Phytophthora palmivora Causing Disease on Theobroma cacao in Hawaii
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogen Isolation
2.2. Molecular Identification
2.3. Temperature Growth Response
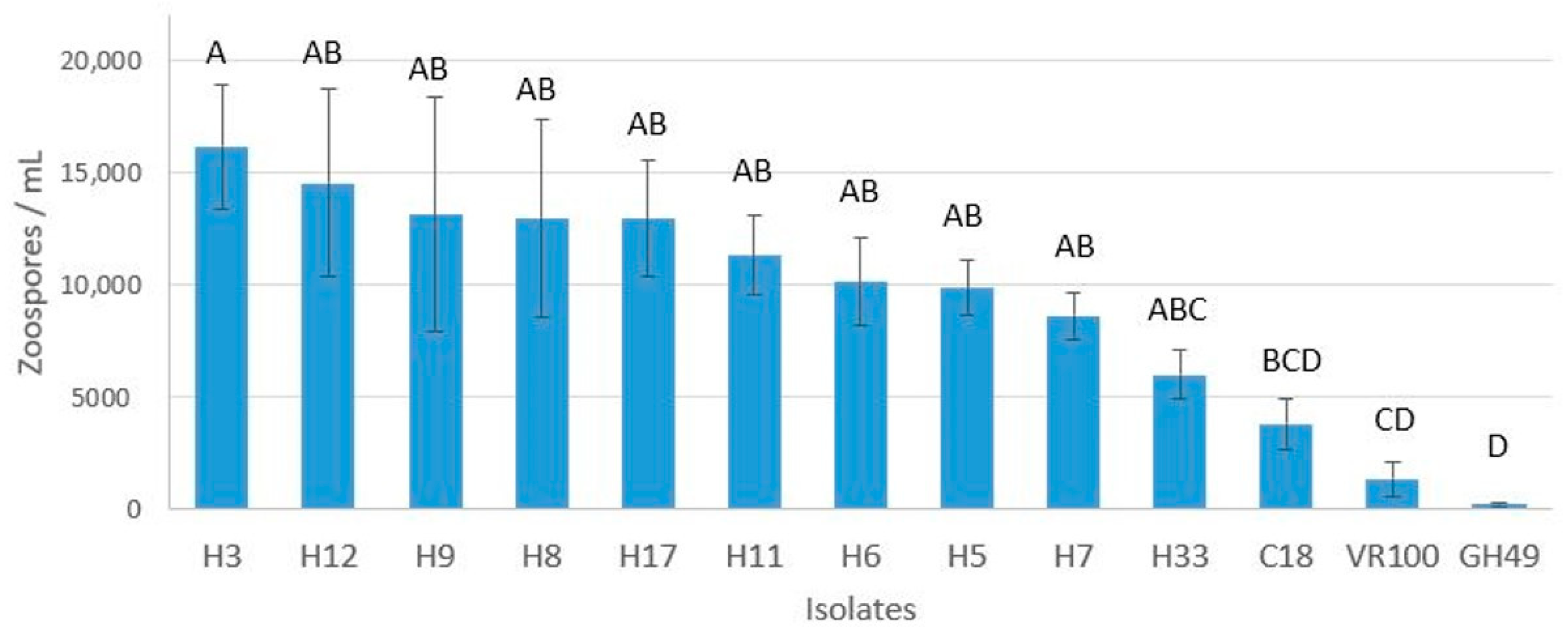
2.4. Zoospore Production
2.5. Virulence Screening
2.6. Fungicide Sensitivity Assay
2.7. Statistical Analyses
3. Results
3.1. Pathogen Isolation
3.2. Molecular Identification
3.3. Temperature Growth Response
3.4. Zoospore Production
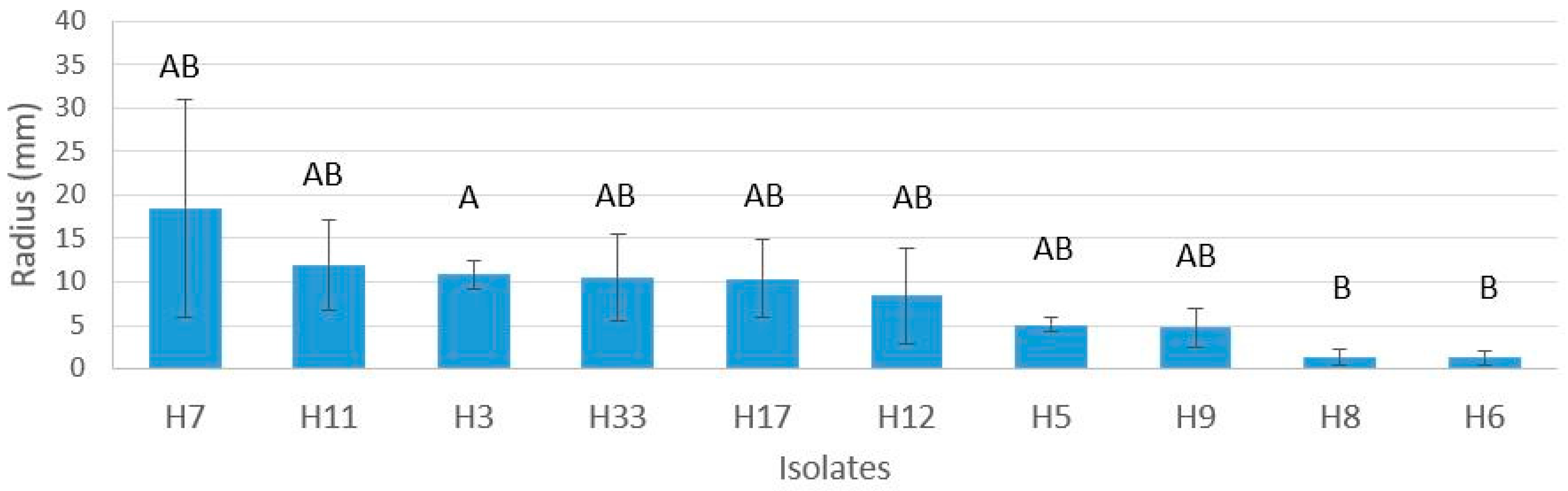
3.5. Virulence Screening
3.6. Fungicide Sensitivity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bittenbender, H.C. History of Cacao in Hawaii; Hawaii Cacao Symposium: Kona, HI, USA, 2005. [Google Scholar]
- Fleming, K.; Smith, V.E.; Bittenbender, H.C. The Economics of Cacao Production in Kona. AgriBusiness 2009, AB-17. Available online: http://hdl.handle.net/10125/13442 (accessed on 19 April 2021).
- Bittenbender, H.C. 2015 Hawaii Cacao Survey. Available online: http://deepdirtcacao.com/2015HawaiiCacaoSurvey.pdf (accessed on 19 April 2021).
- USDA. Hawaii Tropical Fruits and Crops Report 2016. Available online: https://www.nass.usda.gov/Statistics_by_State/Hawaii/Publications/Sugarcane_and_Specialty_Crops/Sugarcane/2017/201709tropicalspecialtiesHI.pdf (accessed on 19 April 2021).
- Hebbar, P.; Bittenbender, H.; O’Doherty, D. Farm and forestry production and marketing profile for cacao (Theobroma cacao). In Specialty crops for Pacific Island Agroforestry; Elevitch, C.R., Ed.; Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2011. [Google Scholar]
- Brasier, C.M. The biosecurity threat to the UK and global environment from international trade in plants. Plant Path. 2008, 57, 792–808. [Google Scholar] [CrossRef]
- Guest, D. Black pod: Diverse pathogens with a global impact on cocoa yield. Phytopathology 2009, 97, 1650–1653. [Google Scholar] [CrossRef]
- Dennis, J.J.; Konam, J.K. Phytophthora palmivora: Cultural control methods and their relationship to disease epidemiology on cocoa in PNG. In Proceedings of the 11th International Cocoa Research Conference, Yamoussoukro, Cote D’Ivoire, 18–24 July 1993; Cocoa Producers Alliance: London, UK, 1994; pp. 953–957. [Google Scholar]
- Gregory, P.H.; Maddison, A. Epidemiology of Phytophthora on Cocoa in Nigeria; Commonwealth Mycological Institute: Kew, UK, 1981. [Google Scholar]
- Opoku, I.Y.; Akrofi, A.Y.; Appiah, A.A. Assessment of sanitation and fungicide application directed at cocoa tree trunks for the control of Phytophthora black pod infections in pods growing in the canopy. Eur. J. Plant Pathol. 2007, 117, 167–175. [Google Scholar] [CrossRef]
- Chee, K.; Foong, K. Use of cacao pod for recovering Phytophthora species pathogenic to Hevea brasiliensis. Plant Dis. Report. 1968, 52, 5. [Google Scholar]
- U.S. Climate Data. Available online: https://www.usclimatedata.com/ (accessed on 27 September 2018).
- Weatherbase. Available online: https://www.weatherbase.com (accessed on 27 September 2018).
- Climate-Data.Org. Available online: https://en.climate-data.org/ (accessed on 27 September 2018).
- Topozone. Available online: https://www.topozone.com/ (accessed on 27 September 2018).
- World Weather and Climate Information. Available online: https://weather-and-climate.com (accessed on 27 September 2018).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hudspeth, D.S.; Nadler, S.A.; Michael, E.S.H. A COX2 Molecular Phylogeny of the Peronosporomycetes. Mycologia 2000, 92, 674–684. [Google Scholar] [CrossRef]
- Ali, S.S.; Amoako-Attah, I.; Bailey, R.A.; Strem, M.D.; Schmidt, M.; Akrofi, A.Y.; Surujdeo-Maharaj, S.; Kolawole, O.O.; Begoude, B.A.D.; Hoopen, G.M.; et al. PCR-based identification of cacao black pod causal agents and identification of biological factors possibly contributing to Phytophthora megakarya’s field dominance in West Africa. Plant Pathol. 2016, 65, 1095–1108. [Google Scholar] [CrossRef]
- Srinivasan, M.R. Probit analysis. In Electronic Manual on Pesticides and Environment; Palaniswamy, S., Kuttalam, S., Chandrasekaran, S., Kennedy, J.S., Srinivasan, M.R., Eds.; Tamil Nadu Agricultural University: Tamil Nadu, India, 2004; Available online: https://www.researchgate.net/publication/312661311_Calculate_LC_50_or_LD_50_with_MS_Excel_worksheet_based_on_Finney%27s_method_of_probit_analysis?chanel=doi&linkId=58879af4aca272b7b452586e&showFulltext=true (accessed on 19 April 2021).
- Schnell, R.J.; Olano, C.T.; Brown, J.S.; Meerow, A.W.; Cervantes-Martinez, C.; Nagai, C.; Motamayor, J.C. Retrospective determination of the parental population of superior cacao (Theobroma cacao L.) seedlings and association of microsatellite alleles with productivity. J. Am. Soc. Hortic. Sci. 2005, 130, 181–190. [Google Scholar] [CrossRef]
- Billock, J. How Hawaii Became the North Pole of Cacao. Smithsonian Magazine. 2018. Available online: https://www.smithsonianmag.com/travel/hawaii-north-pole-cacao-chocolate-tours-180967951/ (accessed on 19 April 2021).
- Puig, A.S.; Marelli, J.P.; Matsumoto, T.; Keith, L.; Gutierrez, O.A. First report of Neofusicoccum parvum causing pod rot on cacao in Hawaii. Plant Dis. 2019, 103, 1416. [Google Scholar] [CrossRef]
- Puig, A.S.; Ali, S.; Strem, M.; Sicher, R.; Gutierrez, O.A.; Bailey, B.A. The differential influence of temperature on Phytophthora megakarya and Phytophthora palmivora pod lesion expansion, mycelia growth, gene expression, and metabolite profiles. Physiol. Mol. Plant Pathol. 2018, 102, 95–112. [Google Scholar] [CrossRef]
- Aragaki, M.; Uchida, J.Y. Morphological Distinctions between Phytophthora capsici and P. tropicalis sp. nov. Mycologia 2001, 93, 137–145. [Google Scholar] [CrossRef]
- Hwu, F.Y.; Lai, M.W.; Liou, R.F. PpMID1 Plays a Role in the Asexual Development and Virulence of Phytophthora parasitica. Front. Microbiol. 2017, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Assinder, S.J. Molecular Genetics and Genomics of Phytophthora. In Applied Mycology and Biotechnology; Arora, D.K., Khachatourians, G.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 4, pp. 137–160. [Google Scholar]
- Russell, P. Sensitivity Baselines in Fungicide Resistance Research and Management; Crop Life International: Brussels, Belgium, 2002. [Google Scholar]
- Bruck, R.; Fry, W.; Apple, A.; Mundt, C. Effect of protectant fungicides on the developmental stages of Phytophthora infestans in potato foliage. Phytopathology 1981, 100, 164–166. [Google Scholar] [CrossRef]
- Miyake, N.; Nagai, H. Efficacy of phosphonate in controlling white powdery rot of fig caused by Phytophthora palmivora. J. Gen. Plant Pathol. 2017, 83, 390–397. [Google Scholar] [CrossRef]
- Groves, C.T.; Ristaino, J.B. Commercial Fungicide Formulations Induce In Vitro Oospore Formation and Phenotypic Change in Mating Type in Phytophthora infestans. Phytopathology 2000, 90, 1201–1208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barak, E.; Edgington, L.V. Cross resistance of Botrytis cinerea to captan, thiram, chlorothalonil and related fungicides. Can. J. Plant Pathol. 1984, 6, 318–320. [Google Scholar] [CrossRef]
- Reis, A.; Ribeiro, F.H.S.; Maffia, L.A.; Mizubuti, E.S.G. Sensitivity of Brazilian Isolates of Phytophthora infestans to Commonly Used Fungicides in Tomato and Potato Crops. Plant Dis. 2005, 89, 1279–1284. [Google Scholar] [CrossRef][Green Version]
- Grünwald, N.J.; Sturbaum, A.K.; Montes, G.R.; Serrano, E.G.; Lozoya-Saldaña, H.; Fry, W.E. Selection for Fungicide Resistance Within a Growing Season in Field Populations of Phytophthora infestans at the Center of Origin. Phytopathology 2006, 96, 1397–1403. [Google Scholar] [CrossRef]
- Tey, C.C.; Wood, R.K.S. Effects of various fungicides in vitro on Phytophthora palmivora from cocoa. Trans. Brit. Mycol. Soc. 1983, 80, 271–282. [Google Scholar] [CrossRef]
- Frac. Fungicide Resistance Action Committee, FRAC Code List 2018: Fungicides Sorted by Mode of Action. Available online: http://www.phi-base.org/images/fracCodeList.pdf (accessed on 19 April 2021).
- Schwinn, F.; Staub, T. Phenylamides and other fungicides against Oomycetes. In Modern Selective Fungicides: Properties, Applications, Mechanisms of Action; Lyr, H., Ed.; Longman Scientific and Technical: London, UK, 1987; pp. 259–274. [Google Scholar]
- Moorman, G.W.; Kang, S.; Geiser, D.M.; Kim, S.H. Identification and Characterization of Pythium Species Associated with Greenhouse Floral Crops in Pennsylvania. Plant Dis. 2002, 86, 1227–1231. [Google Scholar] [CrossRef]
- Lamour, K.H.; Hausbeck, M.K. Mefenoxam Insensitivity and the Sexual Stage of Phytophthora capsici in Michigan Cucurbit Fields. Phytopathology 2000, 90, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Ferrin, D.; Kabashima, J. In vitro insensitivity to metalaxyl of isolates of Phytophthora citricola and P. parasitica from ornamental hosts in southern California. Plant Dis. 1991, 75, 1041–1044. [Google Scholar] [CrossRef]
- Childers, R.; Danies, G.; Myers, K.; Fei, Z.; Small, I.M.; Fry, W.E. Acquired Resistance to Mefenoxam in Sensitive Isolates of Phytophthora infestans. Phytopathology 2015, 105, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Torres-Londono, G.A. Morphological characterization, virulence, and fungicide sensitivity evaluation of Phytophthora palmivora. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2006. Available online: https://d.lib.msu.edu/etd/3824 (accessed on 19 April 2021).
- Fraser, D.E.; Shoemaker, P.B.; Ristaino, J.B. Characterization of Isolates of Phytophthora infestans from Tomato and Potato in North Carolina from 1993 to 1995. Plant Dis. 1999, 83, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Coffey, M.D. Systemic Fungicides and the Control of Oomycetes. Annu. Rev. Phytopathol. 1986, 24, 311–338. [Google Scholar] [CrossRef]
- Mcgrath, M.T. What Are Fungicides? In The Plant Health Instructor. The American Phytopathological Society. 2004. Available online: https://www.apsnet.org/edcenter/disimpactmngmnt/topc/Pages/Fungicides.aspx (accessed on 19 April 2021).
- Vegh, I.; Leroux, P.; Le Berre, A.; Lanen, C. Détection sur Chamaecyparis lawsoniana ‘Ellwoodii’d’une souche de Phytophthora cinnamomi Rands résistante au phoséthyl-Al. PHM Rev. Hortic. 1985, 262, 19–21. [Google Scholar]
- Cohen, Y.; Samoucha, Y. Cross-resistance to four systemic fungicides in metalaxyl-resistant strains of Phytophthora infestans and Pseudoperonospora cubensis. Plant Dis. 1984, 68, 137–139. [Google Scholar] [CrossRef]
- Fenn, M.; Coffey, M.D. Phosphonate fungicides for control of diseases caused by Phytophthora. Calif. Avocado Soc. Yearb. 1987, 71, 241–249. [Google Scholar]
- Matheron, M.E.; Porchas, M. Impact of Azoxystrobin, Dimethomorph, Fluazinam, Fosetyl-Al, and Metalaxyl on Growth, Sporulation, and Zoospore Cyst Germination of Three Phytophthora spp. Plant Dis. 2000, 84, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Farih, A.; Menge, J.A.; Tsao, P.H.; Ohr, H.D. Metalaxyl and efosite aluminium for control of Phytophthora gummosis and root rot on citrus. Plant Dis. 1981, 65, 654–657. [Google Scholar] [CrossRef]
- Chan, L.; Kwee, L. Comparative in vitro sensitivity of selected chemicals on Phytophthora palmivora from cocoa and durian. Pertanika 1986, 9, 183–191. [Google Scholar]
- Dolan, T.E.; Coffey, M.D. Correlative in vitro and in vivo behavior of mutant strains of Phytophthora palmivora expressing different resistances to phosphorous acid and fosetyl-Na. Phytopathology 1988, 78, 974–978. [Google Scholar] [CrossRef]
- Hart, C.; (Manoa, HI, USA). Personal communication, 2018.
- Puig, A.S.; Marelli, J.P.; Suarez-Capello, C.; Gutierrez, O.A. Phytophthora canker affecting CCN51 clones on high productivity cacao farms in Ecuador. In Proceedings of the American Phytopathological Society Annual Meeting, Plant Health, Cleveland, OH, USA, 3–7 August 2019. [Google Scholar]



| Site | Island | Average Temperature | Annual Precipitation | Elevation | Sample # and Type | P. Palmivora Isolates |
|---|---|---|---|---|---|---|
| 1 | Hawaii | 21.8–24.6 °C | 3239 mm | 1 m | 7 pods | H3, H5, H6, H7, H8, H9 |
| 2 | Hawaii | 21.3–24.4 °C | 3365 mm | 46 m | 3 pods | H11, H12, H17 |
| 3 | Hawaii | 21.7–24.6 °C | 3459 mm | 29 m | 7 stems | H33 |
| 4 | Hawaii | 16–27 °C | 467 mm | 493 m | 5 pods | None |
| 5 | Hawaii | 14.3–24.9 °C | 577 mm | 377 m | 1 stem | None |
| 6 | Oahu | 22.1–25.7 °C | 599 mm | 19 m | 6 pods | None |
| 7 | Oahu | 17.7–28.2 °C | 755 mm | 3 m | 0 | None |
| 8 | Oahu | 21.9–27.1 °C | 814 mm | 152 m | 6 pods | None |
| EC50 (µg/mL) | 95% CI a | R² | |||
| C18 | 45.5 | AB | 13.0–159.3 | 0.95 | y = 0.79x + 3.66 |
| VR100 | 44.8 | A | 29.2–68.7 | 0.93 | y = 0.86x + 3.53 |
| H33 | 30.7 | A | 22.0–42.9 | 0.93 | y = 1.02x + 3.43 |
| H5 | 26.9 | A | 18.5–39.2 | 0.99 | y = 1.02x + 3.55 |
| H7 | 25.5 | A | 17.5–37.0 | 0.97 | y = 1.28x + 3.23 |
| H11 | 25 | AB | 9.5–66.4 | 0.99 | y = 0.97x + 3.64 |
| GH49 | 10.5 | B | 7.4–15.0 | 0.98 | y = 1.442x + 3.41 |
| EC50 (µg/mL) | 95% CI a | R² | ||
|---|---|---|---|---|
| C18 | 41.5 | 29.9–57.5 | 0.81 | y = 0.96x + 3.31 |
| H11 | 41.5 | 22.8–75.7 | 0.67 | y = 0.62x + 3.93 |
| H7 | 41.1 | 22.8–74.1 | 0.75 | y = 0.62x + 3.94 |
| VR100 | 40.3 | 30.2–53.8 | 0.90 | y = 1.25x + 2.87 |
| GH49 | 31.6 | 24.7–40.4 | 0.88 | y = 1.30x + 2.97 |
| H5 | 29.7 | 19.3–46.17 | 0.89 | y = 0.78x + 3.83 |
| H33 | 29.5 | 20.2–42.9 | 0.92 | y = 0.90x + 3.66 |
| EC50 (×10–3) a (µg/mL) | 95% CI b (×10–3) | R² | |||
|---|---|---|---|---|---|
| C18 | 168.2 | A | 84.8–333.8 | 0.66 | y = 0.56x + 5.53 |
| GH49 | 75.6 | A | 37.3–153.2 | 0.61 | y = 0.58x + 5.99 |
| VR100 | 38.7 | AB | 12.1–123.6 | 0.57 | y = 0.43x + 5.81 |
| H5 | 1.14 | B | 0.048–27.1 | 0.87 | y = 0.33x + 6.08 |
| H11 | 0.397 | B | 0.006–25.6 | 0.75 | y = 0.27x + 6.08 |
| H33 | 0.319 | B | 0.00496–20.5 | 0.71 | y = 0.29x + 6.20 |
| H7 | 0.234 | B | 0.0026–21.0 | 0.88 | y = 0.28x + 6.12 |
| EC50 (µg/mL) | 95% CI a | R² | |||
|---|---|---|---|---|---|
| C18 | 43.25 | AB | 5.73–326.4 | 0.62 | y = 1.11x + 3.44 |
| VR100 | 19.64 | A | 8.56–45.07 | 0.79 | y = 1.47x + 3.11 |
| H5 | 13.03 | AB | 3.14–54.10 | 0.70 | y = 1.35x + 3.36 |
| GH49 | 6.81 | AB | 1.65–28.05 | 0.82 | y = 0.88x + 4.51 |
| H33 | 4.18 | AB | 0.22–78.41 | 0.54 | y = 1.02x + 3.53 |
| H11 | 2.12 | AB | 0.05–89.92 | 0.58 | y = 1.11x + 3.62 |
| H7 | 0.594 | B | 0.057–6.24 | 0.79 | y = 0.80x + 5.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puig, A.S.; Quintanilla, W.; Matsumoto, T.; Keith, L.; Gutierrez, O.A.; Marelli, J.-P. Phytophthora palmivora Causing Disease on Theobroma cacao in Hawaii. Agriculture 2021, 11, 396. https://doi.org/10.3390/agriculture11050396
Puig AS, Quintanilla W, Matsumoto T, Keith L, Gutierrez OA, Marelli J-P. Phytophthora palmivora Causing Disease on Theobroma cacao in Hawaii. Agriculture. 2021; 11(5):396. https://doi.org/10.3390/agriculture11050396
Chicago/Turabian StylePuig, Alina Sandra, Wilber Quintanilla, Tracie Matsumoto, Lisa Keith, Osman Ariel Gutierrez, and Jean-Philippe Marelli. 2021. "Phytophthora palmivora Causing Disease on Theobroma cacao in Hawaii" Agriculture 11, no. 5: 396. https://doi.org/10.3390/agriculture11050396
APA StylePuig, A. S., Quintanilla, W., Matsumoto, T., Keith, L., Gutierrez, O. A., & Marelli, J.-P. (2021). Phytophthora palmivora Causing Disease on Theobroma cacao in Hawaii. Agriculture, 11(5), 396. https://doi.org/10.3390/agriculture11050396







