Effect of Fertilisation on the Quality of Dried Coriander (Coriandrum sativum L.) and Lovage (Levisticum officinale)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Pot Experiment
2.2. Methods of Drying and Modelling of Drying Kinetics
2.3. Bioactive Compounds Content, Chemical Composition of HS-SPME and Colour
2.4. Statistical Analysis
3. Results and Discussion
3.1. The Kinetics of Drying
3.2. Bioactive Compounds Content and Colour
3.3. Head Space-Solid Phase Microextraction (HS-SPME) of Dried Herbs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Amiri, M.S.; Joharchi, M.R. Ethnobotanical knowledge of Apiaceae family in Iran, A review. Avicenna J. Phytomed. 2016, 6, 621–635. [Google Scholar]
- Pedro, A.G.; Santosa, A.; Figueiredoa, M.C.; Oliveira, M.M.; Barroso, J.G.; Pedro, L.G.; Deans, S.G.; Schefferd, J.J.C. Growth and essential oil composition of hairy root cultures of Levisticum officinale W.D.J. Koch (lovage). Plant Sci. 2005, 168, 1089–1096. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Bioactive Compounds, Antioxidant Activity and Nutritional Quality of Different Culinary Aromatic Herbs. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 179–184. [Google Scholar] [CrossRef]
- González, S.; Fernández, M.; Cuervo, A.; Lasheras, C. Dietary intake of polyphenols and major food sources in an institutionalised elderly population. J. Hum. Nutr. Diet. 2014, 27, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Sakulnarmrat, K.; Konczak, I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012, 134, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kahkonen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Diederichsen, A. Coriander. Promoting the conservation and use of underutilized and neglected crops, 3. In Spices; Purseglove, J.W., Brown, E.G., Green, C.L., Robbins, S.R.J., Eds.; Longman: New York, NY, USA, 1996; Volume 2, pp. 736–788. [Google Scholar]
- Gil, A.; De La Fuente, E.B.; Lenardis, A.E.; Lopez Pereira, M.; Suarez, S.A.; Bandoni, A. Coriander essential oil composition from two genotypes grown in different environmental conditions. J. Agric. Food Chem. 2002, 50, 2870–2877. [Google Scholar] [CrossRef]
- Grosso, C.; Gerraro, V.; Figueiredo, A.C.; Barroso, J.G.; Coelho, J.A.; Palavara, A.M. Supercritical carbon dioxide extraction of volatile oil from Italian coriander seeds. Food Chem. 2002, 111, 197–203. [Google Scholar] [CrossRef]
- Elgayyar, M.; Draughon, F.A.; Golden, D.A.; Mount, J.R. Antimicrobial activity of essential oils from plants against selected pathogenic and saprophytic microorganisms. J. Food Prot. 2001, 64, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Canter, P.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef]
- Stępień, A.E.; Gorzelany, J.; Matłok, N.; Lech, K.; Figiel, A. The effect of drying methods on the energy consumption, bioactive potential and colour of dried leaves of Pink Rock Rose (Cistus creticus). J. Food Sci. Technol. 2019, 56, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Matłok, N.; Gorzelany, J.; Stępień, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef]
- Matłok, N.; Lachowicz, S.; Gorzelany, J.; Balawejder, M. Influence of Drying Method on Some Bioactive Compounds and the Composition of Volatile Components in Dried Pink Rock Rose (Cistus creticus L.). Molecules 2020, 25, 2596. [Google Scholar] [CrossRef]
- Orphanides, A.; Goulas, V.; Geka, V. Drying Technologies: Vehicle to High-Quality Herbs. Food Eng. Rev. 2016, 8, 164–180. [Google Scholar] [CrossRef]
- Danilčenko, H.; Dabkevičius, Z.; Jarienė, E.; Tarasevičienė, Ž.; Televičiūtė, D.; Amošiūnas, A.; Jeznach, M. The effect of stinging nettle and field horsetail extracts on the synthesis of biologically active compounds in germinated leguminous and quinoa seed. Zemdirbyste-Agriculture 2017, 104, 337–344. [Google Scholar] [CrossRef]
- Matłok, N.; Stępień, A.E.; Gorzelany, J.; Wojnarowska-Nowak, R.; Balawejder, M. Effects of Organic and Mineral Fertilization on Yield and Selected Quality Parameters for Dried Herbs of Two Varieties of Oregano (Origanum vulgare L.). Appl. Sci. 2020, 10, 5503. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Pernice, R.; Fogliano, V.; Barbieri, G. Sulphur fertilization may improve the nutritional value of Brassica rapa L. subsp sylvestris. Eur. J. Agron. 2007, 26, 418–424. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Z.; Gerendas, J. Effects of nitrogen and sulfur on total phenolics and antioxidant activity in two genotypes of leaf mustard. J. Plant Nutr. 2008, 31, 1642–1655. [Google Scholar] [CrossRef]
- Barroso, M.R.; Martins, N.; Barros, L.; Antonio, A.L.; Rodrigues, M.A.; Sousa, M.J.; Santos-Buelga, S.; Ferreira, I.C.F.R. Assessment of the nitrogen fertilization effect on bioactive compounds of frozen fresh and dried samples of Stevia rebaudiana Bertoni. Food Chem. 2018, 243, 208–213. [Google Scholar] [CrossRef]
- Dias, A.; Neto, D.A.; Menezes, R.V.; Gheyi, H.R.; Conceição, P.C.; Mitsue, A.; Cova, W.; Ribas, R.F.; Ribeiro, M.D.O. Salt-induced changes in solutes, pigments and essential oil of two basil (Ocimum basilicum L.) genotypes under hydroponic cultivation. Aust. J. Crop Sci. 2019, 13, 1856–1864. [Google Scholar]
- Fraser, D.P.; Sharma, A.; Fletcher, T.; Budge, S.; Moncrieff, C.; Dodd, A.N.; Franklin, K.A. UV-B antagonises shade avoidance and increases levels of the flavonoid quercetin in coriander (Coriandrum sativum). Sci. Rep. 2017, 7, 1–17. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, A.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L. Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Karimi, E.; Ghasemzadeh, A. Impact of Organic and Inorganic Fertilizers Application on the Phytochemical and Antioxidant Activity of Kacip Fatimah (Labisia pumila Benth). Molecules 2013, 18, 10973–10988. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Coriander (Coriandrum sativum): A promising functional food toward the well-being. Food Res. Int. 2018, 105, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Chahal, K.K.; Singh, R.; Kumar, A.; Bhardwaj, U. Chemical composition and biological activity of Coriandrum sativum L.: A review. Indian J. Nat. Prod. Resour. 2017, 8, 193–203. [Google Scholar]
- Nurzyńska-Wierdak, R. Essential oil composition ofthe coriander (Coriandrum sativum L.) herb dependingon the development stage. Acta Agrobotanica 2013, 66, 53–60. [Google Scholar] [CrossRef]
- Telci, I.; Toncer, O.G.; Sahbaz, N. Yield, Essential Oil Content and Composition of Coriandrum sativum Varieties (var. vulgare Alef and var. microcarpum DC.) Grown in Two Different Locations. J. Essent. Oil Res. 2005, 18, 189–193. [Google Scholar] [CrossRef]
- Zeng, Z.; Meng, C.; Ye, X.; Zeng, Z. Analysis of Volatile Components of Adenosma indianum (Lour.) Merr. by Steam Distillation and Headspace Solid-Phase Microextraction. J. Chem. 2013, 2013, 545760. [Google Scholar] [CrossRef]
- Raal, A.; Arak, E.; Orav, A.; Kailas, T.; Müürisepp, M. Composition of the Essential Oil of Levisticum officinale W.D.J. Koch from Some European Countries. J. Essent. Oil Res. 2008, 20, 318–322. [Google Scholar] [CrossRef]
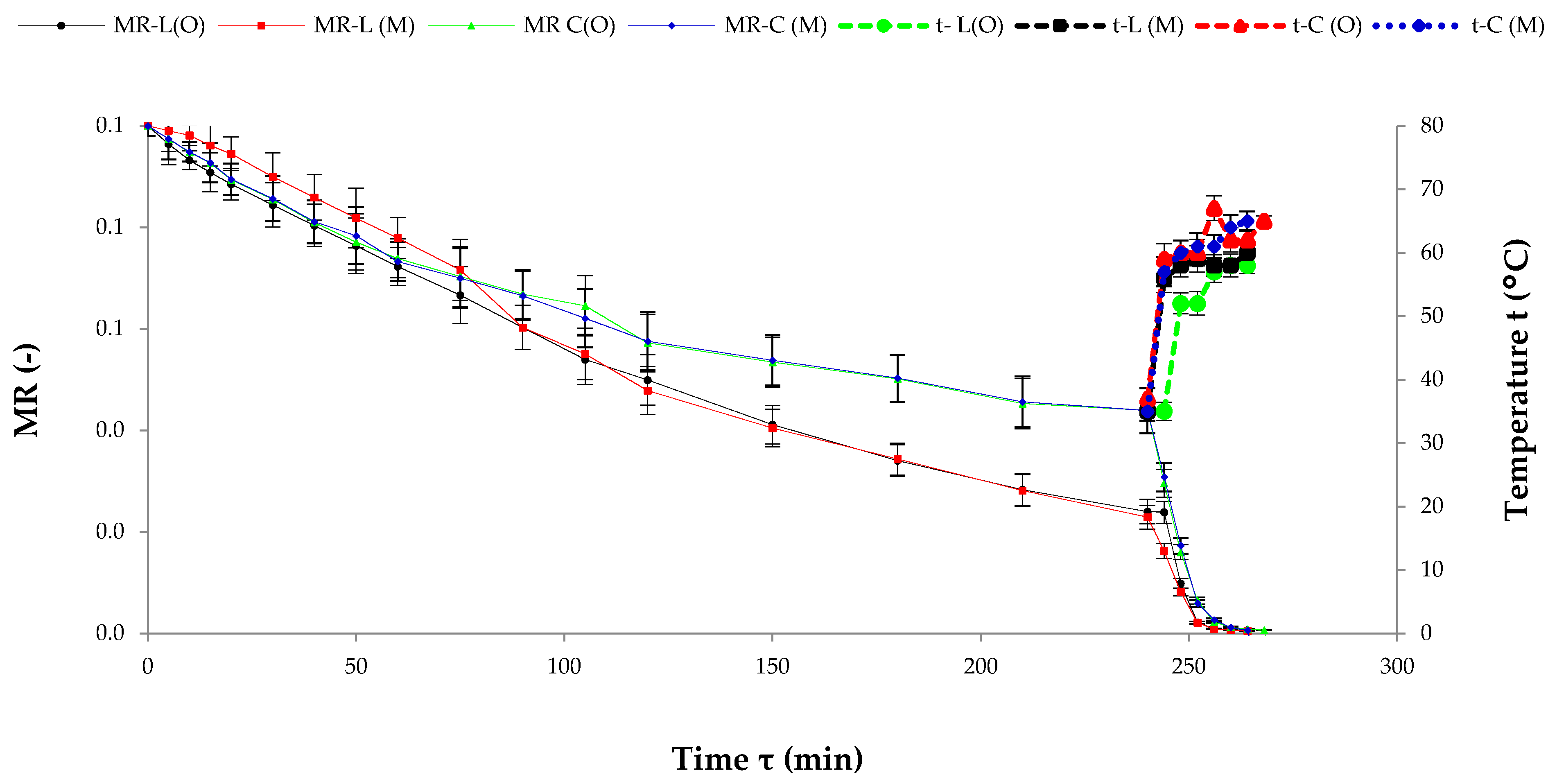
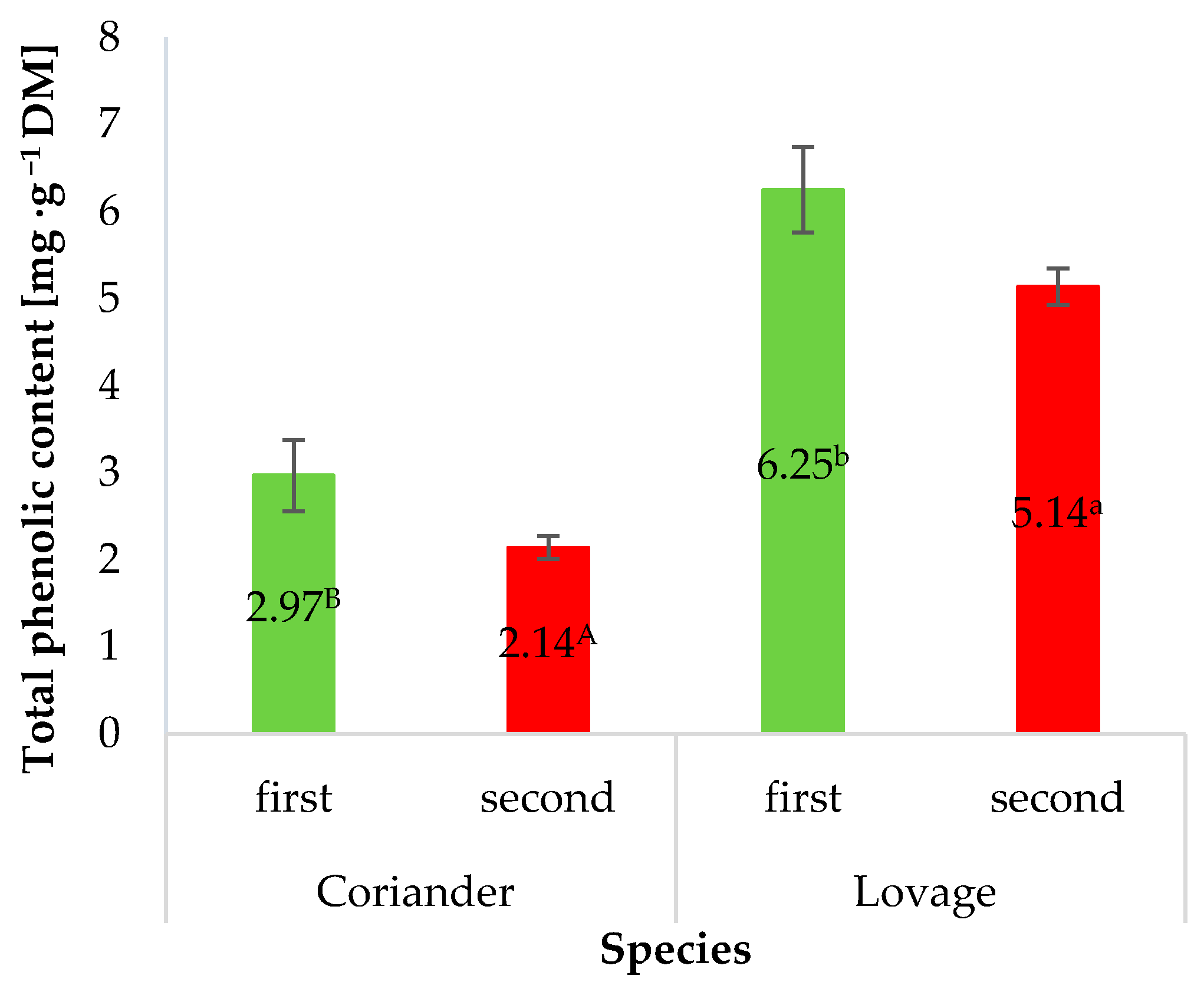

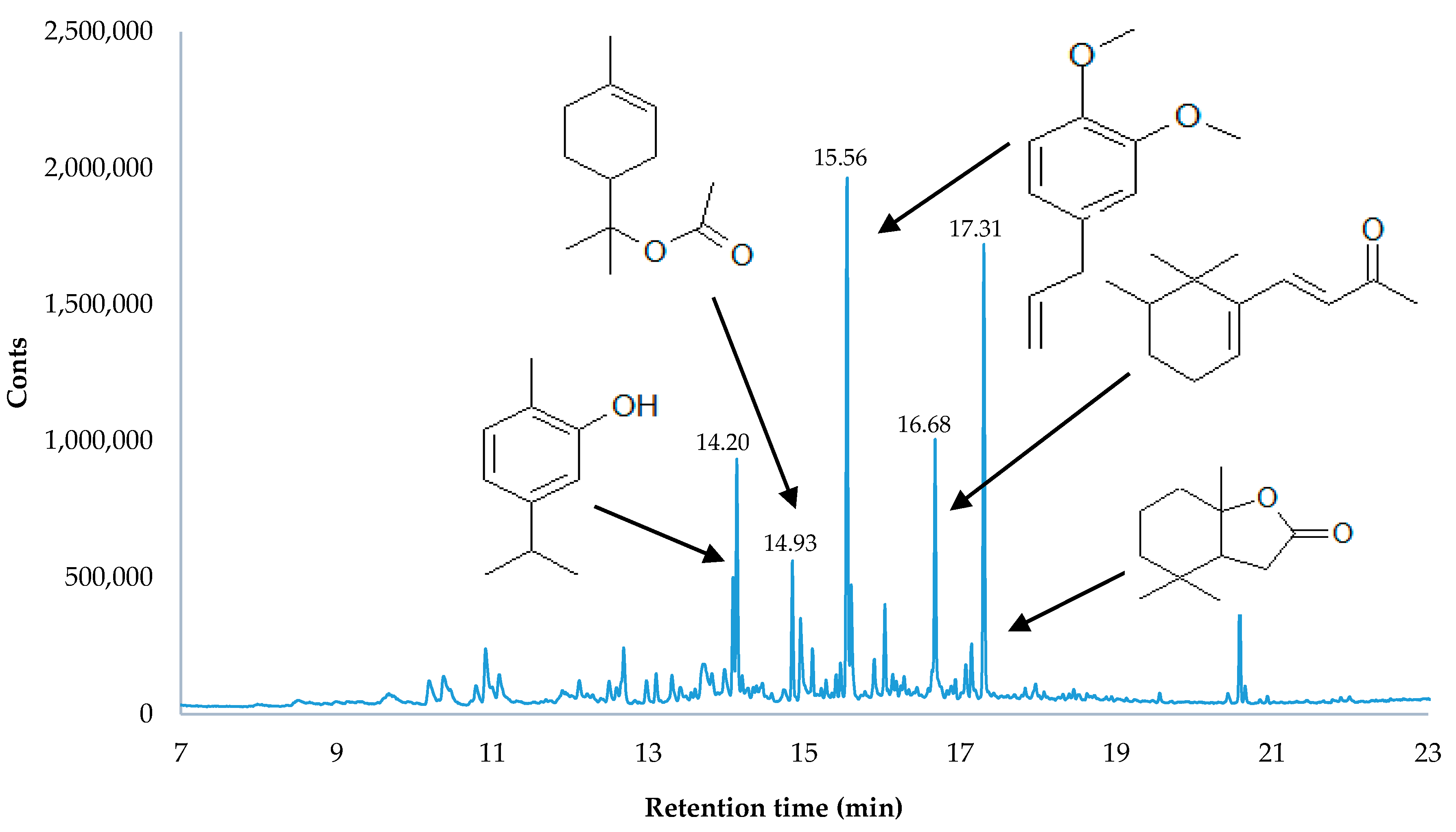

| Species | Fertilisation Method | L* ± SD | a* ± SD | b* ± SD |
|---|---|---|---|---|
| Coriander | first | 49.47 ± 0.52 a | −0.28 ± 0.06 c | 13.08 ± 0.40 d |
| second | 48.21 ± 0.35 a | −0.23 ± 0.21 b | 12.23 ± 0.68 d | |
| Lovage | first | 59.58 ± 0.50 A | −2.42 ± 0.08 C | 12.28 ± 0.20 E |
| second | 57.16 ± 0.49 A | −1.98 ± 0.19 B | 10.46 ± 0.31 D |
| No. | RT (min) | Peak Share in the Chromatogram [%] | Ordinary Substance Name | Systematic Substance Name | |||
|---|---|---|---|---|---|---|---|
| L(O) | L(M) | C(O) | C(M) | ||||
| 1 | 9.53 | 12.58 b ± 2.11 | 8.44 a ± 1.76 | - | - | cymene | methyl(1-methylethyl)benzene |
| 2 | 9.69 | 3.35 b ± 0.34 | 2.44 a ± 0.12 | - | - | β-phellandrene | 3-Isopropyl-6-methylenecyclohex-1-ene |
| 3 | 10.16 | 0.87 b ± 0.16 | 0.33 a ± 0.06 | - | - | trans-sabinene hydrate | 2-methyl-5-propan-2-ylbicyclo[3.1.0]hexan-2-ol |
| 4 | 10.37 | - | - | 4.25 ± 1.38 | trace | - | octa-3,5-dien-2-one |
| 5 | 10.91 | - | - | trace | 3.78 ± 0.97 | linalool | 3,7-Dimethyl-1,6-octadien-3-ol |
| 6 | 11.45 | trace | 0.43 | - | - | fenchol | 1,3,3-trimethylbicyclo[2.2.1]heptan-2-ol |
| 7 | 12.45 | 0.76 b ± 0.14 | 0.54 a ± 0.11 | - | - | (-)-terpinen-4-ol | (1R)-4-methyl-1-propan-2-ylcyclohex-3-en-1-ol |
| 8 | 12.51 | 1.41 b ± 0.23 | 1.33 a ± 0.17 | - | - | α-terpinenol | 2-[(1S)-4-methyl-1-cyclohex-3-enyl]propan-2-ol |
| 9 | 13.28 | 0.80 a ± 0.02 | 0.59 b ± 0.08 | trace | trace | carvacryl methyl ether | 4-isopropyl-2-methoxy-1-methylbenzene |
| 10 | 13.83 | 1.76 b ± 0.49 | 0.91 a ± 0.31 | trace | trace | phellandral | 4-propan-2-ylcyclohexene-1-carbaldehyde |
| 11 | 14.06 | trace | 0.81 ± 0.2 | trace | 5.54 | thymol | 2-isopropyl-5-methylphenol |
| 12 | 14.21 | 1.26 b ± 0.41 | 0.68 a ± 0.27 | 13.47 b ± 1.26 | 11.74 a ± 0.95 | carvacrol | 2-methyl-5-(propan-2-yl)phenol |
| 13 | 14.93 | 66.87 a ± 5.70 | 75.35 b ± 3.32 | 10.95 b ± 2.81 | 6.28 a ± 1.26 | α-terpinyl acetate | (±)-2-(4-Methyl-3-cyclohexenyl)isopropyl acetate |
| 14 | 14.98 | - | - | 8.25 ± 1.05 | trace | eugenol | 2-methoxy-4-(2-propenyl)phenol |
| 15 | 15.11 | - | - | 10.78 ± 2.16 | trace | piperitenone oxide | (1S)-1,2-epoxy-p-menth-4(8)-en-3-on |
| 16 | 15.26 | trace | 0.31 ± 0.07 | - | - | lavandulyl acetate | (5-methyl-2-prop-1-en-2-ylhex-4-enyl) acetate |
| 17 | 15.31 | 0.12 a ± 0.04 | 0.28 b ± 0.06 | - | - | α-copaene | 1,3-dimethyl-8-(1-methyl ethyl) tricyclo(4.4.0.0.02,7-)dec-3-ene stereoisomer |
| 18 | 15.56 | 1.16 b ± 0.31 | 0.49 a ± 0.11 | 13.65 a ± 2.98 | 26.65 b ± 6.87 | methyl eugenol | 4-Allyl-1,2-dimethoxybenzene, Eugenol methyl ether, eugenyl methyl ether |
| 19 | 15.60 | - | - | trace | 6.78 ± 1.28 | nothosyrnol | 1,3-bis(1-methoxyprop-1-enyl)benzene |
| 20 | 15.98 | trace | trace | - | - | β-caryophyllene | (−)-trans-caryophyllene, trans-(1R,9S)-8-Methylene-4,11,11-trimethylbicyclo[7.2.0]undec-4-ene |
| 21 | 16.60 | trace | trace | - | - | ƴ-muurolene | (1R,4aR,8aS)-7-methyl-4-methylidene-1-propan-2-yl-2,3,4a,5,6,8a-hexahydro-1H-naphthalene |
| 22 | 16.68 | 0.79 a ± 0.21 | 0.77 a ± 0.28 | 9.97 a ± 2.64 | 9.80 a ± 3.71 | (E)-β-ionone | (E)-4-[(5R)-5,6,6-trimethylcyclohexen-1-yl]but-3-en-2-one |
| 23 | 16.77 | trace | trace | - | - | β-selinene | 28(3S,4aR,8aS)-8a-methyl-5-methylidene-3-prop-1-en-2-yl-1,2,3,4,4a,6,7,8-octahydronaphthalene |
| 24 | 17.31 | 1.59 b ± 0.16 | 0.93 a ± 0.11 | 28.65 b ± 2.07 | 23.51 a ± 1.31 | (S)-dihydroactinidiolide | (2,6,6-trimethyl-2-hydroxycyclohexylidene)acetic acid lactone |
| TOTAL | 93.32 a | 94.63 b | 99.97 b | 93.81 a | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matłok, N.; Gorzelany, J.; Figiel, A.; Balawejder, M. Effect of Fertilisation on the Quality of Dried Coriander (Coriandrum sativum L.) and Lovage (Levisticum officinale). Agriculture 2021, 11, 386. https://doi.org/10.3390/agriculture11050386
Matłok N, Gorzelany J, Figiel A, Balawejder M. Effect of Fertilisation on the Quality of Dried Coriander (Coriandrum sativum L.) and Lovage (Levisticum officinale). Agriculture. 2021; 11(5):386. https://doi.org/10.3390/agriculture11050386
Chicago/Turabian StyleMatłok, Natalia, Józef Gorzelany, Adam Figiel, and Maciej Balawejder. 2021. "Effect of Fertilisation on the Quality of Dried Coriander (Coriandrum sativum L.) and Lovage (Levisticum officinale)" Agriculture 11, no. 5: 386. https://doi.org/10.3390/agriculture11050386
APA StyleMatłok, N., Gorzelany, J., Figiel, A., & Balawejder, M. (2021). Effect of Fertilisation on the Quality of Dried Coriander (Coriandrum sativum L.) and Lovage (Levisticum officinale). Agriculture, 11(5), 386. https://doi.org/10.3390/agriculture11050386










