Ciano, S.; et al. Chemico-biological characterization of Torpedino Di Fondi® tomato fruits: A comparison with San Marzano Cultivar at two ripeness stages. Antioxidants 2020, 9, 1027. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Sumalan, R.L. The antioxidant profile evaluation of some tomato Landraces with soil salinity tolerance correlated with high nutraceuticaland functional value. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as potential source of natural additives for meat industry. A review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.S.N.; Ali, M.; Lee, W.H.; Cho, S.I.; Chung, S.O. Physicochemical quality changes in tomatoes during delayed cooling and storage in a controlled chamber. Agriculture 2020, 10, 196. [Google Scholar] [CrossRef]
- Javanmardi, J.; Kubota, C. Variation of lycopene, antioxidant activity, total soluble solids and weight loss of tomato during postharvest storage. Postharvest Biol. Technol. 2006, 41, 151–155. [Google Scholar] [CrossRef]
- Sachadyn-Król, M.; Materska, M.; Chilczuk, B. Ozonation of hot red pepper fruits increases their antioxidant activity and changes some antioxidant contents. Antioxidants 2019, 8, 356. [Google Scholar] [CrossRef] [PubMed]
- Marchica, A.; Cotrozzi, L.; Detti, R.; Lorenzini, G.; Pellegrini, E.; Petersen, M.; Nali, C. The biosynthesis of phenolic compounds is an integrated defence mechanism to prevent ozone injury in salvia officinalis. Antioxidants 2020, 9, 1274. [Google Scholar] [CrossRef]
- Matłok, N.; Piechowiak, T.; Zardzewiały, M.; Gorzelany, J.; Balawejder, M. Effects of ozone treatment on microbial status and the contents of selected bioactive compounds in Origanum majorana L. plants. Plants 2020, 9, 1637. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, J.; Yin, C.; Li, G.; Jiang, Y.; Shan, Y. Effect of ozone treatment on flavonoid accumulation of Satsuma mandarin (Citrus unshiu Marc.) during ambient storage. Biomolecules 2019, 9, 821. [Google Scholar] [CrossRef]
- Zardzewiały, M.; Matlok, N.; Piechowiak, T.; Gorzelany, J.; Balawejder, M. Ozone treatment as a process of quality improvement method of Rhubarb (Rheum rhaponticum L.) petioles during storage. Appl. Sci. 2020, 10, 8282. [Google Scholar] [CrossRef]
- Aguayo, E.; Escalona, V.; Silveira, A.C.; Artés, F. Quality of tomato slices disinfected with ozonated water. Food Sci. Technol. Int. 2013, 20, 227–235. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. J. Food Compos. Anal. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Lee, H.S. Characterization of carotenoids in juice of red navel orange (Cara Cara). J. Agric. Food Chem. 2001, 49, 2563–2568. [Google Scholar] [CrossRef]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvent. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef]
- Onopiuk, A.; Półtorak, A.; Wyrwisz, J.; Moczkowska, M.; Stelmasiak, A.; Lipińska, A.; Szpicer, A.; Zalewska, M.; Zaremba, R.; Kuboń, M.; et al. Impact of ozonisation on pro-health properties and antioxidant capacity of ‘Honeoye’ strawberry fruit. CyTA–J. Food 2017, 15, 58–64. [Google Scholar]
- Lu, C.; Ding, J.; Park, H.K.; Feng, H. High intensity ultrasound as a physical elicitor affects secondary metabolites and antioxidant capacity of tomato fruits. Food Control 2020, 113, 107176. [Google Scholar] [CrossRef]
- Mauro, R.P.; Agnello, M.; Onofri, A.; Leonardi, C.; Giuffrida, F. Scion and rootstock differently influence growth, yield and quality characteristics of cherry tomato. Plants 2020, 9, 1725. [Google Scholar] [CrossRef]
- Aragüez, L.; Colombo, A.; Borneo, R.; Aguirre, A. Active packaging from triticale flour films for prolonging storage life of cherry tomato. Food Packag. Shelf Life 2020, 25, 100520. [Google Scholar] [CrossRef]
- Onopiuk, A.; Półtorak, A.; Moczkowska, M.; Szpicer, A.; Wierzbicka, A. The impact of ozone on health-promoting, microbiological and colour properties of Rubus ideaus raspberries. CyTA–J. Food. 2017, 15, 563–573. [Google Scholar] [CrossRef]
- López-Palestina, C.U.; Aguirre-Mancilla, C.L.; Raya-Pérez, J.C.; Ramírez-Pimentel, J.G.; Gutiérrez-Tlahque, J.; Hernández-Fuentes, A.D. The effect of an edible coating with tomato oily extract on the physicochemical and antioxidant properties of garambullo (Myrtillocactus geometrizans) fruits. Agronomy 2018, 8, 248. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Xyrafis, E.; Polyzos, N.; Antoniadis, V.; Barros, L.; Ferreira, I.C.F.R. The optimization of nitrogen fertilization regulates crop performance and quality of processing tomato (Solanum lycopersicum L. cv. Heinz 3402). Agronomy 2020, 10, 715. [Google Scholar] [CrossRef]
- Inestroza-Lizardo, C.; Mattiuz, B.H.; da Silva, J.P.; Galati, V.C.; Voigt, V. Hyperbaric pressure at room temperature increases post-harvest preservation of the tomato cultivar ‘Débora’. Sci. Hortic. 2018, 228, 103–112. [Google Scholar] [CrossRef]
- Liu, L.H.; Zabaras, D.; Bennett, L.E.; Aguas, P.; Woonton, B.W. Effects of UV-C, red light and sun light on the carotenoid content and physical qualities of tomatoes during post-harvest storage. Food Chem. 2009, 115, 495–500. [Google Scholar] [CrossRef]
- Odriozola-Serrano, J.; Soliva-Fortuny, R.; Martin-Bellono, O. Effect of minimal processing on bioactive compounds and colour attributes of fresh-cut tomatoes. LWT-Food Sci. Technol. 2008, 41, 217–226. [Google Scholar] [CrossRef]
- Zou, J.; Chen, J.; Tang, N.; Gao, Y.; Hong, M.; Wei, W.; Cao, H.; Jian, W.; Li, N.; Deng, W.; et al. Transcriptome analysis of aroma volatile metabolism change in tomato (Solanum lycopersicum) fruit under different storage temperatures and 1-MCP treatment. Postharvest Biol. Technol. 2018, 135, 57–67. [Google Scholar] [CrossRef]
- Buendía-Moreno, L.; Soto-Jover, S.; Ros-Chumillas, M.; Antolinos, V.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; López-Gómez, A. Innovative cardboard active packaging with a coating including encapsulated essential oils to extend cherry tomato shelf life. LWT-Food Sci. Technol. 2019, 116, 108584. [Google Scholar] [CrossRef]
- Tigist, A.; Workneh, T.S.; Woldetsadik, K. Effects of variety on the quality of tomato stored under ambient conditions. J. Food Sci. Technol. 2011, 50, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Bravo, S.; García-Alonso, J.; Martín-Pozuelo, G.; Gomez, V.; Santaella, M.; Navarro-Gonzalez, I.; Periago, M.J. The influence of post-harvest UV-C hormesis on lycopene, b-carotene, and phenolic content and antioxidant activity of breaker tomatoes. Food Res. 2012, 49, 296–302. [Google Scholar] [CrossRef]
- Del Giudice, R.; Raiola, A.; Tenore, G.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Ding, X.X.; Guo, Y.; Ni, Y.N.; Kokot, S.A. Novel NIR spectroscopic method for rapid analyses of lycopene, total acid, sugar, phenols and antioxidant activity in dehydrated tomato samples. Vib. Spectrosc. 2016, 82, 1–9. [Google Scholar] [CrossRef]
- Glowacz, M.; Rees, D. Exposure to ozone reduces postharvest quality loss in red and green chilli peppers. Food Chem. 2016, 210, 305–310. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Borland, A.; Singleton, I.; Barnes, J. Impact of atmospheric ozone-enrichment on quality-related attributes of tomato fruit. Postharvest Biol. Technol. 2007, 45, 317–325. [Google Scholar] [CrossRef]
- Gonzali, S.; Perata, P. Anthocyanins from purple tomatoes as novel antioxidants to promote human health. Antioxidants 2020, 9, 1017. [Google Scholar] [CrossRef]
- Onopiuk, A.; Półtorak, A.; Wojtasik-Kalinowska, I.; Szpicer, A.; Marcinkowska-Lesiak, M.; Pogorzelski, G.; Wierzbicka, A. Impact of the storage atmosphere enriched with ozone on the quality of Lycopersicon esculentum tomatoes. J. Food Process. Pres. 2019, 43, e14252. [Google Scholar] [CrossRef]
- Klunklin, W.; Savage, G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods 2017, 6, 56. [Google Scholar] [CrossRef]
- Park, M.H.; Sangwanangkul, P.; Baek, D.R. Changes in carotenoid and chlorophyll content of black tomatoes (Lycopersicone sculentum L.) during storage at various temperatures. Saudi J. Biol. Sci. 2018, 25, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kopec, R.E.; Riedel, K.M.; Harrison, E.H.; Curley, R.W.; Hruszkewycz, D.P.; Clinton, S.K.; Schwartz, S.J. Identification and quantification of apo-lycopenals in fruits, vegetables and human plasma. J. Agric. Food Chem. 2010, 58, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Ölmez, H.; Akbas, M.Y. Optimization of ozone treatment of fresh-cut green leaf lettuce. J. Food Eng. 2009, 90, 487–494. [Google Scholar] [CrossRef]
- Georgé, S.; Tourniaire, F.; Gautier, H.; Goupy, P.; Rock, E.; Caris-Veyrat, C. Changes in the contents of carotenoids, phenolic compounds and vitamin C during technical processing and lyophilisation of red and yellow tomatoes. Food Chem. 2011, 124, 1603–1611. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sérino, S.; Costagliola, G.; Gomez, L. Lyophilized tomato plant material: Validation of a reliable extraction method for the analysis of vitamin C. J. Food Compos. Anal. 2019, 81, 37–45. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Mirdehghan, S.H.; Valero, D. Bioactive compounds in tomato fruit and its antioxidant activity as affected by incorporation of aloe, eugenol, and thymol in fruit package during storage. Int. J. Food Prop. 2017, 20, 1798–1806. [Google Scholar]
- Rodoni, L.; Casadei, N.; Concellon, A.; Alicia, A.R.C.; Vicente, A.R. Effect of short-term ozone treatments on tomato (Solanum lycopersicum L.) fruit quality and cell wall degradation. J. Agric. Food Chem. 2010, 58, 594–599. [Google Scholar]
- Chen, H.; Zhang, Y.; Zhong, Q. Potential of acidified sodium benzoate as an alternative wash solution of cherry tomatoes: Changes of quality, background microbes, and inoculated pathogens during storage at 4 and 21 °C post-washing. Food Microbiol. 2019, 82, 111–118. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Xiao, Y.; Joung, J.; Kim, M.; Singh, S.; Lee, Y.S. Alkaline halloysite nanotubes/low density polyethylene nanocomposite films with increased ethylene absorption capacity: Applications in cherry tomato packaging. Food Packag. Shelf Life 2020, 25, 100533. [Google Scholar] [CrossRef]
- Causse, M.; Friguet, C.; Coiret, C.; Lépicier, M.; Navez, B.; Lee, M.; Holthuysen, N.; Sinesio, F.; Moneta, E.; Grandillo, S. Consumer preferences for fresh tomato at the European scale: A common segmentation on taste and firmness. J. Food Sci. 2010, 75, S531–S541. [Google Scholar] [CrossRef] [PubMed]
- Bertin, N.; Génard, M. Tomato quality as influenced by preharvest factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- García, M.; Casariego, A.; Díaz, R.; Roblejo, L. Effect of edible chitosan/zeolite coating on tomatoes quality during refrigerated storage. Emir. J. Food Agric. 2014, 26, 238–246. [Google Scholar]
- Wani, S.; Barnes, J.; Singleton, I. Investigation of potential reasons for bacterial survival on ‘ready-to-eat’ leafy produce during exposure to gaseous ozone. Postharvest Biol. Technol. 2016, 111, 185–190. [Google Scholar] [CrossRef]
| Chemical Quality Parameters | Concentration and Ozonisation Time | Storage Period (Days) | ||||
|---|---|---|---|---|---|---|
| D0 | D5 | D10 | D15 | |||
| Total soluble solids (%) | control | 4.10 A ± 0.10 | 4.27 Aa ± 0.15 | 4.83 Ba ± 0.12 | 4.40 Aa ± 0.10 | |
| 0.9 mg L−1 | 30 min | 4.23 A ± 0.06 | 4.50 ABab ± 0.10 | 5.10 Cb ± 0.10 | 4.81 BCb ± 0.17 | |
| 120 min | 4.07 A ± 0.15 | 4.57 Bab ± 0.15 | 5.27 Cb ± 0.06 | 4.80 Bb ± 0.10 | ||
| 2.5 mg L−1 | 30 min | 4.13 A ± 0.06 | 4.77 Bbc ± 0.12 | 5.33 Cbc ± 0.12 | 5.03 BCb ± 0.15 | |
| 120 min | 4.27 A ± 0.15 | 4.93 Bc ± 0.06 | 5.57 Cc ± 0.06 | 5.10 Bb ± 0.10 | ||
| Titratable acidity (%) | control | 0.40 C ± 0.02 | 0.37 BCb ± 0.04 | 0.31 Bc ± 0.01 | 0.25 Ab ± 0.02 | |
| 0.9 mg L−1 | 30 min | 0.41 C ± 0.02 | 0.34 Bab ± 0.02 | 0.29 ABbc ± 0.02 | 0.26 Ab ± 0.02 | |
| 120 min | 0.41 C ± 0.03 | 0.32 Bab ± 0.01 | 0.28 ABbc ± 0.03 | 0.23 Ab ± 0.02 | ||
| 2.5 mg L−1 | 30 min | 0.43 C ± 0.01 | 0.29 Ba ± 0.03 | 0.24 ABab ± 0.02 | 0.21 Aab ± 0.02 | |
| 120 min | 0.43 C ± 0.01 | 0.29 Bab ± 0.04 | 0.21 Aa ± 0.01 | 0.17 Aa ± 0.02 | ||
| Chemical Quality Parameters | Concentration and Ozonisation Time | Storage Period (Days) | ||||
|---|---|---|---|---|---|---|
| D0 | D5 | D10 | D15 | |||
| Total phenolic content (TPC) mg kg−1 GAE | control | 296.9 A ± 6.9 | 331.4 Ba ± 6.4 | 321.4 Ba ± 10.8 | 288.9 Aa ± 8.1 | |
| 0.9 mg L−1 | 30 min | 287.5 A ± 11.6 | 349.2 Cab ± 11.7 | 331.0 BCa ± 6.7 | 306.7 ABab ± 5.9 | |
| 120 min | 289.3 A ± 11.3 | 357.7 Cb ± 7.9 | 354.0 Cb ± 6.7 | 325.2 Bb ± 3.7 | ||
| 2.5 mg L−1 | 30 min | 276.9 A ± 12.1 | 334.1 Bab ± 12.8 | 328.7 Ba ± 2.3 | 299.4 Aa ± 11.9 | |
| 120 min | 279.2 A ± 14.1 | 334.6 Cab ± 8.1 | 329.7 BCa ± 9.9 | 304.5 ABab ± 8.0 | ||
| Total flavonoid content (TFC) mg kg−1 RE | control | 170.4 B ± 8.2 | 151.6 Ba ± 8.1 | 121.9 Aa ± 8.0 | 101.2 Aa ± 8.2 | |
| 0.9 mg L−1 | 30 min | 167.6 C ± 3.8 | 160.3 BCab ± 5.5 | 148.4 Bb ± 8.4 | 120.8 Aab ± 7.3 | |
| 120 min | 174.2 C ± 6.6 | 171.1 Cb ± 4.3 | 151.2 Bb ± 6.2 | 134.6 Ab ± 6.5 | ||
| 2.5 mg L−1 | 30 min | 179.5 B ± 8.9 | 166.3 Bab ± 6.8 | 142.7 Ab ± 7.4 | 127.0 Ab ± 8.4 | |
| 120 min | 170.6 B ± 7.7 | 159.0 Bab ± 4.2 | 137.9 Aab ± 6.2 | 122.9 Ab ± 8.9 | ||
| Lycopene (mg kg−1) | control | 159.7 Bb ± 4.2 | 173.0 Ca ± 3.6 | 152.3 Bb ± 3.8 | 129.3 Aa ± 3.2 | |
| 0.9 mg L−1 | 30 min | 143.7 Aa ± 3.2 | 190.0 Cbc ± 3.0 | 165.3 Bc ± 3.5 | 143.0 Ab ± 5.2 | |
| 120 min | 153.3 Ab ± 3.1 | 193.7 Cc ± 4.0 | 175.0 Bc ± 5.2 | 158.3 Ac ± 4.5 | ||
| 2.5 mg L−1 | 30 min | 157.8 Bb ± 4.8 | 181.3 Cab ± 4.2 | 138.0 Aa ± 6.6 | 128.3 Aa ± 3.5 | |
| 120 min | 150.5 Cab ± 3.5 | 173.0 Da ± 2.6 | 141.0 Bab ± 3.5 | 125.7 Aa ± 3.8 | ||
| Total carotenoids content (mg kg−1 β-CaE) | control | 231.7 Bab ± 2.5 | 258.7 Da ± 4.5 | 243.0 Cab ± 3.6 | 206.7 Acd ± 3.1 | |
| 0.9 mg L−1 | 30 min | 238.7 Bbc ± 3.5 | 270.7 Db ± 3.8 | 249.3 Cb ± 5.1 | 212.3 Ad ± 3.1 | |
| 120 min | 243.3 Bc ± 3.8 | 283.3 Cc ± 4.0 | 275.7 Cc ± 2.9 | 193.3 Aab ± 5.1 | ||
| 2.5 mg L−1 | 30 min | 232.3 Bab ± 4.0 | 269.0 Db ± 2.6 | 250.0 Cb ± 4.4 | 199.3 Abc ± 4.5 | |
| 120 min | 228.7 Ba ± 3.5 | 256.7 Ca ± 3.8 | 234.3 Ba ± 3.8 | 188.0 Aa ± 4.6 | ||
| Vitamin C (mg kg−1) | control | 210.8 A ± 3.2 | 229.2 B ± 3.2 | 210.8 A ± 6.4 | 201.7 A ± 8.4 | |
| 0.9 mg L−1 | 30 min | 216.3 B ± 6.4 | 234.7 C ± 8.4 | 203.5 AB ± 5.5 | 190.7 A ± 6.4 | |
| 120 min | 207.2 AB ± 3.2 | 225.5 B ± 9.5 | 205.3 A ± 6.4 | 192,5 A ± 9.5 | ||
| 2.5 mg L−1 | 30 min | 214.5 BC ± 5.5 | 232.8 C ± 3.2 | 205.3 AB ± 11.4 | 188.8 A ± 8.4 | |
| 120 min | 205.3 B ± 3.2 | 229.2 C ± 8.4 | 198.0 AB ± 5.5 | 185.2 A ± 8.4 | ||
| Group | Days in Storage | L* | a* | b* |
|---|---|---|---|---|
| Control | 0 | 44.40 d ± 1.06 | 25.56 ABa ± 1.05 | 20.22 c ± 0.65 |
| 5 | 42.01 c ± 1.69 | 27.64 b ± 0.53 | 18.32 b ± 0.63 | |
| 10 | 38.81 b ± 1.65 | 28.71 BCc ± 0.80 | 18.34 Cb ± 0.52 | |
| 15 | 36.83 ABa ± 0.62 | 30.32 Bd ± 0.75 | 16.96 Ba ± 0.42 | |
| 0.9 mg L−1; 30 min | 0 | 43.75 c ± 1.34 | 25.91 Ba ± 0.56 | 21.09 c ± 0.77 |
| 5 | 42.49 c ± 1.51 | 27.28 b ± 0.71 | 18.17 b ± 0.50 | |
| 10 | 39.08 b ± 1.07 | 28.35 ABCc ± 0.63 | 17.90 BCb ± 0.42 | |
| 15 | 36.22 Aa ± 0.62 | 29.61 ABd ± 1.03 | 16.20 Aa ± 0.50 | |
| 0.9 mg L−1; 120 min | 0 | 44.23 d ± 1.48 | 25.52 ABa ± 0.72 | 20.69 d ± 0.66 |
| 5 | 42.21 c ± 1.74 | 27.28 b ± 0.74 | 18.35 c ± 0.58 | |
| 10 | 39.18 b ± 1.17 | 27.81 ABb ± 0.66 | 17.51 Bb ± 0.47 | |
| 15 | 36.35 ABa ± 0.91 | 29.33 ABc ± 0.85 | 16.04 Aa ± 0.41 | |
| 2.5 mg L−1; 30 min | 0 | 45.03 c ± 1.63 | 25.42 ABa ± 0.79 | 20.57 c ± 0.70 |
| 5 | 42.37 b ± 1.28 | 27.06 b ± 0.46 | 18.79 b ± 0.50 | |
| 10 | 38.52 a ± 0.62 | 27.53 Ab ± 0.90 | 16.79 Aa ± 0.44 | |
| 15 | 37.38 Ba ± 1.17 | 29.31 ABc ± 0.96 | 16.58 ABa ± 0.72 | |
| 2.5 mg L−1; 120 min | 0 | 44.34 c ± 1.95 | 24.68 Aa ± 0.86 | 20.86 c ± 0.89 |
| 5 | 41.88 b ± 1.76 | 27.57 b ± 0.87 | 18.19 b ± 0.34 | |
| 10 | 38.58 a ± 0.80 | 29.25 Cc ± 0.53 | 17.87 BCb ± 0.62 | |
| 15 | 37.10 ABa ± 0.60 | 29.05 Ac ± 0.69 | 16.41 ABa ± 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onopiuk, A.; Szpicer, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A.; Półtorak, A. Impact of Ozonisation Time and Dose on Health Related and Microbiological Properties of Rapanui Tomatoes. Agriculture 2021, 11, 428. https://doi.org/10.3390/agriculture11050428
Onopiuk A, Szpicer A, Wojtasik-Kalinowska I, Wierzbicka A, Półtorak A. Impact of Ozonisation Time and Dose on Health Related and Microbiological Properties of Rapanui Tomatoes. Agriculture. 2021; 11(5):428. https://doi.org/10.3390/agriculture11050428
Chicago/Turabian StyleOnopiuk, Anna, Arkadiusz Szpicer, Iwona Wojtasik-Kalinowska, Agnieszka Wierzbicka, and Andrzej Półtorak. 2021. "Impact of Ozonisation Time and Dose on Health Related and Microbiological Properties of Rapanui Tomatoes" Agriculture 11, no. 5: 428. https://doi.org/10.3390/agriculture11050428
APA StyleOnopiuk, A., Szpicer, A., Wojtasik-Kalinowska, I., Wierzbicka, A., & Półtorak, A. (2021). Impact of Ozonisation Time and Dose on Health Related and Microbiological Properties of Rapanui Tomatoes. Agriculture, 11(5), 428. https://doi.org/10.3390/agriculture11050428









