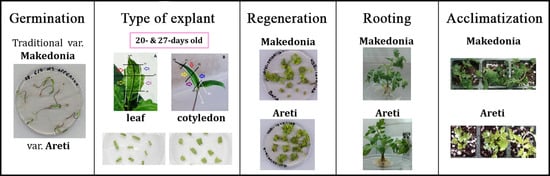
Development of a Simple and Low-Resource Regeneration System of Two Greek Tomato Varieties
Abstract
1. Introduction
2. Materials and Methods
3. Results
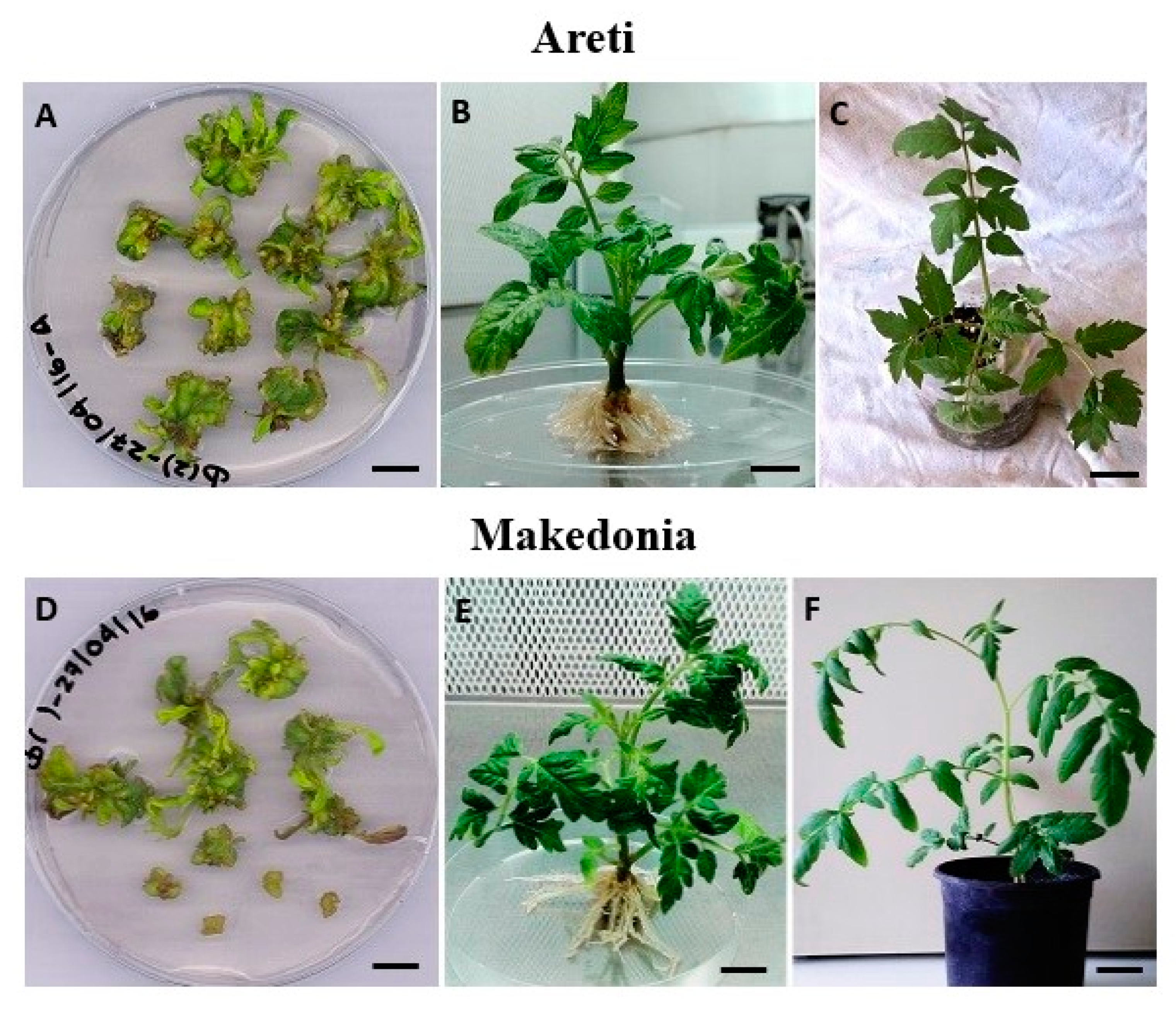
3.1. In Vitro Regeneration
3.2. In Vitro Rooting and Acclimatization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gebhardt, C. The historical role of species from the Solanaceae plant family in genetic research. Theor. Appl. Genet. 2016, 129, 2281–2294. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K. Tomato tolerance to abiotic stress: A review of most often engineered target sequences. Plant. Growth Regul. 2017, 83, 175–198. [Google Scholar] [CrossRef]
- Terzopoulos, P.J.; Bebeli, P.J. Phenotypic diversity in Greek tomato (Solanum lycopersicum L.) landraces. Sci. Hortic. 2010, 126, 138–144. [Google Scholar] [CrossRef]
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The tomato genome sequence provides insights into fleshy fruit evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Bolger, A.; Scossa, F.; Bolger, M.E.; Lanz, C.; Maumus, F.; Tohge, T.; Quesneville, H.; Alseekh, S.; Sørensen, I.; Lichtenstein, G.; et al. The genome of the stress-tolerant wild tomato species Solanum pennellii. Nat. Genet. 2014, 46, 1034–1038. [Google Scholar] [CrossRef]
- Terzopoulos, P.J.; Bebeli, P.J. DNA and morphological diversity of selected Greek tomato (Solanum lycopersicum L.) landraces. Sci. Hortic. 2008, 116, 354–361. [Google Scholar] [CrossRef]
- Cebolla-Cornejo, J.; Roselló, S.; Nuez, F. Phenotypic and genetic diversity of Spanish tomato landraces. Sci. Hortic. 2013, 162, 150–164. [Google Scholar] [CrossRef]
- Corrado, G.; Caramante, M.; Piffanelli, P.; Rao, R. Genetic diversity in Italian tomato landraces: Implications for the development of a core collection. Sci. Hortic. 2014, 168, 138–144. [Google Scholar] [CrossRef]
- García-Gonzáles, R.; Quiroz, K.; Carrasco, B.; Caligari, P. Plant tissue culture: Current status, opportunities and challenges. Cienc. Investig. Agrar. 2010, 37, 5–30. [Google Scholar] [CrossRef]
- Vikram, G.; Madhusudhan, K.; Srikanth, K.; Laxminarasu, M.; Swamy, N.R. Zeatin induced direct multiple shoots development and plant regeneration from cotyledon explants of cultivated tomato (Solanum lycopersicum L.). Aust. J. Crop. Sci. 2012, 6, 31–35. [Google Scholar]
- Koeda, S.; Takisawa, R.; Nabeshima, T.; Tanaka, Y.; Kitajima, A. Production of Tomato yellow leaf curl virus-free parthenocarpic tomato plants by leaf primordia-free shoot apical meristem culture combined with In vitro grafting. Hortic. J. 2015, 84, 327–333. [Google Scholar] [CrossRef]
- Koeda, S.; Matsumoto, S.; Matsumoto, Y.; Takisawa, R.; Nishikawa, K.; Kataoka, K. Medium-term in vitro conservation of virus-free parthenocarpic tomato plants. Vitr. Cell. Dev. Biol. Plant. 2018, 54, 392–398. [Google Scholar] [CrossRef]
- Fan, P.; Miller, A.M.; Schilmiller, A.L.; Liu, X.; Ofner, I.; Jones, A.D.; Zamir, D.; Last, R.L. In vitro reconstruction and analysis of evolutionary variation of the tomato acylsucrose metabolic network. Proc. Natl. Acad. Sci. USA 2016, 113, E239–E248. [Google Scholar] [CrossRef] [PubMed]
- Us-Camas, R.; Rivera-Solís, G.; Duarte-Aké, F.; De-la-Peña, C. In vitro culture: An epigenetic challenge for plants. Plant Cell. Tissue Organ Cult. 2014, 118, 187–201. [Google Scholar] [CrossRef]
- Kulus, D. Genetic resources and selected conservation methods of tomato. J. Appl. Bot. Food Qual. 2018, 91, 135–144. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. An introduction to plant cell culture: The future ahead. Methods Mol. Biol. 2012, 877, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dong, O.X.; Ronald, P.C. Genetic engineering for disease resistance in plants: Recent progress and future perspectives. Plant. Physiol. 2019, 180, 26–38. [Google Scholar] [CrossRef]
- Stavridou, E.; Τzioutziou, N.A.; Madesis, P.; Labrou, N.E.; Nianiou-Obeidat, I. Effect of different factors on regeneration and transformation efficiency of tomato (Lycopersicum esculentum) hybrids. Czech. J. Genet. Plant. Breed. 2019, 55, 120–127. [Google Scholar] [CrossRef]
- Senapati, S.K. A review on research progress on in vitro regeneration and transformation of tomato. Annu. Res. Rev. Biol. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Grigoriadis, I.; Nianiou-Obeidat, I.; Tsaftaris, A.S. Shoot regeneration and micrografting of micropropagated hybrid tomatoes. J. Hortic. Sci. Biotechnol. 2005, 80, 183–186. [Google Scholar] [CrossRef]
- Calinski, T.; Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach. Biometrics 1981, 37, 859. [Google Scholar] [CrossRef]
- Toothaker, L. Multiple Comparison Procedures. In Paper Series on Quantitative Applications in the Social Sciences, Series No. 07-089; Sage University: Newbury Park, CA, USA, 1993. [Google Scholar]
- Liza, L.N.; Ashrafuzzaman, M. In Vitro Growth Media Effect for Regeneration of Tomato (Lycopersicon esculentum) and Evaluation of the Salt Tolerance Activity of Callus. J. Agric. Sustain. 2013, 3, 132–143. [Google Scholar]
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T.; Kononowicz, A.K. Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant. Cell. Tissue Organ. Cult. 2015, 120, 881–902. [Google Scholar] [CrossRef]
- Harish, M.C.; Rajeevkumar, S.; Sathishkumar, R. Efficient in vitro callus induction and regeneration of different Tomato landraces of India. Asian J. Biotechnol. 2010, 2, 178–184. [Google Scholar] [CrossRef][Green Version]
- Shah, S.H.; Ali, S.; Jan, S.A.; Din, J.; Ali, G.M. Callus induction, in vitro shoot regeneration and hairy root formation by the assessment of various plant growth regulators in tomato (Solanum lycopersicum Mill.). J. Anim. Plant. Sci. 2015, 25, 528–538. [Google Scholar]
- Schaller, G.E.; Bishopp, A.; Kieber, J.J. The yin-yang of hormones: Cytokinin and auxin interactions in plant development. Plant. Cell 2015, 27, 44–63. [Google Scholar] [CrossRef]
- Skoog, F.; Miller, C. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 54, 118–130. [Google Scholar]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Khuong, T.T.H.; Crété, P.; Robaglia, C.; Caffarri, S. Optimisation of tomato Micro-tom regeneration and selection on glufosinate/Basta and dependency of gene silencing on transgene copy number. Plant Cell Rep. 2013, 32, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Ajenifujah-Solebo, S.; Isu, N.; Olorode, O.; Ingelbrecht, I.; Abiade, O. Tissue culture regeneration of three Nigerian cultivars of tomatoes. Afr. J. Plant. Sci. 2012, 6, 370–375. [Google Scholar] [CrossRef]
- Kantor, M.; Sestras, R.; Chowdhury, K. Identification of the most organogenic-responsive variety of tomato using the variety × medium interaction. Rom. Biotechnol. Lett. 2010, 15, 5640–5645. [Google Scholar]
- Pawar, B.; Jadhav, A.; Kale, A.; Chimote, V.; Pawar, S. Zeatin induced direct in vitro shoot regeneration in tomato (Solanum lycopersicum L.). Bioscan 2012, 7, 247–250. [Google Scholar]
- Pino, L.E.; Lombardi-Crestana, S.; Azevedo, M.S.; Scotton, D.C.; Borgo, L.; Quecini, V.; Figueira, A.; Peres, L.E.P. The Rg1 allele as a valuable tool for genetic transformation of the tomato ‘Micro-Tom’ model system. Plant Methods 2010, 6, 1–11. [Google Scholar] [CrossRef]
- Gupta, S.; Van Eck, J. Modification of plant regeneration medium decreases the time for recovery of Solanum lycopersicum cultivar M82 stable transgenic lines. Plant Cell. Tissue Organ. Cult. 2016, 127, 417–423. [Google Scholar] [CrossRef]
- Tadeu De Faria, R.; Destro, D.; Filho, J.C.B.; Illg, R.D. Introgression of in vitro regeneration capability of Lycopersicon pimpinellifolium Mill. into recalcitrant tomato cultivars. Euphytica 2002, 124, 59–63. [Google Scholar] [CrossRef]
- Prihatna, C.; Chen, R.; Barbetti, M.J.; Barker, S.J. Optimisation of regeneration parameters improves transformation efficiency of recalcitrant tomato. Plant Cell. Tissue Organ. Cult. 2019, 137, 473–483. [Google Scholar] [CrossRef]
- Motte, H.; Vereecke, D.; Geelen, D.; Werbrouck, S. The molecular path to in vitro shoot regeneration. Biotechnol. Adv. 2014, 32, 107–121. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhang, X.S. The Hormonal Control of Regeneration in Plants, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 108, ISBN 9780123914989. [Google Scholar]
- Edesi, J.; Maria, A.; Hely, P. Modified light spectral conditions prior to cryopreservation alter growth characteristics and cryopreservation success of potato (Solanum tuberosum L.) shoot tips in vitro. Plant Cell Tissue Organ. Cult. 2017, 128, 409–421. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Mikos, V.; Brussaard, L.; Delbaere, B.; Pulleman, M.M. Opportunities and limitations for functional agrobiodiversity in the European context. Environ. Sci. Policy 2013, 27, 223–231. [Google Scholar] [CrossRef]
- Al Shaye, N.; Migdadi, H.; Charbaji, A.; Alsayegh, S.; Daoud, S.; AL-Anazi, W.; Alghamdi, S. Genetic variation among Saudi tomato (Solanum lycopersicum L.) landraces studied using SDS-PAGE and SRAP markers. Saudi J. Biol. Sci. 2018, 25, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Sardaro, M.L.S.; Marmiroli, M.; Maestri, E.; Marmiroli, N. Genetic characterization of Italian tomato varieties and their traceability in tomato food products. Food Sci. Nutr. 2013, 1, 54–62. [Google Scholar] [CrossRef] [PubMed]

| Source of Variation | Significance | ||
|---|---|---|---|
| Shoot Regeneration | Rooting | Acclimatization | |
| Variety (V) | * | * | * |
| Type of Explant (E) | * | * | * |
| Age of explant (A) | * | * | * |
| Regeneration medium (M) | ns | ns | ns |
| (V) × (E) | ns | ns | ns |
| (V) × (A) | ns | ns | ns |
| (E) × (A) | ns | ns | ns |
| (A) × (M) | ns | ns | ns |
| (V) × (M) | ns | ns | ns |
| (E) × (M) | ns | ns | ns |
| (V) × (E) × (M) | ns | ns | ns |
| (V) × (E) × (A) | ns | ns | ns |
| (V) × (A) × (M) | * | ns | ns |
| (E) × (A) × (M) | ns | ns | ns |
| (V) × (E) × (A) × (M) | ns | ns | ns |
| Variety | Type of Explant | Age of Explants | Regeneration Media | |||
|---|---|---|---|---|---|---|
| MSR1 | MSR2 | MSR3 | MSR4 | |||
| Areti | Cotyledon | 20 days | 4.7 (0.72) | 4.0 (0.46) | 11.7 (1.10) | 1.3 (0.23) |
| 27 days | 0.7 (0.16) | 1.3 (0.23) | 1.3 (0.30) | 1.0 (0.20) | ||
| Leaf | 20 days | 5.3 (0.76) | 4.0 (0.69) | 28.7 (1.44) | 8.3 (0.92) | |
| 27 days | 6.7 (0.85) | 4.7 (0.54) | 4.3 (0.57) | 9.3 (1.00) | ||
| Makedonia | Cotyledon | 20 days | 3.7 (0.62) | 0.0 (0.00) | 0.3 (0.10) | 0.0 (0.00) |
| 27 days | 0.0 (0.00) | 0.7 (0.16) | 0.3 (0.10) | 0.0 (0.00) | ||
| Leaf | 20 days | 4.7 (0.68) | 2.3 (0.42) | 3.0 (0.46) | 5.0 (0.74) | |
| 27 days | 1.3 (0.30) | 1.0 (0.39) | 1.7 (0.36) | 4.0 (0.46) | ||
| Factors | Regeneration | Rooting | Acclimatization | |
|---|---|---|---|---|
| Variety | Areti | 6.1 (0.63a) | 4.6 (0.58a) | 4.0 (0.53a) |
| Makedonia | 1.8 (0.30b) | 1.7 (0.29b) | 1.4 (0.27b) | |
| Type of explant | Leaf | 5.9 (0.66a) | 4.7 (0.61a) | 3.9 (0.55a) |
| Cotyledon | 1.9 (0.27b) | 1.6 (0.26b) | 1.5 (0.25a) | |
| Age of explants | 20 days | 5.4 (0.58a) | 4.3 (0.54a) | 3.0 (0.51a) |
| 27 days | 2.4(0.35b) | 2.0 (0.33b) | 1.6 (0.29b) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titeli, V.S.; Zafeiriou, I.; Laskaridou, A.; Menexes, G.; Madesis, P.; Stavridou, E.; Nianiou-Obeidat, I. Development of a Simple and Low-Resource Regeneration System of Two Greek Tomato Varieties. Agriculture 2021, 11, 412. https://doi.org/10.3390/agriculture11050412
Titeli VS, Zafeiriou I, Laskaridou A, Menexes G, Madesis P, Stavridou E, Nianiou-Obeidat I. Development of a Simple and Low-Resource Regeneration System of Two Greek Tomato Varieties. Agriculture. 2021; 11(5):412. https://doi.org/10.3390/agriculture11050412
Chicago/Turabian StyleTiteli, Vaia Styliani, Ioannis Zafeiriou, Angeliki Laskaridou, Georgios Menexes, Panagiotis Madesis, Evangelia Stavridou, and Irini Nianiou-Obeidat. 2021. "Development of a Simple and Low-Resource Regeneration System of Two Greek Tomato Varieties" Agriculture 11, no. 5: 412. https://doi.org/10.3390/agriculture11050412
APA StyleTiteli, V. S., Zafeiriou, I., Laskaridou, A., Menexes, G., Madesis, P., Stavridou, E., & Nianiou-Obeidat, I. (2021). Development of a Simple and Low-Resource Regeneration System of Two Greek Tomato Varieties. Agriculture, 11(5), 412. https://doi.org/10.3390/agriculture11050412