Relationship between Antioxidant Components and Oxidative Stability of Peanut Oils as Affected by Roasting Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Peanut Oils
2.3. Fatty Acid and Quality Indicesanalysis
2.4. Antioxidant Components and Antioxidant Capacity Analysis
2.5. Volatile Compound Analysis
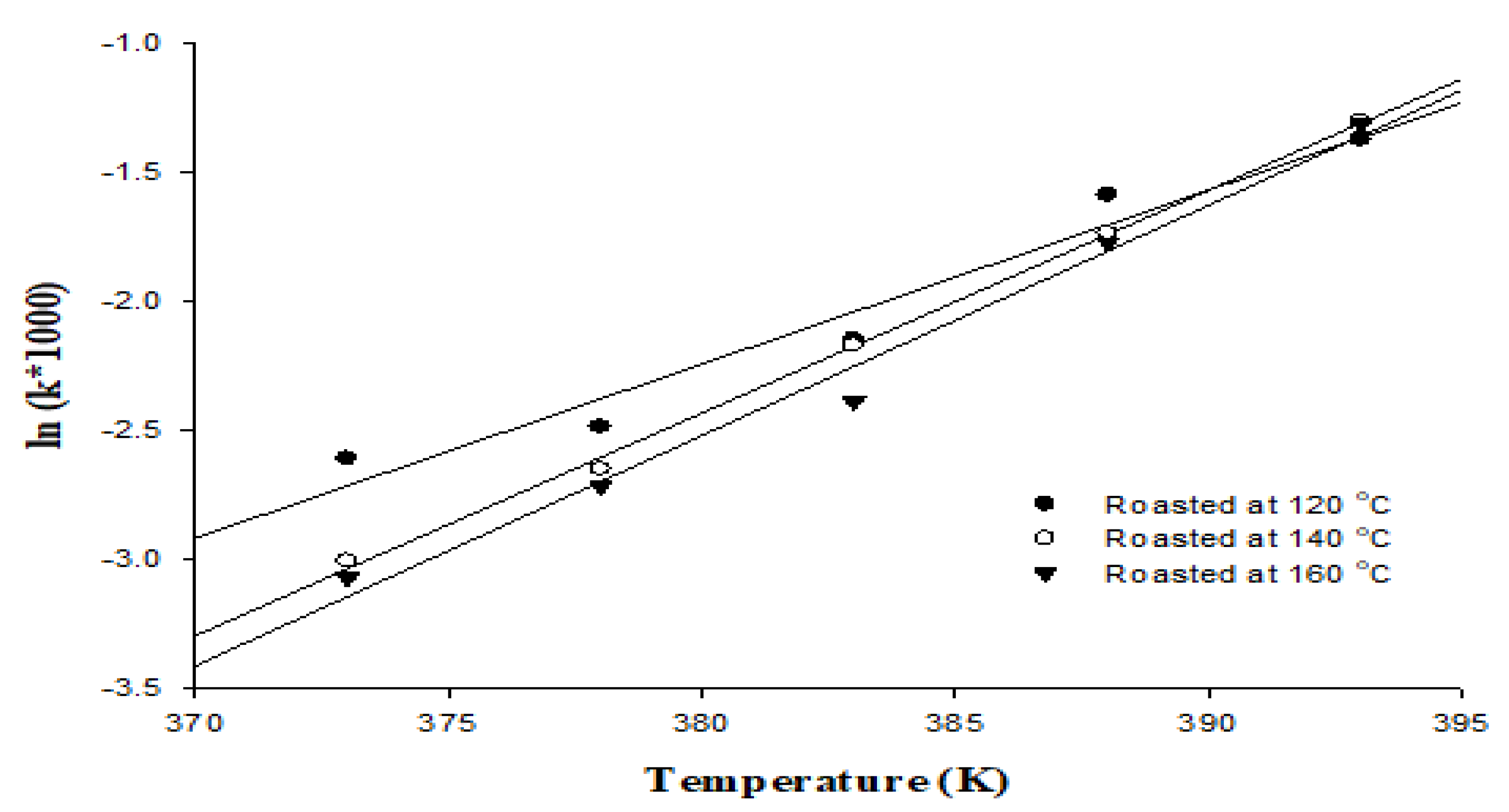
2.6. Kinetic Parameters of Rancimat Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Quality Indices
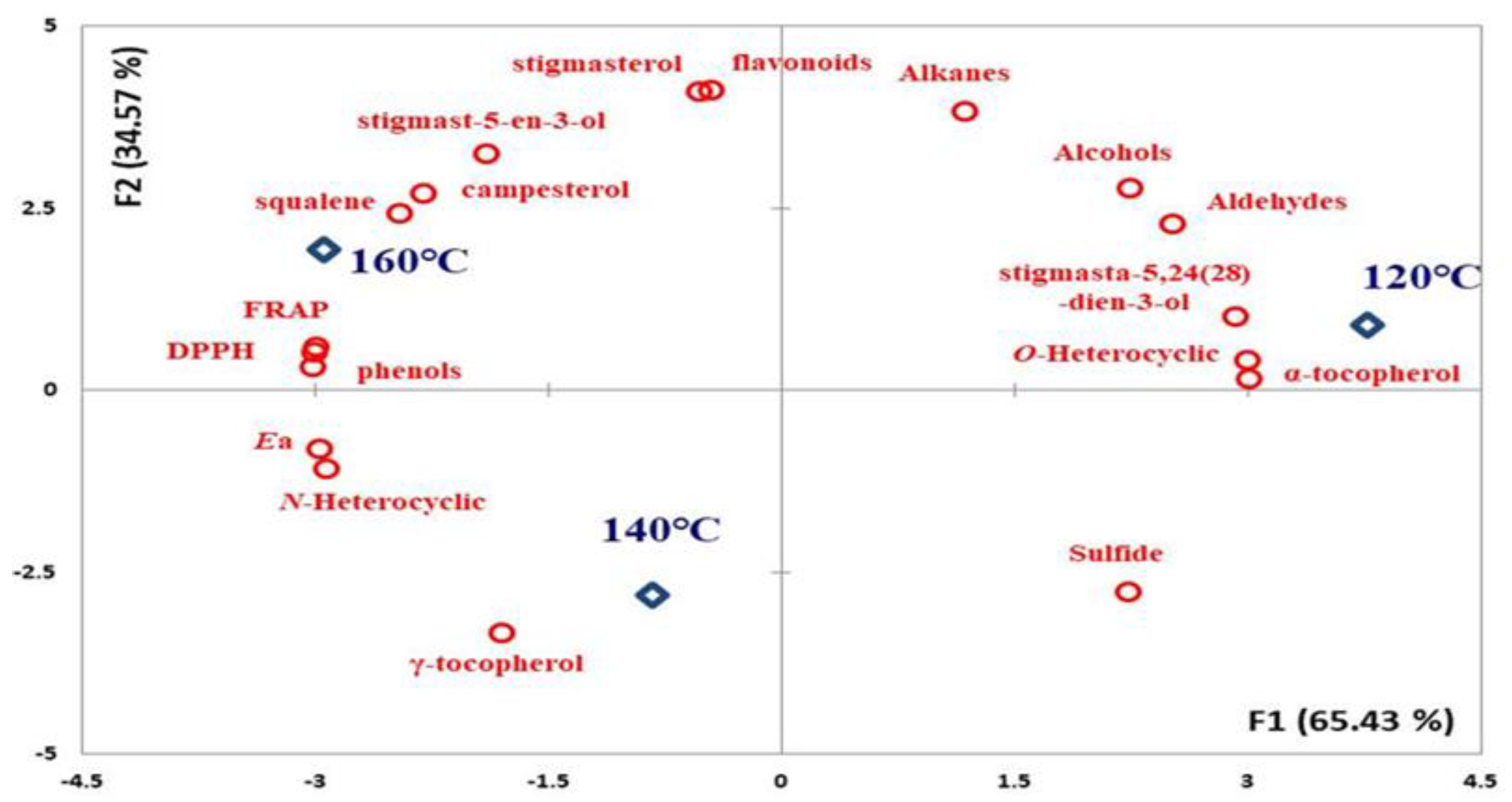
3.2. Antioxidant Components Change
3.3. Oxidation Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCA | principal component analysis |
| SFA | saturated fatty acid |
| MUFA | monounsaturated fatty acid |
| PUFA | polyunsaturated fatty acid |
| U/S | unsaturated fatty acid/saturated fatty acids |
| CVD | cardiovascular disease |
| LDL | low-density lipoprotein |
| GC/FID | gas chromatography/flame ionization detector |
| HPLC | high performance liquid chromatography |
| SPME | solid phase microextraction |
| DVB/CAR/PDMS | Divinylbenzene/Carboxen/Polydimethylsiloxane |
| BI | browning index |
| IP | induction period |
| Ea | activation energies |
| POV | peroxide value |
| p-AV | anisidine value |
| AV | acid value |
| TOTOX | total oxidation value |
| DPPH | 1,1-diphenyl-2-picrylhydrazylFRA |
| FRAP | Ferric ion reducing antioxidant power |
| TPTZ | 2,4,6-Tripyridyl-S-Triazine |
| GAE | gallic acid equivalents |
| QE | quercetin equivalents |
| RI | retention indices |
References
- Athar, M.; Nasir, S.M. Taxonomic perspective of plant species yielding vegetable oils used in cosmetics and skin care products. J. Biotechnol. 2005, 4, 36–44. [Google Scholar]
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef]
- Nawade, B.; Mishra, G.P.; Radhakrishnan, T.; Dodia, S.M.; Ahmad, S.; Kumar, A.; Kundu, R. High oleic peanut breeding: Achievements, perspectives, and prospects. Trends Food Sci. Technol. 2018, 78, 107–119. [Google Scholar] [CrossRef]
- Chukwumah, Y.; Walker, L.; Vogler, B.; Verghese, M. Changes in the phytochemical composition and profile of raw, boiled, and roasted peanuts. J. Agric. Food Chem. 2007, 55, 9266–9273. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, Y.; Chen, F. Effect of gamma irradiation on the physicochemical properties and nutrient contents of peanut. LWT 2018, 96, 535–542. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, S.; Li, M.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Evaluation on the formation of lipid free radicals in the oxidation process of peanut oil. LWT 2019, 104, 24–29. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Vegetable oil blending: A review of physico-chemical, nutritional and health effects. Trends Food Sci. Technol. 2016, 57, 52–58. [Google Scholar] [CrossRef]
- Chen, C.Y.O.; Blumberg, J.B. Phytochemical composition of nuts. Asia Pac. J. Clin. Nutr. 2008, 17, 329–332. [Google Scholar] [PubMed]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef]
- Mingyai, S.; Srikaeo, K.; Kettawan, A.; Singanusong, R.; Nakagawa, K.; Kimura, F.; Ito, J. Effects of extraction methods on phytochemicals of rice bran oils produced from colored rice. J. Oleo Sci. 2018, 67, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Chiang, P.Y. Variation quality and kinetic parameter of commercial n-3 PUFA-rich oil during oxidation via Rancimat. Mar. Drugs 2017, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Bolling, B.W.; Shahidi, F. Nuts and their co-products: The impact of processing (roasting) on phenolics, bioavailability, and health benefits–A comprehensive review. J. Funct. Foods 2016, 26, 88–122. [Google Scholar]
- Taş, N.G.; Gökmen, V. Phenolic compounds in natural and roasted nuts and their skins: A brief review. Curr. Opin. Food Sci. 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Różańska, M.B.; Kowalczewski, P.Ł.; Tomaszewska-Gras, J.; Dwiecki, K.; Mildner-Szkudlarz, S. Seed-Roasting Process Affects Oxidative Stability of Cold-Pressed Oils. Antioxidants 2019, 8, 313. [Google Scholar] [CrossRef]
- Rostami, M.; Farzaneh, V.; Boujmehrani, A.; Mohammadi, M.; Bakhshabadi, H. Optimizing the extraction process of sesame seed’s oil using response surface method on the industrial scale. Ind. Crop. Prod. 2014, 58, 160–165. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Ce 2-66: Preparation of methyl esters of fatty acids. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; Firestone, D., Ed.; American Oil Chemists’ Society: Champaign, IL, USA, 1997. [Google Scholar]
- Yang, K.M.; Hsu, F.L.; Chen, C.W.; Hsu, C.L.; Cheng, M.C. Quality Characterization and Oxidative Stability of Camellia Seed Oils Produced with Different Roasting Temperatures. J. Oleo Sci. 2018, 67, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, Y.; Feng, M. Contents of phytosterols in vegetables and fruits commonly con-sumed in China. Biomed. Environ. Sci. 2008, 21, 449–453. [Google Scholar] [CrossRef]
- Lin, L.Y.; Chuang, C.H.; Chen, H.C.; Yang, K.M. Lime (Citrus aurantifolia (Christm.) Swingle) Essential Oils: Volatile Com-pounds, Antioxidant Capacity, and Hypolipidemic Effect. Foods 2019, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arroyo, S.; Rodríguez-Medina, I.C.; Beltrán-Debón, R.; Pasini, F.; Joven, J.; Micol, V.; Segura-Carretero, A.; Fer-nández-Gutiérrez, A. Quantification of the polyphenolic fraction and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sabdariffa aqueous extract. Food Res. Int. 2011, 44, 1490–1495. [Google Scholar] [CrossRef]
- Yang, K.M.; Cheng, M.C.; Chen, C.W.; Tseng, C.Y.; Lin, L.Y.; Chiang, P.Y. Characterization of volatile compounds with HS-SPME from oxidized n-3 PUFA rich oils via Rancimat tests. J. Oleo Sci. 2017, 66, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Niazmand, R.; Rezaei, M.; Sarabi, M. Kinetic parameter determination of vegetable oil oxidation under Ranci-mat test conditions. Eur. J. Lipid Sci. Technol. 2008, 110, 587–592. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Özcan, M.M.; Aadiamo, O.Q.; Alsawmahi, O.N. Influence of Storage and Roasting on the Quality Properties of Kernel and Oils of Raw and Roasted Peanuts. J. Oleo Sci. 2018, 67, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Madhujith, T.; Sivakanthan, S. Oxidative Stability of Edible Plant Oils. Bioact. Mol. Food 2018, 7, 1–23. [Google Scholar]
- Xu, T.T.; Li, J.; Fan, Y.W.; Zheng, T.W.; Deng, Z.Y. Comparison of oxidative stability among edible oils under continuous frying conditions. Int. J. Food Prop. 2015, 18, 1478–1490. [Google Scholar] [CrossRef]
- Peng, C.Y.; Lan, C.H.; Lin, P.C.; Kuo, Y.C. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes. J. Hazard. Mater. 2017, 324, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Cicero, N.; Albergamo, A.; Salvo, A.; Bua, G.D.; Bartolomeo, G.; Mangano, V.; Dugo, G. Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market. Food Res Int. 2018, 109, 517–525. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the relationship between vegetable oil composition and oxidative stability: A multifactorial approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Dun, Q.; Yao, L.; Deng, Z.; Li, H.; Li, J.; Fan, Y.; Zhang, B. Effects of hot and cold-pressed processes on volatile compounds of peanut oil and corresponding analysis of characteristic flavor components. LWT 2019, 112, 107648. [Google Scholar] [CrossRef]
- Siegmund, B.; Murkovic, M. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil (Part 2: Volatile compounds). Food Chem. 2004, 84, 367–374. [Google Scholar] [CrossRef]
- Haiyan, Z.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem. 2007, 100, 1544–1551. [Google Scholar] [CrossRef]
- Robertson, G.L. Shelf life of packaged foods: Its measurements and prediction. Dev. New Food Prod. Chang. Marketpl. 2000, 329–353. [Google Scholar]
- Kochhar, S.P.; Henry, C.J.K. Oxidative stability and shelf-life evaluation of selected culinary oils. Int. J. Food Sci. Nutr. 2009, 60, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Hoseini-Yazdi, S.Z. Evolution of oxidative values during kinetic studies on olive oil oxidation in the Rancimat test. J. Am. Oil Chem. Soc. 2014, 91, 281–293. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Cao, Y.; Wang, C.; Xue, Y. Effect of roasting on the chemical components of peanut oil. LWT 2020, 125, 109249. [Google Scholar] [CrossRef]
| Peanut Oil Roasted At | |||
|---|---|---|---|
| 120 °C | 140 °C | 160 °C | |
| BI | 37.94 ± 1.96 | 62.39 ± 4.58 | 195.71 ± 9.61 |
| POV | 5.48 ± 0.34 | 7.13 ± 0.56 | 8.67 ± 0.44 |
| AV | 1.12 ± 0.14 | 1.74 ± 0.21 | 1.94 ± 0.24 |
| p–AV | 5.47 ± 0.69 | 12.44 ± 1.15 | 10.42 ± 0.96 |
| TOTOX | 16.43 ± 1.34 | 26.70 ± 2.33 | 27.76 ± 1.98 |
| Peanut Oil Roasted At | |||
|---|---|---|---|
| 120 °C | 140 °C | 160 °C | |
| C16:0 | 14.36 ± 0.06 | 13.68 ± 0.04 | 14.18 ± 0.06 |
| C18:0 | 3.01 ± 0.01 | 3.07 ± 0.02 | 3.63 ± 0.02 |
| C18:1 | 37.23 ± 0.15 | 36.67 ± 0.20 | 38.44 ± 0.11 |
| C18:2 | 40.65 ± 0.24 | 40.80 ± 0.19 | 39.00 ± 0.21 |
| C20:0 | 1.25 ± 0.01 | 1.53 ± 0.02 | 1.29 ± 0.01 |
| C20:1 | 0.75 ± 0.01 | 0.83 ± 0.01 | 0.73 ± 0.01 |
| C22:0 | 2.00 ± 0.03 | 2.48 ± 0.01 | 2.10 ± 0.01 |
| C24:0 | 0.75 ± 0.01 | 0.94 ± 0.01 | 0.64 ± 0.01 |
| U/S | 3.68 ± 0.01 | 3.61 ± 0.02 | 3.58 ± 0.02 |
| Peanut Oil Roasted at | |||
|---|---|---|---|
| 120 °C | 140 °C | 160 °C | |
| Antioxidant Components | |||
| α–tocopherol (μg/g) | 72.33 ± 3.24 c | 60.39 ± 3.64 b | 55.72 ± 2.84 a |
| γ–tocopherol (μg/g) | 67.31 ± 2.48 a | 72.91 ± 3.11 a | 70.51 ± 3.18 a |
| squalene1 (μg/g) | 8.58 ± 1.83 a | 9.15 ± 1.41 ab | 13.31 ± 2.52 b |
| campesterol (μg/g) | 2.48 ± 1.17 a | 2.51 ± 0.73 a | 3.20 ± 0.89 b |
| stigmasterol (μg/g) | 2.55 ± 0.19 a | 2.01 ± 0.22 a | 2.88 ± 0.17 b |
| stigmast–5–en–3–ol (μg/g) | 7.34 ± 0.34 a | 6.73 ± 0.55 a | 10.23 ± 0.48 b |
| stigmasta–5,24(28)–dien–3–ol (μg/g) | 2.72 ± 0.11 a | 2.27 ± 0.20 a | 2.23 ± 0.16 a |
| total phenol (GAE μg/g) | 18.31 ± 1.03 a | 29.63 ± 1.62 b | 36.64 ± 2.83 c |
| total flavonoid (QE μg/g) | 4.27 ± 0.15 a | 3.96 ± 0.19 a | 4.44 ± 0.21 a |
| Antioxidant Capacity | |||
| DPPH (%) | 42.02 ± 1.35 a | 47.97 ± 1.26 b | 52.34 ± 1.96 c |
| FRAP (TrE μg/g) | 151.22 ± 12.62 a | 250.52 ± 20.38 b | 328.64 ± 26.14 c |
| Compound | RI | Peanut Oil Roasted at | ||
|---|---|---|---|---|
| 120 °C | 140 °C | 160 °C | ||
| hexanal | 778 | 9.86 | 9.11 | 9.49 |
| 2–methylpyrazine | 794 | 20.65 | 38.31 | 26.61 |
| fruanmethanol | 844 | 3.8 | 2.45 | 0.89 |
| methylbutyloxirane | 878 | 2.17 | 1.06 | 0.53 |
| 3,5–dimethylcyclohexene | 882 | 1.43 | 1.7 | 1.32 |
| 2,5–dimethylpyrazine | 884 | 21.39 | 23.33 | 20.86 |
| benzaldehyde | 925 | 6.58 | 4.53 | 3.19 |
| 2,3,4–trithiapentane | 942 | 0.38 | 0.38 | 0.13 |
| 2–hepten–1–ol | 948 | 0.42 | 0.15 | 0.26 |
| 2–ethyl–6–methylpyrazine | 964 | 13.61 | 11.39 | 12.15 |
| 2,5–dimethylheotane | 981 | 4.23 | 2.02 | 2.52 |
| benzeneacetaldhyde | 1008 | 10.16 | 1.53 | 0.88 |
| acthylpyrrole | 1032 | ND | 0.07 | 13.69 |
| 2–ethyl–3,5–dimethylpyrazine | 1056 | 2.51 | 2.51 | 3.11 |
| nonanal | 1100 | 1.83 | 0.4 | 0.61 |
| benzeneethanol | 1108 | 0.65 | 0.1 | ND |
| 2–acetyl–3–methylpyrzaine | 1120 | ND | 0.14 | 0.31 |
| undecane | 1132 | 0.33 | 0.46 | 0.38 |
| 3,5–diethyl–2–ethylpyrazine | 1184 | ND | 0.1 | 0.2 |
| tetradecane | 1744 | ND | 0.26 | 2.87 |
| Peanut Oil Roasted at | |||
|---|---|---|---|
| 120 °C | 140 °C | 160 °C | |
| ln(k) = a(1/T) + b | |||
| a | −9.8725 | −12.653 | −13.064 |
| b | 23.74 | 30.87 | 31.87 |
| R2 | 0.956 | 0.997 | 0.983 |
| Ea (kJ/mol) | 82.08 | 105.2 | 108.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciou, J.-Y.; Chen, H.-C.; Chen, C.-W.; Yang, K.-M. Relationship between Antioxidant Components and Oxidative Stability of Peanut Oils as Affected by Roasting Temperatures. Agriculture 2021, 11, 300. https://doi.org/10.3390/agriculture11040300
Ciou J-Y, Chen H-C, Chen C-W, Yang K-M. Relationship between Antioxidant Components and Oxidative Stability of Peanut Oils as Affected by Roasting Temperatures. Agriculture. 2021; 11(4):300. https://doi.org/10.3390/agriculture11040300
Chicago/Turabian StyleCiou, Jhih-Ying, Hsin-Chun Chen, Chih-Wei Chen, and Kai-Min Yang. 2021. "Relationship between Antioxidant Components and Oxidative Stability of Peanut Oils as Affected by Roasting Temperatures" Agriculture 11, no. 4: 300. https://doi.org/10.3390/agriculture11040300
APA StyleCiou, J.-Y., Chen, H.-C., Chen, C.-W., & Yang, K.-M. (2021). Relationship between Antioxidant Components and Oxidative Stability of Peanut Oils as Affected by Roasting Temperatures. Agriculture, 11(4), 300. https://doi.org/10.3390/agriculture11040300








