Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review
Abstract
1. Introduction
2. Categories and Procedure
2.1. Discretion of Two Categories
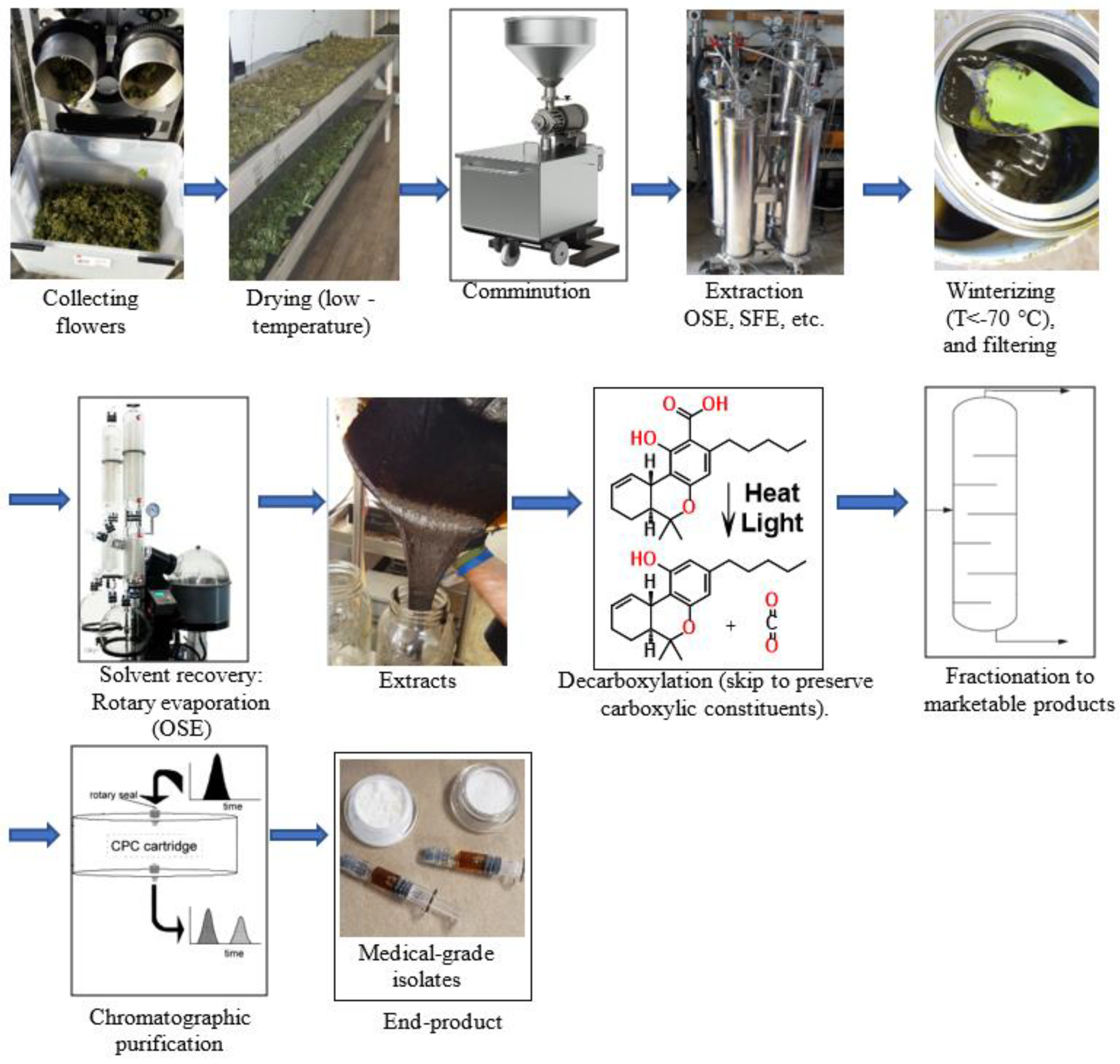
2.2. Downstream Process in Cannabinoid/Terpene Extraction
3. Extraction
3.1. Basics
3.2. Solubility Parameters
3.3. Chemically Assisted Extraction
3.3.1. Soxhlet Extraction Method
3.3.2. Maceration (Immersion) Method
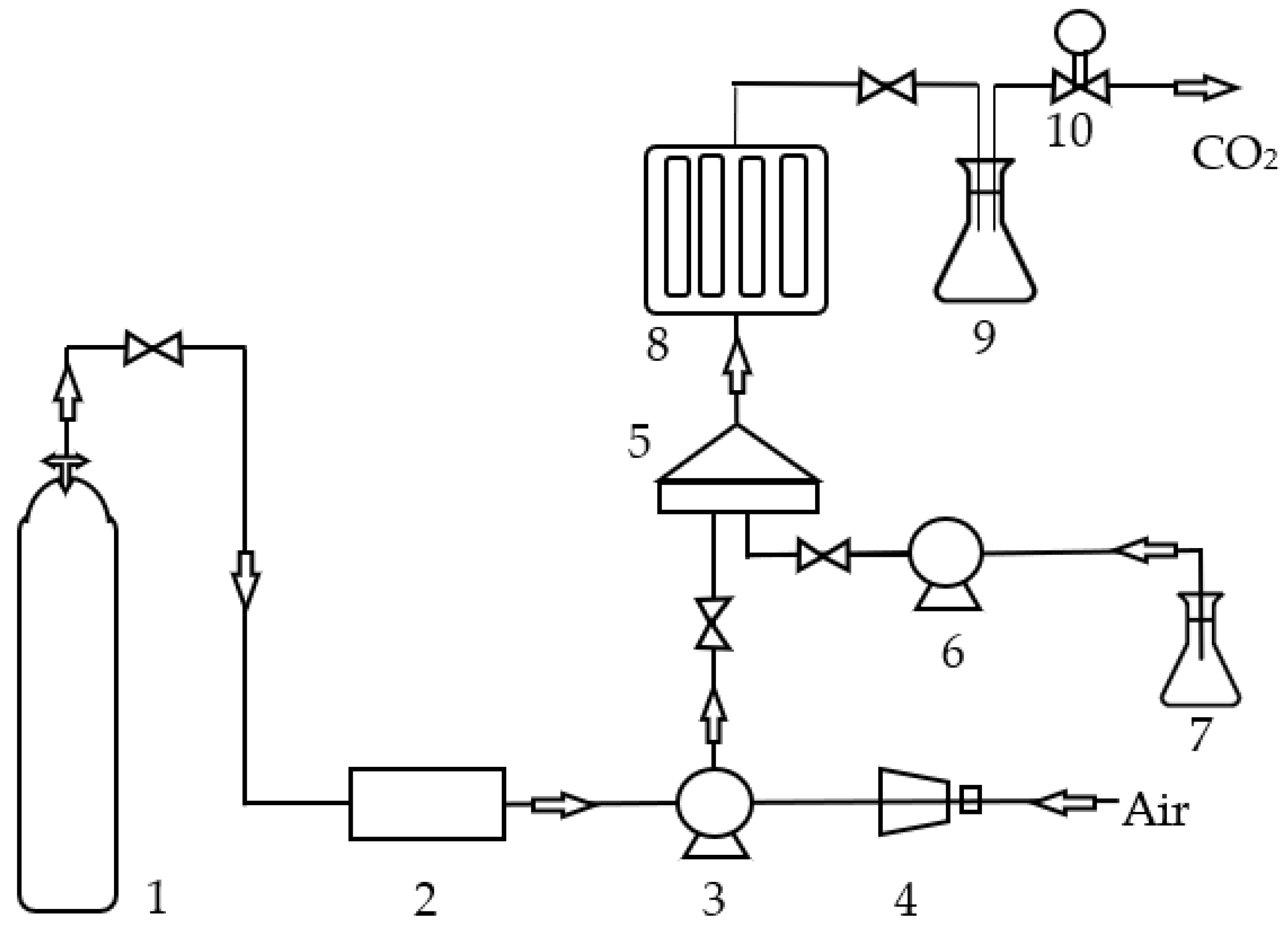
3.3.3. Extraction by Supercritical Fluids
3.3.4. Comparison between Organic Solvent and SC-CO2 Extraction Methods
3.4. Effect of Pretreatment on Oil Extraction
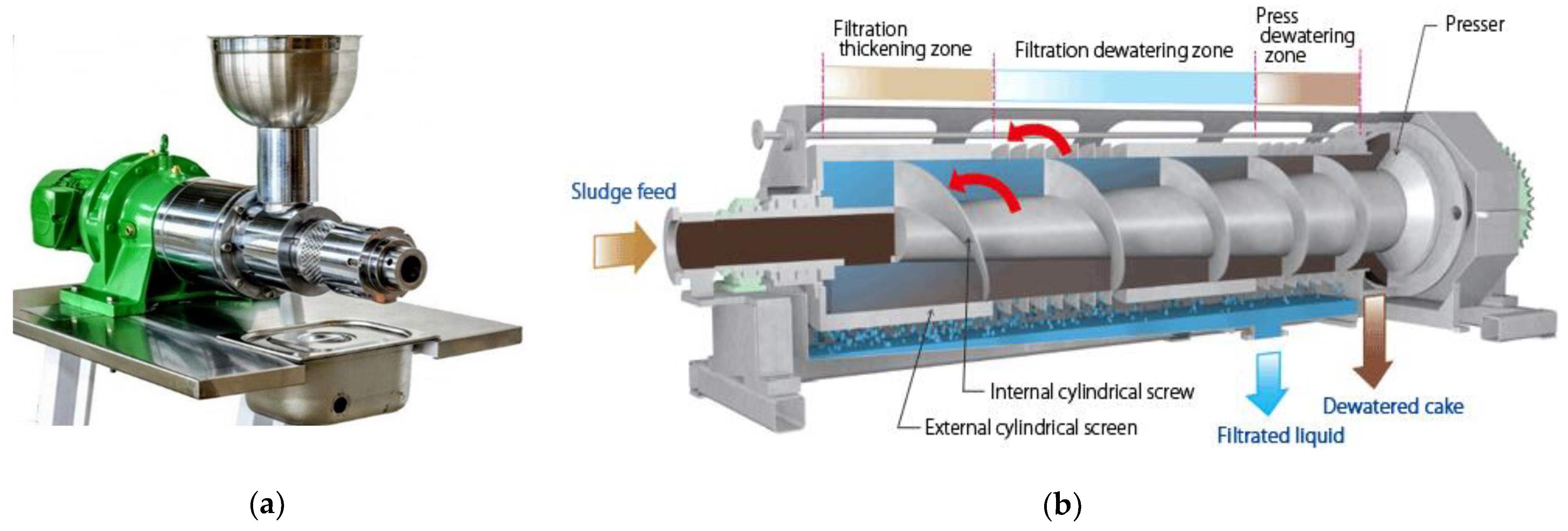
3.4.1. Mechanical (Press) Extraction
3.4.2. Microwave-Assisted Extraction
3.5. Supercritical Hot Water Extraction
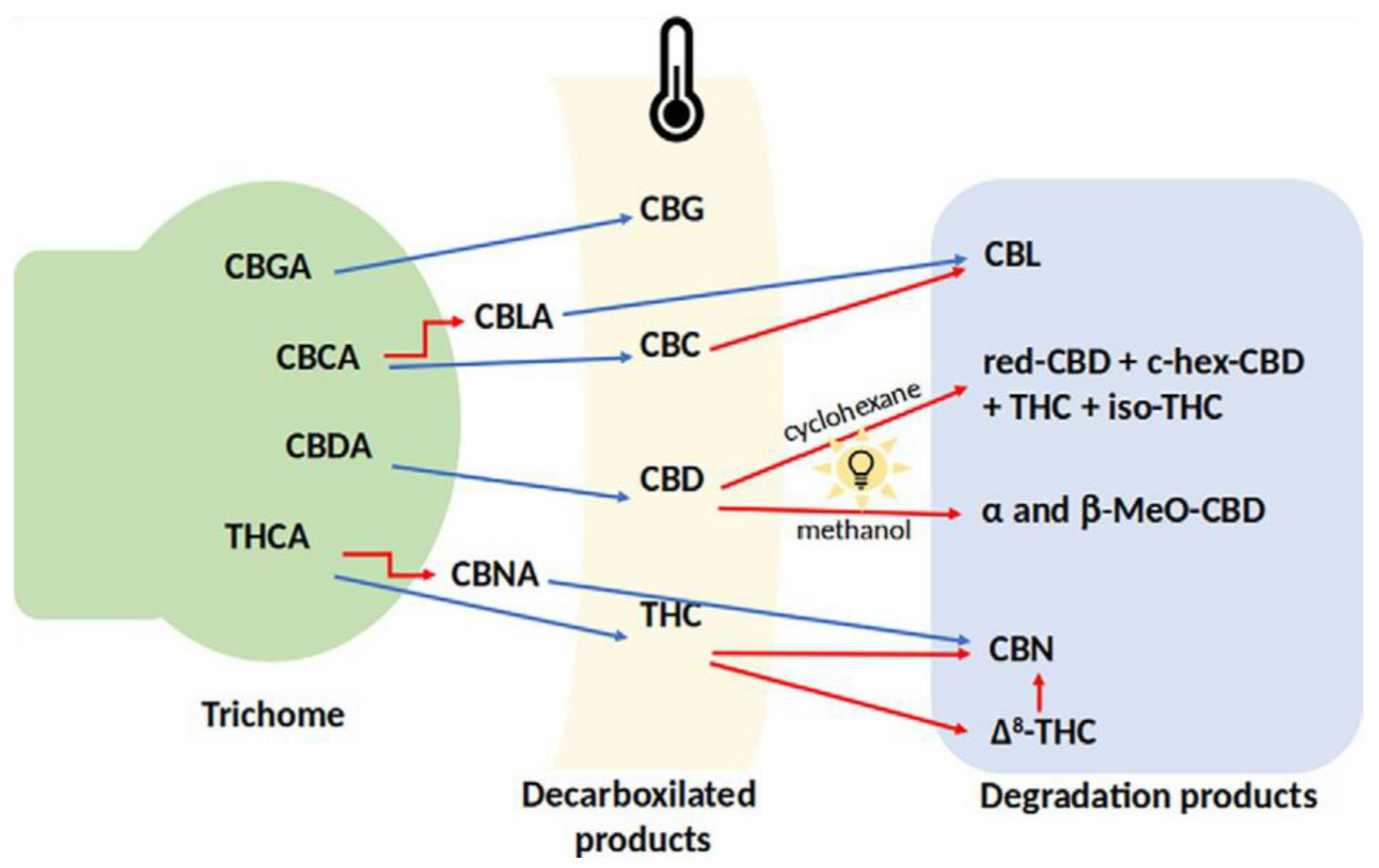
4. Transformation of Constituents
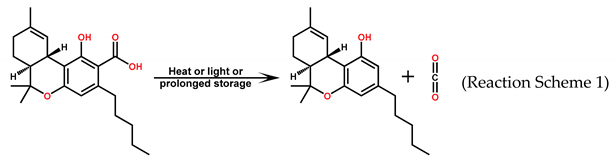
Effect of Light on Transformation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peters, H.; Nahas, G.G. A Brief History of Four Millennia (B.C. 2000—A.D. 1974). In Marihuana and Medicine; Humana Press: New York, NY, USA, 1999; pp. 3–7. [Google Scholar]
- Linnaeus, C. Species Plantarum; Impensis Laurentii Salvii; Holmiae: Stockholm, Sweden, 1753. [Google Scholar]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial Cannabinoids from Cannabis sativa: A Structure−Activity Study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Touw, M. The religious and medicinal uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef]
- Yamauchi, T.; Shoyama, Y.; Matsuo, Y.; Nishioka, I. Cannabigerol Monomethyl Ether, a New Component of Hemp. Chem. Pharm. Bull. 1968, 16, 1164–1165. [Google Scholar] [CrossRef]
- ITIS. ITIS Standard Report Page: Cannabis sativa. Available online: https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=19109#null (accessed on 7 July 2019).
- Pollio, A. The Name of Cannabis: A Short Guide for Nonbotanists. Cannabis Cannabinoid Res. 2016, 1, 234–238. [Google Scholar] [CrossRef]
- Aiello, G.; Fasoli, E.; Boschin, G.; Lammi, C.; Zanoni, C.; Citterio, A.; Arnoldi, A. Proteomic characterization of hempseed (Cannabis sativa L.). J. Proteom. 2016, 147, 187–196. [Google Scholar] [CrossRef]
- Congressional Research Service. SEC. 7606. Legitimacy of Industrial Hemp Research; Congressional Research Service USA: Washington, DC, USA, 2008; Volume 3.
- Grotenhermen, F.; Karus, M. Industrial Hemp Is Not Marijuana. Available online: http://www.internationalhempassociation.org/jiha/jiha5210.html (accessed on 13 January 2020).
- Johnson, R. Hemp as an Agricultural Commodity Renée Johnson Specialist in Agricultural Policy; Congressional Research Service: Washington, DC, USA, 2018.
- Grotenhermen, F.; Karus, M.; Lohmeyer, D. THC-limits for food: A scientific study. J. Int. Hemp Assoc. 1998, 5. [Google Scholar]
- DISA. Global Solutions Map of Marijuana Legality by States. Available online: https://disa.com/map-of-marijuana-legality-by-state (accessed on 3 January 2021).
- US Food and Drug Administration. FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. Case Med. Res. 2018. [Google Scholar] [CrossRef]
- Koturbash, I.; MacKay, D. Cannabidiol and Other Cannabinoids: From Toxicology and Pharmacology to the Development of a Regulatory Pathway. J. Diet. Suppl. 2020, 17, 487–492. [Google Scholar] [CrossRef]
- Dussy, F.E.; Hamberg, C.; Luginbühl, M.; Schwerzmann, T.; Briellmann, T.A. Isolation of Δ9-THCA-A from hemp and analytical aspects concerning the determination of Δ9-THC in cannabis products. Forensic Sci. Int. 2005, 149, 3–10. [Google Scholar] [CrossRef]
- Europäischer Rat. Amtsblatt der Europäischen Gemeinschaften, Germany, 2000.
- Arcview Global Legal Cannabis Markets to Grow 36% in 2019 Despite 2018 Challenges; To Break $40 Billion by 2024. Available online: https://bdsa.com/new-report-global-legal-cannabis-markets-to-grow-36-in-2019-despite-2018-challenges-to-break-40-billion-by-2024/ (accessed on 20 April 2020).
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A Review on the Current State of Knowledge of Growing Conditions, Agronomic Soil Health Practices and Utilities of Hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Molleken, H.; Theimer, R. Survey of minor fatty acids in Cannabis sativa L. fruits. J. Int. Hemp Assoc. 1997, 4, 13–17. [Google Scholar]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the Quality of Protein from Hemp Seed (Cannabis sativa L.) Products Through the use of the Protein Digestibility-Corrected Amino Acid Score Method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef] [PubMed]
- Afif, M.K.; Biradar, C.H. Production of Biodiesel from Cannabis sativa (Hemp) Seed oil and its Performance and Emission Characteristics on DI Engine Fueled with Biodiesel Blends. Int. Res. J. Eng. Technol. 2019, 6, 246–253. [Google Scholar]
- Da Porto, C.; Decorti, D.; Natolino, A. Separation of aroma compounds from industrial hemp inflorescences (Cannabis sativa L.) by supercritical CO2 extraction and on-line fractionation. Ind. Crop. Prod. 2014, 58, 99–103. [Google Scholar] [CrossRef]
- Pojić, M.; Dapčević Hadnadev, T.; Hadnadev, M.; Rakita, S.; Brlek, T. Bread Supplementation with Hemp Seed Cake: A By-Product of Hemp Oil Processing. J. Food Qual. 2015, 38, 431–440. [Google Scholar] [CrossRef]
- Small, E. Cannabis; CRC Press: Boca Raton, FL, USA; Taylor & Francis: New York, NY, USA, 2017; ISBN 9781315367583. [Google Scholar]
- Whittle, B.C.A.; Hill, I.R.; Flockhart, D.V.D.; Gibson, P.; Whittle, G.W.W.; Brian, A.; Hill, C.A.; Flockhart, I.R.; Downs, D.V.; Gibson, P.; et al. Extraction of Pharmaceutically Active Components from Plant Materials. U.S. Patent 7344736 B2, 18 March 2008. [Google Scholar]
- Rosenthal, E. Beyond Buds: Marijuana Extracts—Hash, Vaping, Dabbing, Edibles & Medicines; Quick Trading Company: Oakland, CA, USA, 2014; ISBN 9781936807239. [Google Scholar]
- Rutz, A. CPC Distribution Chromatography of Cannabinoids. U.S. Patent WO2016135346A1, 1 September 2016. [Google Scholar]
- Wang, M.; Wang, Y.H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; Van Antwerp, J.; Parcher, J.F.; Elsohly, M.A.; Khan, I.A. Decarboxylation Study of Acidic Cannabinoids: A Novel Approach Using Ultra-High-Performance Supercritical Fluid Chromatography/Photodiode Array-Mass Spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef]
- Nahar, L.; Uddin, S.J.; Alam, M.A.; Sarker, S.D. Extraction of naturally occurring cannabinoids: An update. Phytochem. Anal. 2020, 2987. [Google Scholar] [CrossRef]
- King, J.W. The relationship between cannabis/hemp use in foods and processing methodology. Curr. Opin. Food Sci. 2019, 28, 32–40. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis sativa L.—Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-54563-9. [Google Scholar]
- Brenneisen, R. Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents. In Marijuana and the Cannabinoids; Humana Press: New York, NY, USA, 2007; pp. 17–49. [Google Scholar]
- Mark, T.; Shepherd, J.; Olson, D.; Snell, W.; Proper, S.; Thornsbury, S. Economic Viability of Industrial Hemp in the United States: A Review of State Pilot Programs; United States Department of Agriculture: Erie, KS, USA, 2020. [Google Scholar]
- Pieter Sikkel PYXUS international 2019 Annual Report; PYXUS International, Inc.: New York, NY, USA, 2019; Volume 61.
- Deferne, J.; Pate, D.W. Hemp seed oil: A source of valuable essential fatty acids. J. Int. Hemp Assoc. 1996, 3, 4–7. [Google Scholar]
- Al Mamun, M.A. Safe Storage Guidelines for Industrial Hemp (Cannabis sativa) Seeds; University of Manitoba: Winnipeg, MB, Canada, 2018. [Google Scholar]
- Aladić, K. Cold Pressing and Supercritical CO2 Extraction of Hemp (Cannabis sativa) Seed Oil. Chem. Biochem. Eng. Q. J. 2015, 28, 481–490. [Google Scholar] [CrossRef]
- Shin, S.J.; Sung, Y.J. Improving enzymatic hydrolysis of industrial hemp (Cannabis sativa L.) by electron beam irradiation. Radiat. Phys. Chem. 2008, 77, 1034–1038. [Google Scholar] [CrossRef]
- Subratti, A.; Lalgee, L.J.; Jalsa, N.K. Liquified dimethyl ether (DME): A green solvent for the extraction of hemp (Cannabis sativa L.) seed oil. Sustain. Chem. Pharm. 2019, 12, 100144. [Google Scholar] [CrossRef]
- Grijó, D.R.; Vieitez Osorio, I.A.; Cardozo-Filho, L. Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers. J. Co2 Util. 2018, 28, 174–180. [Google Scholar] [CrossRef]
- Kitrytė, V.; Bagdonaitė, D.; Rimantas Venskutonis, P. Biorefining of industrial hemp (Cannabis sativa L.) threshing residues into cannabinoid and antioxidant fractions by supercritical carbon dioxide, pressurized liquid and enzyme-assisted extractions. Food Chem. 2018, 267, 420–429. [Google Scholar] [CrossRef]
- Drinić, Z.; Vladić, J.; Koren, A.; Zeremski, T.; Stojanov, N.; Kiprovski, B.; Vidović, S. Microwave-assisted extraction of cannabinoids and antioxidants from Cannabis sativa aerial parts and process modeling. J. Chem. Technol. Biotechnol. 2020, 95, 831–839. [Google Scholar] [CrossRef]
- Kostić, M.D.; Joković, N.M.; Stamenković, O.S.; Rajković, K.M.; Milić, P.S.; Veljković, V.B. The kinetics and thermodynamics of hempseed oil extraction by n-hexane. Ind. Crop. Prod. 2014, 52, 679–686. [Google Scholar] [CrossRef]
- Srinivas, K.; King, J.W.; Monrad, J.K.; Howard, L.R.; Hansen, C.M. Optimization of Subcritical Fluid Extraction of Bioactive Compounds Using Hansen Solubility Parameters. J. Food Sci. 2009, 74, E342–E354. [Google Scholar] [CrossRef] [PubMed]
- Soxhlet, F. Scopus—Document details. Dingler’s Polytech. J. 1879, 232, 461–465. [Google Scholar]
- Dutta, R.; Sarkar, U.; Mukherjee, A. Extraction of oil from Crotalaria Juncea seeds in a modified Soxhlet apparatus: Physical and chemical characterization of a prospective bio-fuel. Fuel 2014, 116, 794–802. [Google Scholar] [CrossRef]
- Nakahara, Y.; Sekine, H. Studies on Confirmation of Cannabis Use. I. Determination of the Cannabinoid Contents in Marijuana Cigarette, Tar, and Ash Using High Performance Liquid Chromatography with Electrochemical Detection. J. Anal. Toxicol. 1985, 9, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Marchini, M.; Charvoz, C.; Dujourdy, L.; Baldovini, N.; Filippi, J.-J. Multidimensional analysis of cannabis volatile constituents: Identification of 5,5-dimethyl-1-vinylbicyclo[2.1.1]hexane as a volatile marker of hashish, the resin of Cannabis sativa L. J. Chromatogr. A 2014, 1370, 200–215. [Google Scholar] [CrossRef]
- Cheng, H.-F. Green Engineering: Green Composite Material, Biodiesel from Waster Coffee Grounds, and Polyurethane Bio-Foam; California State University: Long Beach, CA, USA, 2012. [Google Scholar]
- Jadhav, D.; Rekha, B.N.; Gogate, P.R.; Rathod, V.K. Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. J. Food Eng. 2009, 93, 421–426. [Google Scholar] [CrossRef]
- Dmytryk, A.; Jacek, O.; Edward, R.; Katarzyna, C. Supercritical Fluid Applications—SC-CO2 Spirulina Platensis Extract as Plant Growth Biostimulant; New Chemical Syntheses Institute: Puławy, Poland, 2016; ISBN 978-83-935354-1-5. [Google Scholar]
- Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Özcan, M.M.; Osman, M.A.; Gassem, M.A.; Salih, H.A.A. Effects of Cold-Press and Soxhlet Extraction Systems on Antioxidant Activity, Total Phenol Contents, Fatty Acids, and Tocopherol Contents of Walnut Kernel Oils. J. Oleo Sci. 2019, 68, 167–173. [Google Scholar] [CrossRef]
- Thomas, D.A.; Virtanen, T.; Wiebe, M.G. Soxhlet extraction of mucic acid from fungal biomass. Sep. Sci. Technol. 2018, 53, 903–909. [Google Scholar] [CrossRef]
- Valizadeh Derakhshan, M.; Nasernejad, B.; Abbaspour-Aghdam, F.; Hamidi, M. Oil extraction from algae: A comparative approach. Biotechnol. Appl. Biochem. 2015, 62, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh Derakhshan, M.; Nasernejad, B.; Dadvar, M.; Hamidi, M. Pretreatment and kinetics of oil extraction from algae for biodiesel production. Asia-Pac. J. Chem. Eng. 2014, 9, 629–637. [Google Scholar] [CrossRef]
- Chawankul, N.; Chuaprasert, S.; Douglas, P.; Luewisutthichat, W. Simulation of an agitated thin film evaporator for concentrating orange juice using AspenPlus. J. Food Eng. 2001, 47, 247–253. [Google Scholar] [CrossRef]
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Martínez Ramírez, J.A.; Rivera Monroy, Z.J.; King, J.W.; Parada-Alfonso, F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208–216. [Google Scholar] [CrossRef]
- Kianimanesh, H.R.; Abbaspour-Aghdam, F.; Derakhshan, M.V. Biodiesel production from vegetable oil: Process design, evaluation and optimization. Pol. J. Chem. Technol. 2017, 19, 49–55. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lipid Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crop. Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind. Crop. Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Wianowska, D.; Dawidowicz, A.L.; Kowalczyk, M. Transformations of Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Cannabinol During Their Extraction from Cannabis sativa L. J. Anal. Chem. 2015, 70, 805–810. [Google Scholar] [CrossRef]
- Crescente, G.; Piccolella, S.; Esposito, A.; Scognamiglio, M.; Fiorentino, A.; Pacifico, S. Chemical composition and nutraceutical properties of hempseed: An ancient food with actual functional value. Phytochem. Rev. 2018, 17, 733–749. [Google Scholar] [CrossRef]
- Pandohee, J.; Holland, B.J.; Li, B.; Tsuzuki, T.; Stevenson, P.G.; Barnett, N.W.; Pearson, J.R.; Jones, O.A.H.; Conlan, X.A. Screening of cannabinoids in industrial-grade hemp using two-dimensional liquid chromatography coupled with acidic potassium permanganate chemiluminescence detection. J. Sep. Sci. 2015, 38, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Da Porto, C.; Voinovich, D.; Decorti, D.; Natolino, A. Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J. Supercrit. Fluids 2012, 68, 45–51. [Google Scholar] [CrossRef]
- Chang, C.W.; Yen, C.C.; Wu, M.T.; Hsu, M.C.; Wu, T.Y. Microwave-assisted extraction of cannabinoids in hemp nut using response surface methodology: Optimization and comparative study. Molecules 2017, 22, 1894. [Google Scholar] [CrossRef] [PubMed]
- Attard, T.M.; Bainier, C.; Reinaud, M.; Lanot, A.; McQueen-Mason, S.J.; Hunt, A.J. Utilisation of supercritical fluids for the effective extraction of waxes and Cannabidiol (CBD) from hemp wastes. Ind. Crop. Prod. 2018, 112, 38–46. [Google Scholar] [CrossRef]
- Marques, G.; del Río, J.C.; Gutiérrez, A. Lipophilic extractives from several nonwoody lignocellulosic crops (flax, hemp, sisal, abaca) and their fate during alkaline pulping and TCF/ECF bleaching. Bioresour. Technol. 2010, 101, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Tegen, M.G.; Cho, J.; Sutterlin, W.R. Processes for Solvent Extraction of Cannabinoids, Terpenes and Flavonoids from Biomass. U.S. Patent No 10,414,709, 17 September 2019. [Google Scholar]
- Franklin, R.M.; Rosenthal, E. Method for Conducing Concentrated Cannabis Oil To Be Stable, Emulsifiable and Flavorless for Use in Hot Beverages and Resulting Powderized Cannabis Oil. U.S. Patent No 20,170,188,605, 6 July 2017. [Google Scholar]
- Delmoral, R.K.; Hughes, B.; Pal, K.; Samuelsson, A. Cannabinoid Extraction Process Using Brin. U.S. Patent No 10,406,453, 10 September 2019. [Google Scholar]
- Cunha, V.M.B.; da Silva, M.P.; da Costa, W.A.; de Oliveira, M.S.; Bezerra, F.W.F.; de Melo, A.C.; Pinto, R.H.H.; Machado, N.T.; Araujo, M.E.; de Junior, R.N.C. Carbon Dioxide Use in High-Pressure Extraction Processes. In Carbon Dioxide Chemistry, Capture and Oil Recovery; InTechOpen: London, UK, 2018. [Google Scholar]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- Hong, G.T. Supercritical Fluid Extraction. J. Nat. Prod. 1996, 59, 1215. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibañez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae—A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Moslavac, T.; Jokić, S.; Šubarić, D.; Aladić, K.; Vukoja, J.; Prce, N. Pressing and supercritical CO2 extraction of Camelina sativa oil. Ind. Crop. Prod. 2014, 54, 122–129. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Smith, R.; Inomata, H.; Peters, C. Introduction to Supercritical Fluids, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2013; ISBN 9780080931302. [Google Scholar]
- Kraujalis, P.; Kraujalienė, V.; Kazernavičiūtė, R.; Venskutonis, P.R. Supercritical carbon dioxide and pressurized liquid extraction of valuable ingredients from Viburnum opulus pomace and berries and evaluation of product characteristics. J. Supercrit. Fluids 2017, 122, 99–108. [Google Scholar] [CrossRef]
- Gallego, R.; Bueno, M.; Herrero, M. Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. Trac Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Mueller, A. Method for Producing an Extract from Cannabis Plant Matter, Containing a Tetrahydrocannabinol and a Cannabidiol and Cannabis Extracts. U.S. Patent No 8,895,078, 25 November 2014. [Google Scholar]
- Apeks Apeks Supercritical. Available online: https://www.apekssupercritical.com/wp-content/uploads/2020/01/Force-Spec-Sheet-10.19.pdf (accessed on 30 October 2020).
- Edenlabs Hi-Flo FX2 High Performance Series (CO2) Extraction Systems). Available online: https://www.edenlabs.com/wp-content/uploads/2019/09/EDL-Product_Flyer-Hi-FloFX2-071519.pdf (accessed on 30 October 2020).
- May, M.A. Guide to Cannabis Extraction Equipment and Machines. Available online: https://www.analyticalcannabis.com/news/a-guide-to-cannabis-extraction-equipment-and-machines-311552 (accessed on 30 October 2020).
- Teräsvalli, H. Extraction and Purification of Cannabidiol. Ph.D. Thesis, Lappeenranta University of Technology, Lappeenranta, Finland, 2020. [Google Scholar]
- Apeks Supercritical Introductory: The Bambino®—Apeks Supercritical. Available online: https://www.apekssupercritical.com/extraction-systems/introductory-the-bambino/ (accessed on 30 October 2020).
- Extractor Extrakt Lab. E-180. Supercritical CO2 Extractor. Available online: https://extraktlab.com/wp-content/uploads/2020/03/supercritical-co2-extraction-180.pdf (accessed on 30 October 2020).
- Lindy, J. Supercritical Fluid Extraction: Technology, Applications and Limitations; Nova Science: New York, NY, USA, 2014; ISBN 978-1-63463-310-9. [Google Scholar]
- Arranz, E.; Jaime, L.; Lopez de la Hazas, M.C.; Vicente, G.; Reglero, G.; Santoyo, S. Supercritical sage extracts as anti-inflammatory food ingredients. Ind. Crop. Prod. 2014, 54, 159–166. [Google Scholar] [CrossRef]
- Perrotin-Brunel, H.; Kroon, M.C.; Van Roosmalen, M.J.E.; Van Spronsen, J.; Peters, C.J.; Witkamp, G.J. Solubility of non-psychoactive cannabinoids in supercritical carbon dioxide and comparison with psychoactive cannabinoids. J. Supercrit. Fluids 2010, 55, 603–608. [Google Scholar] [CrossRef]
- Kotra, L.; Lewis, M.; Wasilewski, E.; Grover, H. Decarboxylated Cannabis Resins, Use Thereof and Methods of Making Same. U.S. Patent No 10,383,892, 20 August 2019. [Google Scholar]
- Perrotin-Brunel, H.; Perez, P.C.; van Roosmalen, M.J.E.; van Spronsen, J.; Witkamp, G.J.; Peters, C.J. Solubility of Δ9-tetrahydrocannabinol in supercritical carbon dioxide: Experiments and modeling. J. Supercrit. Fluids 2010, 52, 6–10. [Google Scholar] [CrossRef]
- Eggers, R. Extraktion von Fettrohstoffen mit überkritischem CO2. Fett Wiss. Technol. Sci. Technol. 1994, 96, 513–518. [Google Scholar] [CrossRef]
- Tomita, K.; Machmudah, S.; Quitain, A.T.; Sasaki, M.; Fukuzato, R.; Goto, M. Extraction and solubility evaluation of functional seed oil in supercritical carbon dioxide. J. Supercrit. Fluids 2013, 79, 109–113. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus, B. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Pojić, M.; Mišan, A.; Sakač, M.; Dapčević Hadnađev, T.; Šarić, B.; Milovanović, I.; Hadnađev, M.; HadnaCrossed D Signev, T.D.; Šarić, B.; Milovanović, I.; et al. Characterization of Byproducts Originating from Hemp Oil Processing. J. Agric. Food Chem. 2014, 62, 12436–12442. [Google Scholar] [CrossRef]
- Oseyko, M.; Sova, N.; Lutsenko, M.; Kalyna, V. Chemical aspects of the composition of industrial hemp seed products. Ukr. Food J. 2019, 8, 544–559. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Menexis, N.; Iakovidis, D.; Makris, D.; Goula, A. A Green Extraction Process to Recover Polyphenols from Byproducts of Hemp Oil Processing. Recycling 2018, 3, 15. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Ramirez, C.L.; Fanovich, M.A.; Churio, M.S. Cannabinoids: Extraction Methods, Analysis, and Physicochemical Characterization. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 61, pp. 143–173. ISBN 9780444641830. [Google Scholar]
- Xie, D.T.; Wang, Y.Q.; Kang, Y.; Hu, Q.F.; Su, N.Y.; Huang, J.M.; Che, C.T.; Guo, J.X. Microwave-assisted extraction of bioactive alkaloids from Stephania sinica. Sep. Purif. Technol. 2014, 130, 173–181. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess. Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction. J. Food Sci. Technol. 2019, 56, 4198–4210. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Máthé, K.; Hofmann, T.; Csóka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis sativa L. Optimized by Response Surface Methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef]
- Serna-Loaiza, S.; Adamcyk, J.; Beisl, S.; Kornpointner, C.; Halbwirth, H.; Friedl, A. Pressurized Liquid Extraction of Cannabinoids from Hemp Processing Residues: Evaluation of the Influencing Variables. Processes 2020, 8, 1334. [Google Scholar] [CrossRef]
- Teh, S.S.; Niven, B.E.; Bekhit, A.E.D.A.; Carne, A.; Birch, E.J. The Use of Microwave and Pulsed Electric Field as a Pretreatment Step in Ultrasonic Extraction of Polyphenols from Defatted Hemp Seed Cake (Cannabis sativa) Using Response Surface Methodology. Food Bioprocess. Technol. 2014, 7, 3064–3076. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Liang, J. Value-Addition of Cold Pressed Hemp Seed Oil and Oil By-Products through Ultrasonic Bleaching and Heat Treatment: Evaluation of Chlorophyll, Oxidative Stability and Antioxidant Activity. Ph.D. Thesis, University of Manitoba, Winnipeg, MB, Canada, 2017. [Google Scholar]
- Plaza, M.; Turner, C. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Nuapia, Y.; Tutu, H.; Chimuka, L.; Cukrowska, E. Selective extraction of cannabinoid compounds from cannabis seed using pressurized hot water extraction. Molecules 2020, 25, 1335. [Google Scholar] [CrossRef]
- Lang, Q.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar] [CrossRef]
- Iffland, K.; Carus, M.; Franjo Grotenhermen, M. Decarboxylation of Tetrahydrocannabinolic acid (THCA) to active THC. EIHA 2016, 1–3. [Google Scholar] [CrossRef]
- Perrotin-Brunel, H.; Buijs, W.; van Spronsen, J.; van Roosmalen, M.J.E.V.; Peters, C.J.; Verpoorte, R.; Witkamp, G.-J.J. Decarboxylation of Δ9-tetrahydrocannabinol: Kinetics and molecular modeling. J. Mol. Struct. 2011, 987, 67–73. [Google Scholar] [CrossRef]
- Taschwer, M.; Schmid, M.G. Determination of the relative percentage distribution of THCA and δ9-THC in herbal cannabis seized in Austria—Impact of different storage temperatures on stability. Forensic Sci. Int. 2015, 254, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Gryczka, U.; Migdal, W.; Chmielewska, D.; Antoniak, M.; Kaszuwara, W.; Jastrzebska, A.; Olszyna, A. Examination of changes in the morphology of lignocellulosic fibers treated with e-beam irradiation. Radiat. Phys. Chem. 2014, 94, 226–230. [Google Scholar] [CrossRef]
- Sung, Y.J.; Shin, S.-J. Compositional changes in industrial hemp biomass (Cannabis sativa L.) induced by electron beam irradiation Pretreatment. Biomass Bioenergy 2011, 35, 3267–3270. [Google Scholar] [CrossRef]
- Cardenia, V.; Gallina Toschi, T.; Scappini, S.; Rubino, R.C.; Rodriguez-Estrada, M.T. Development and validation of a Fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018, 26, 1283–1292. [Google Scholar] [CrossRef]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef]
- Eichhorn Bilodeau, S.; Wu, B.-S.S.; Rufyikiri, A.-S.S.; MacPherson, S.; Lefsrud, M. An Update on Plant Photobiology and Implications for Cannabis Production. Front. Plant. Sci. 2019, 10, 296. [Google Scholar] [CrossRef]
- Kazem-Rostami, M. Design and Synthesis of Ʌ-Shaped Photoswitchable Compounds Employing Tröger’s Base Scaffold. Synthesis 2016, 49, 1214–1222. [Google Scholar] [CrossRef][Green Version]
- Kazem-Rostami, M. Optically active and photoswitchable Tröger’s base analogs. New J. Chem. 2019, 43, 7751–7755. [Google Scholar] [CrossRef]
- Zamengo, L.; Bettin, C.; Badocco, D.; Di Marco, V.; Miolo, G.; Frison, G. The role of time and storage conditions on the composition of hashish and marijuana samples: A four-year study. Forensic. Sci. Int. 2019, 298, 131–137. [Google Scholar] [CrossRef]
- Trofin, I.G.; Vlad, C.C.; Dabija, G.; Filipescu, L. Influence of storage conditions on the chemical potency of herbal cannabis. Rev. Chim. 2011, 62, 639–645. [Google Scholar]
- Fairbairn, J.W.; Liebmann, J.A.; Rowan, M.G. The stability of cannabis and its preparations on storage. J. Pharm. Pharmacol. 1976, 28, 1–7. [Google Scholar] [CrossRef]
- Park, Y.; Mackie, A.L.; Macisaac, S.A.; Gagnon, G.A. Photo-oxidation of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol using medium-pressure UV and UV/H2O2-a kinetic study. Environ. Sci. Water Res. Technol. 2018, 4, 1262–1271. [Google Scholar] [CrossRef]
- Pipelzadeh, E.; Babaluo, A.A.; Haghighi, M.; Tavakoli, A.; Derakhshan, M.V.; Behnami, A.K. Silver doping on TiO2 nanoparticles using a sacrificial acid and its photocatalytic performance under medium pressure mercury UV lamp. Chem. Eng. J. 2009, 155, 660–665. [Google Scholar] [CrossRef]
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef]


| Trichome Category | Hempseed Category | |
|---|---|---|
| Target chemicals | Cannabinoids and terpenes | Fatty acids |
| Occurrence in plant | Female flower buds before pollination | Hempseed |
| Solvent used | Mainly polar solvents | nonpolar and polar solvents |
| Mass transfer driving force | ~(ΔCtrichome-media) = Concentration gradient between the solvent in contact with the trichome and that of the media | ~ (C* − C) = Concentration gradient between the saturated solution in contact with the seed particles and that of the media. [46] |
| Extraction method | OSE, SFE, and Soxhlet (small scale only) | Cold press, screw expeller followed by OSE, SFE, or Soxhlet (small scale only) |
| Co-extracts | Waxes, pigments, etc. | Defatted oil, oil sludges, etc. |
| Other application | pharmaceutical, recreational supplements, Electronics | Bioenergy production, abrasive fluids, food supplements, and cosmetics |
| Typical structures of chemicals of interest | 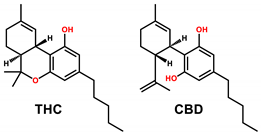 | 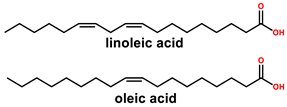 |
| Pre-extraction treatment | Mild comminution is sufficient; intense grinding leads to the co-extraction of undesirable substances [40] | Crushing and grinding the seed to smaller pieces contribute to an enhanced mass transfer |
| Biomass sp. | Dry Weight | Pretreatment | Solvent (s) | Extract | Target Analytes | Ref |
|---|---|---|---|---|---|---|
| Industrial-grade hemp (cannabis) | 0.5 g | Ground and sifted 368 μm | Consecutive batches of ethyl acetate 2 × 300 mL | batch 1, 1.5 h at 78 °C batch 2, 1 h at 78 °C | Cannabinoids | [68] |
| Seized Cannabis sativa L. plants | 2 g | Ground | n-hexane or methanol 75 mL | 1–3 h | Δ9-THC, THCA, CBN | [66] |
| Hemp (Cannabis sativa) seed oil | 10 g | Desiccated seeds | n-hexane 100 mL | 24 h at 70 °C | ω-6 and ω-3 fatty acids | [60] |
| Cannabis sativa L. seeds | 30 g | Ground hempseed | n-hexane 240 mL | 8 h at 70 °C | Fatty acids, e.g., ω-3 fatty acids | [64,71] |
| Hemp seed | 15 g | Extraction | Methanol 300 mL | 8 h at 90 °C | Cannabinoids | [72] |
| Industrial hemp dust residue | 11 g | Finely powdered hemp | Heptane 200 mL | 4 h | Waxes and cannabidiol (CBD) C16 was predominating | [73] |
| Hemp raw material and hemp cellulose pulps | - | - | Acetone | 8 h | Lipophilic extractives | [74] |
| Hemp seed oil | 5 g | Ground hemp seeds | n-hexane 120 mL | 2 h, 45 min at 180 °C | Tocopherols, fatty acids, and pigments | [65] |
| Cannabis sativa L. plant | 4 g | NA | Solvent polarity gradient:hexanes, ethyl acetate, and ethanol | Reduced pressure, 6 h | Total THC content | [61] |
| State of the Material | Density (kg m–3) | Diffusion (mm2 s–1) | Viscosity (μPa s) |
|---|---|---|---|
| Liquid phase | ~1000 | ~0.001 | ~(500–1000) |
| Supercritical state | ~100 to 1000 | ~0.01 to 0.1 | ~(50–100) |
| Gas state | ~1 | ~(1–10) | ~10 |
| Product Name | The Force® | The Bambino® | E-180 | Hi-FloTM FX2 |
|---|---|---|---|---|
| Manufacturer | Apeks Supercritical (Columbus, OH, USA) | Apeks Supercritical (Columbus, OH, USA) | ExtraktLAB (St Croix Falls, WI, USA) | Eden Labs (Seattle, WA, USA) |
| Production scale | Large-scale commercial, industrial | Small scale commercial, R&D | Large-scale commercial, industrial | Commercial |
| Extraction vessel volume | 80 L | 5 L | 80 L | 20 L |
| Per run dry biomass capacity | 18 kg | 1.4 kg | 10–16 kg | 4.5 kg |
| Max vessel pressure | 344 bars | 137 bars | 344 bars | 344 bars |
| Extraction temperature | Max 71 °C | Max 71 °C | 25–100 °C | −60 °C–60 °C |
| Flow rate of CO2 | 3.5–4.2 kg.min–1 | 0.4–0.8 kg.min–1 | Not disclosed | 2.2 L.min–1 |
| CO2 recovery | 95% | 95% | Not disclosed | Up to 95% |
| Run time | Not disclosed | Not disclosed | Not disclosed | 3–7 h |
| Remarks | Fully automated, subcritical extraction possible | Fully automated, subcritical extraction possible | Automated process control, possible subcritical extraction | Extensive automation |
| Ref. | [88] | [92] | [93] | [89,90] |
| Biomass Form | Pretreatment | Operating Temperature and Pressure | Objective of Study | Ref |
|---|---|---|---|---|
| Hemp flower | NA | 42, 54, 61, and 72 °C; 13.2–25.1 MPa | Solubility of cannabinoids using analytical methods | [98] |
| Hemp flower | NA | 42, 53, and 61 °C; 11.3–20.6 MPa | Comparing the solubility of psychoactive and non-psychoactive compounds | [96] |
| Hemp | N.A | 110–140 bar, 40–60 °C | Comparison of the molar solubility of the different cannabinoids in SC-CO2 | [99] |
| Hemp inflorescence | Ground | 10 and 14 MPa, 40 °C | Recovery volatile compounds from the inflorescences and comparison with the hydro-distillation performance | [25] |
| Hemp | Ground | 60 MPa, 35 °C | Method of preparing an herbal drug extract from medicinal cannabis | [28] |
| Leaves and buds | Ground | 17, 24, and 34 MPa at 55 °C CO2 flow rate of 200 g min–1 | Exploring the effects of pressure, initial cannabinoid plant composition, time, and the use of ethanol as a co-solvent | [83] |
| Hemp inflorescence | Ground | 25 MPa, 60 °C | Proposing a method for extraction of CBD-rich and THC-rich product from cannabis plant materials | [87] |
| Biomass | Pretreatment | Parameters | Objective of study | Ref. |
|---|---|---|---|---|
| Hempseed | Desiccated ground hempseed sieved (24-mesh tray) | 40–80 °C pressures of 20–40 MPa CO2 flow rate 3 mL min–1 | Determine fatty acids, tocopherols, and pigment content (chlorophyll a and b and total carotene) | [100] |
| Hempseed | Ground for 10, 30, and 60 s | Temperature (40, 50, and 60 °C), pressure (250, 300, and 350 bar) and particle diameter (0.59, 0.71, and 0.83 mm) | Total extraction and oxidation stability | [71] |
| Hempseed | Finely ground | Temperatures of 40, 60, and 80 °C and pressures of 300 and 40 MPa | Extraction yields, fatty-acid composition of the oil, and oxidation stability | [64] |
| 51 different genotypes of hemp | Finely ground | Optimized extraction temperature, 40 °C restricted heating, and a total volume of parameters were: 51.7 MPa, 100 °C extraction temp 120 mL carbon dioxide for each extraction | Fatty-acid composition and tocopherol content | [101] |
| Hempseed | Pressed | Pressure of 20 MPa and temperature of 40 °C with a CO2 mass flow rate of 4.9 kg h–1 | Evaluate the influence of extraction conditions on concentration of tocopherols, fatty acids, and pigments | [65] |
| Hemp stem fiber | Fine powder | 35 MPa and 50 °C (40 MPa and 65 °C, highest yield of crude wax extraction) | Extraction of fatty acids, policosanols (fatty alcohols), fatty aldehydes, triterpenoids, hydrocarbons, sterols, and cannabinoids | [73] |
| Pretreatment Method | Flower vs. Seed (Cannabinoids vs. Lipids) | Proper Scale | Remark | Selected Publications |
|---|---|---|---|---|
| Press and screw expeller | Fatty-acid | Small and large | Slow, cheap | [40,55,65,82,112] |
| Grind | Both | Small and large | Fast, cheap | [40,64,66,71,106] |
| Microwave | Fatty-acid | Small and large | Fast, cheap | [45,110,113,114] |
| High-pressure homogenization | Both | Small | Fast | [65] |
| Ultrasonication | Both | Small | Fast, expensive | [65,72,115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture 2021, 11, 384. https://doi.org/10.3390/agriculture11050384
Valizadehderakhshan M, Shahbazi A, Kazem-Rostami M, Todd MS, Bhowmik A, Wang L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture. 2021; 11(5):384. https://doi.org/10.3390/agriculture11050384
Chicago/Turabian StyleValizadehderakhshan, Mehrab, Abolghasem Shahbazi, Masoud Kazem-Rostami, Matthew Scott Todd, Arnab Bhowmik, and Lijun Wang. 2021. "Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review" Agriculture 11, no. 5: 384. https://doi.org/10.3390/agriculture11050384
APA StyleValizadehderakhshan, M., Shahbazi, A., Kazem-Rostami, M., Todd, M. S., Bhowmik, A., & Wang, L. (2021). Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture, 11(5), 384. https://doi.org/10.3390/agriculture11050384








