Bread Wheat Quality under Limiting Environmental Conditions: I-Molecular Properties of Storage Proteins and Starch Constituents in Mature Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Wheat Grain Quality Assessment
2.3. Wheat Flour Preparation
2.4. Quantification of Grain Proteins by Size Exclusion–High Performance Liquid Chromatography (SE-HPLC)
2.5. Determination of the Molecular Distribution of Flour Protein by Asymmetrical Flow Field Flow Fractionation (A4F)
2.6. Quantitation of High Molecular Weight Glutenin Subunits (HMW-GS) by Lab-on-a-Chip
2.7. Extraction of Starch Granules of the Wheat Flours and Determination of Their Size
2.8. The Molecular Weight Distribution of Starch Polysaccharides by Asymmetrical Flow Field Flow Fractionation (A4F)
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Grain Filling Period
3.2. Prolamin Content of Wheat Grains and Their Molecular Weight Distribution
3.3. Composition and Content of Glutenin Subunits of Wheat Grains
3.4. Starch Content of Wheat Grains and Their Molecular Weight Distribution
3.5. Starch Granule Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Indexmundi. 2019. Available online: https://www.indexmundi.com/agriculture/?country=lb&commodity=wheat&graph (accessed on 20 February 2019).
- Triboi, E. Modèle d’élaboration du poids du grain chez le blé tendre (Triticum aestivum em Thell). Agronomie 1990, 10, 191–200. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat stress in wheat during reproductive and grain-filling phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Satorre, E.H.; Slafer, G.A. Wheat: Ecology and Physiology of Yield Determination; Food Products Press: New York, NY, USA, 1999. [Google Scholar]
- Dupont, F.M.; Altenbach, S.B. Molecular and biochemical impacts of environmental factors on wheat grain development and protein synthesis. J. Cereal Sci. 2003, 38, 133–146. [Google Scholar] [CrossRef]
- Farooq, J.; Khaliq, I.; Mahmood, A. Evaluation of some wheat hybrids under normal and heat stress conditions. Triticeae Genom. Genet. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Narayanan, S.; Prasad, P.V.V.; Fritz, A.K.; Boyle, D.L.; Gill, B.S. Impact of high night-time and high daytime temperature stress on winter wheat. J. Agro. Crop Sci. 2015, 201, 206–218. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Finney, K.F.; Barmore, M.A. Loaf volume and protein content of hard winter and spring wheats. Cereal Chem. 1948, 25, 291–312. [Google Scholar]
- Schofield, J.D. Wheat proteins: Structure and functionality in milling and breadmaking. In Wheat Production, Properties and Quality; Bushuk, W., Rasper, V.F., Eds.; Chapman & Hall: London, UK, 1994; pp. 72–106. [Google Scholar]
- Shewry, P.R.; Halford, N.G.; Belton, P.S. The structure and properties of gluten: An elastic protein from wheat grain. Philos. Trans. R. Soc. B 2002, 357, 133–142. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S.; Barro, F.; Barcelo, P.; Lazzeri, P. Biotechnology of breadmaking: Unraveling and manipulating the multi-protein gluten complex. Nat. Biotechnol. 1995, 13, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Keck, B.; Köhler, P.; Wieser, H. Disulphide bonds in wheat gluten: Cystine peptides derived from gluten proteins following peptic and thermolytic digestion. Z. Lebensm. Unters. Forsch. 1995, 200, 432–439. [Google Scholar] [CrossRef]
- D’Ovidio, R.; Masci, S. The low-molecular-weight glutenin subunits of wheat gluten. J. Cereal Sci. 2004, 39, 321–339. [Google Scholar] [CrossRef]
- Tatham, A.S.; Shewry, P.R. The conformation of wheat gluten proteins. The secondary structures and thermal stabilities of α-, β, γ and ω-Gliadins. J. Cereal Sci. 1995, 3, 103–113. [Google Scholar] [CrossRef]
- Bietz, J.A.; Huebner, F.R. Structure of glutenin: Achievements at the Northern Regional Research Center. Ann. Technol. Agric. 1980, 29, 249–277. [Google Scholar]
- Lemelin, E.; Branlard, G.; Salvo, L.; Lein, V.; Aussenac, T.; Daydé, J. Breadmaking stability of wheat flours: Relation between mixing properties and molecular weight distribution of polymeric glutenins. J. Cereal Sci. 2005, 42, 317–326. [Google Scholar] [CrossRef]
- Barrera, G.; Pérez, G.; Ribotta, P.; León, A. Influence of Damaged Starch on Cookie and Bread-making Quality. Eur. Food Res. Technol. 2007, 225, 1–7. [Google Scholar] [CrossRef]
- Dodds, N.J.H. Damaged Starch Determination in Wheat Flours in Relation to Dough Water Absorption. Starch Stärke 1971, 23, 23–27. [Google Scholar] [CrossRef]
- Ghodke, S.K.; Ananthanarayan, L.; Rodrigues, L. Use of Response Surface Methodology to Investigate the Effects of Milling Conditions on Damaged Starch, Dough Stickiness and Chapatti Quality. Food Chem. 2009, 112, 1010–1015. [Google Scholar] [CrossRef]
- Kaletunc, G.; Breslauer, K.J. Characterization of Cereals and Flours: Properties, Analysis and Applications; Marcel Dekker, Inc.: New York, NY, USA, 2003; p. 496. [Google Scholar]
- Hug-Iten, S.; Handschin, S.; Conde-Petit, B.; Escher, F. Changes in Starch Microstructure on Baking and Staling of Wheat Bread. LWT Food Sci. Technol. 1999, 32, 255–260. [Google Scholar] [CrossRef]
- Massaux, C.; Bodson, B.; Lenartz, J.; Sindic, M.; Sinnaeve, G.; Dardenne, P.; Falisse, A.; Deroanne, C. L’amidon Natif du Grain de blé: Un Composé Naturel à Valoriser Par la Connaissance de ses Propriétés Techno-Fonctionnelles; Livre blanc « céréales »; F.U.S.A et CRAW: Gembloux, Belgium, 2006. [Google Scholar]
- Hoseney, R.C.; Finney, K.F.; Pomeranz, Y.; Shogren, M.D. Functional (breadmaking) and biochemical properties of wheat flour components. VIII. Starch. Cereal Chem. 1971, 48, 191–201. [Google Scholar]
- Sakr, N.; Rhazi, L.; Aussenac, T. Bread wheat quality under limiting environmental conditions: II—Rheological properties of Lebanese wheat genotypes. J. Saudi Soc. Agric. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Baum, M.; Tadesse, W.; Nachit, M.; Abdalla, O.; Rajaram, S.; Singh, R.; Payne, T.; Ammar, K.; Morgounov, A.; Braun, H. Global Crop Improvement Networks to Bridge Technology Gaps. In Advances in Wheat Genetics: From Genome to Field; Ogihara, Y., Takumi, S., Handa, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 387–399. [Google Scholar]
- Fellahi, Z.; Hannachi, A.; Ferras, K.; Oulmi, A.; Boutalbi, W.; Bouzerzour, H.; Benmahammed, A. Analysis of the phenotypic variability of twenty f 3 biparental populations of bread wheat (Triticum aestivum L.) evaluated under semi-arid environment. J. Fundam. Appl. Sci. 2017, 9, 102–118. [Google Scholar] [CrossRef][Green Version]
- AACC. (76.13.01) Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- Hajas, L.; Scherf, K.A.; Török, K.; Bugyi, Z.; Schall, E.; Poms, R.E.; Koehler, P.; Tömösközi, S. Variation in protein composition among wheat (Triticum aestivum L.) cultivars to identify cultivars suitable as reference material for wheat gluten analysis. Food Chem. 2018, 267, 387–394. [Google Scholar] [CrossRef]
- Rhazi, L.; Bodard, A.-L.; Fathollahi, B.; Aussenac, T. High throughput microchip-based separation and quantitation of high-molecular-weight glutenin subunits. J. Cereal Sci. 2009, 49, 272–277. [Google Scholar] [CrossRef]
- Bancel, E.; Rogniaux, H.; Debiton, C.; Chambon, C.; Branlard, G. Extraction and proteome analysis of starch granule-associated proteins in mature wheat kernel (Triticum aestivum L.). J. Proteome Res. 2010, 9, 3299–3310. [Google Scholar] [CrossRef]
- Chiaramonte, E.; Rhazi, L.; Aussenac, T.; Richard White, D., Jr. Amylose and amylopectin in starch by asymmetric flow field-flow fractionation with multi-angle light scattering and refractive index detection (AF4-MALS-RI). J. Cereal Sci. 2012, 56, 457–463. [Google Scholar] [CrossRef]
- Cook, B.I.; Anchukaitis, K.J.; Touchan, R.; Meko, D.M.; Cook, E.R. Spatiotemporal drought variability in the Mediterranean over the last 900 years. J. Geophys. Res. Atmos. 2016, 121, 2060–2074. [Google Scholar] [CrossRef]
- Carceller, J.L.; Aussenac, T. Accumulation and changes in molecular size distribution of polymeric proteins in developing grains of hexaploid wheats: Role of the desiccation phase. Aust. J. Plant Physiol. 1999, 26, 301–310. [Google Scholar] [CrossRef]
- El-Kareem, T.H.A.A.; El-Saidy, A.E. Evaluation of yield and grain quality of some bread wheat genotypes under normal irrigation and drought stress conditions in calcareous soils. J. Biol. Sci. 2011, 11, 156–164. [Google Scholar]
- Jenner, C. Starch Synthesis in the Kernel of Wheat under High Temperature Conditions. Aust. J. Plant Physiol. 1994, 21, 791. [Google Scholar] [CrossRef]
- Branlard, G.; Lesage, V.S.; Bancel, E.; Martre, P.; Méleard, B.; Rhazi, L. Coping with wheat quality in a changing environment: Proteomics evidence for stress caused by environmental changes. In Advances in Wheat Genetics: From Genome to Field; Ogihara, Y., Takumi, S., Handa, H., Eds.; Springer: Tokyo, Japan, 2015; pp. 255–264. [Google Scholar]
- Graybosch, R.A.; Peterson, C.J.; Baenziger, P.S.; Shelton, D.R. Environmental modification of hard red winter wheat flour protein composition. J. Cereal Sci. 1995, 22, 45–51. [Google Scholar] [CrossRef]
- Williams, R.M.; O’Brien, L.; Eagles, H.A.; Solah, V.A.; Jayasena, V. The influences of genotype, environment, and genotype × environment interaction on wheat quality. Aust. J. Agric. Res. 2008, 59, 95. [Google Scholar] [CrossRef]
- Aussenac, T.; Rhazi, L. Storage Proteins Accumulation and Aggregation in Developing Wheat Grains. In Global Wheat Production; Fahad, S., Basir, A., Adnan, M., Eds.; IntechOpen: London, UK, 2018; pp. 134–163. [Google Scholar]
- Southan, M.; MacRitchie, F. Molecular Weight Distribution of Wheat Proteins. Cereal Chem. 1999, 76, 827–836. [Google Scholar] [CrossRef]
- MacRitchie, F.; Lafiandra, D. Structure-fonction relationships of wheat proteins. In Food Proteins and Their Applications; CRC Press: New York, NY, USA, 1997; pp. 293–323. [Google Scholar]
- Stone, B.; Morell, M.K. Carbohydrates. In Wheat: Chemistry and Technology; Khan, K., Shewry, P.R., Eds.; AACC: St. Paul, MN, USA, 2009; pp. 299–362. [Google Scholar]
- Bechtel, D.B.; Zargas, I.; Kaliekau, L.; Pomeranz, Y. Size distribution of wheat starch granules during endosperm development. Cereal Chem. 1990, 67, 59–63. [Google Scholar]
- Shi, Y.C.; Seib, P.A.; Bernardin, J.E. Effects of temperature during grain-filling on starches from six wheat cultivars. Cereal Chem. 1994, 71, 369–383. [Google Scholar]
- Park, S.-H.; Chung, O.K.; Seib, P.A. Effects of varying weight ratios of large and small wheat starch granules on experimental straight-dough bread. Cereal Chem. 2005, 82, 166–172. [Google Scholar] [CrossRef]
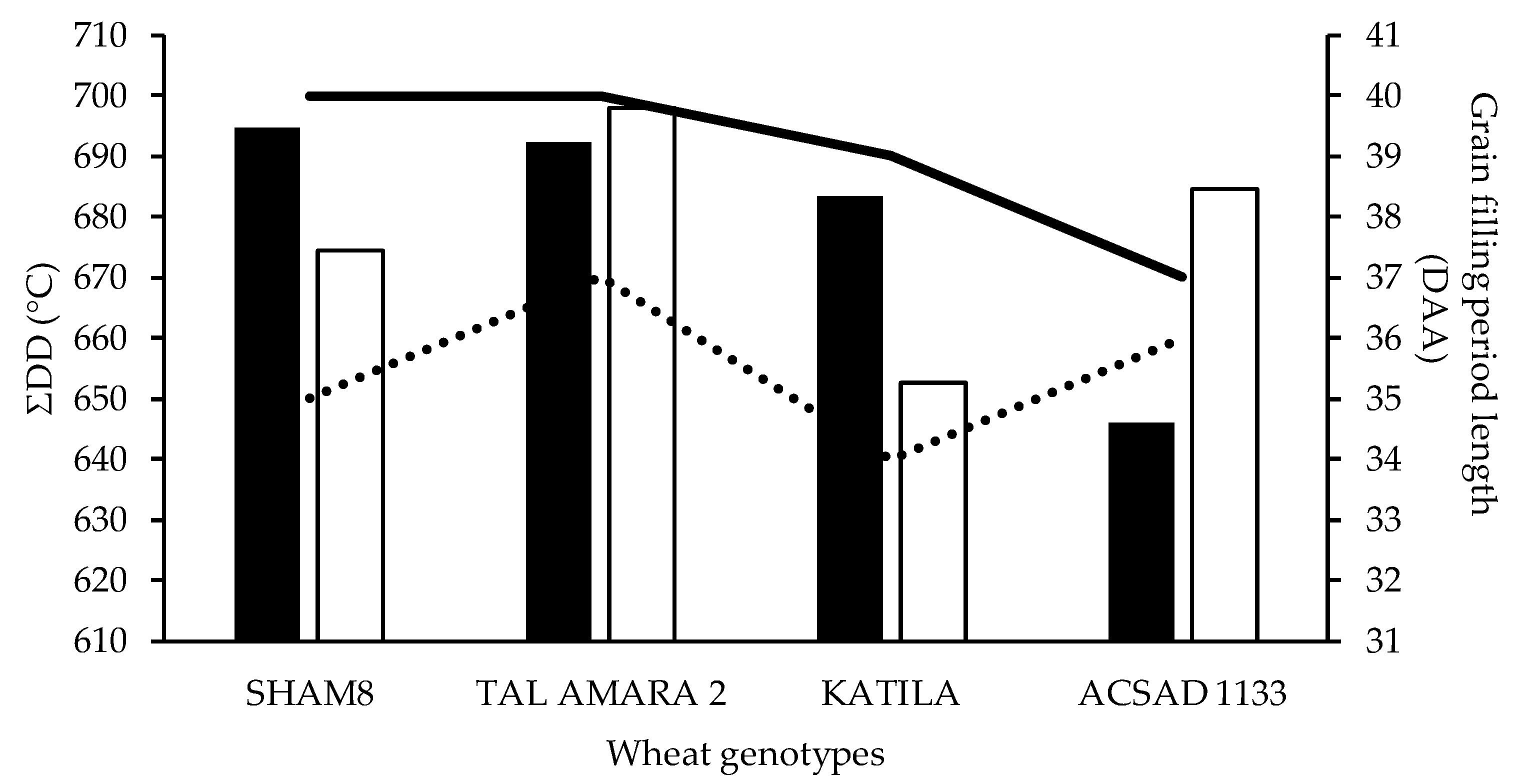
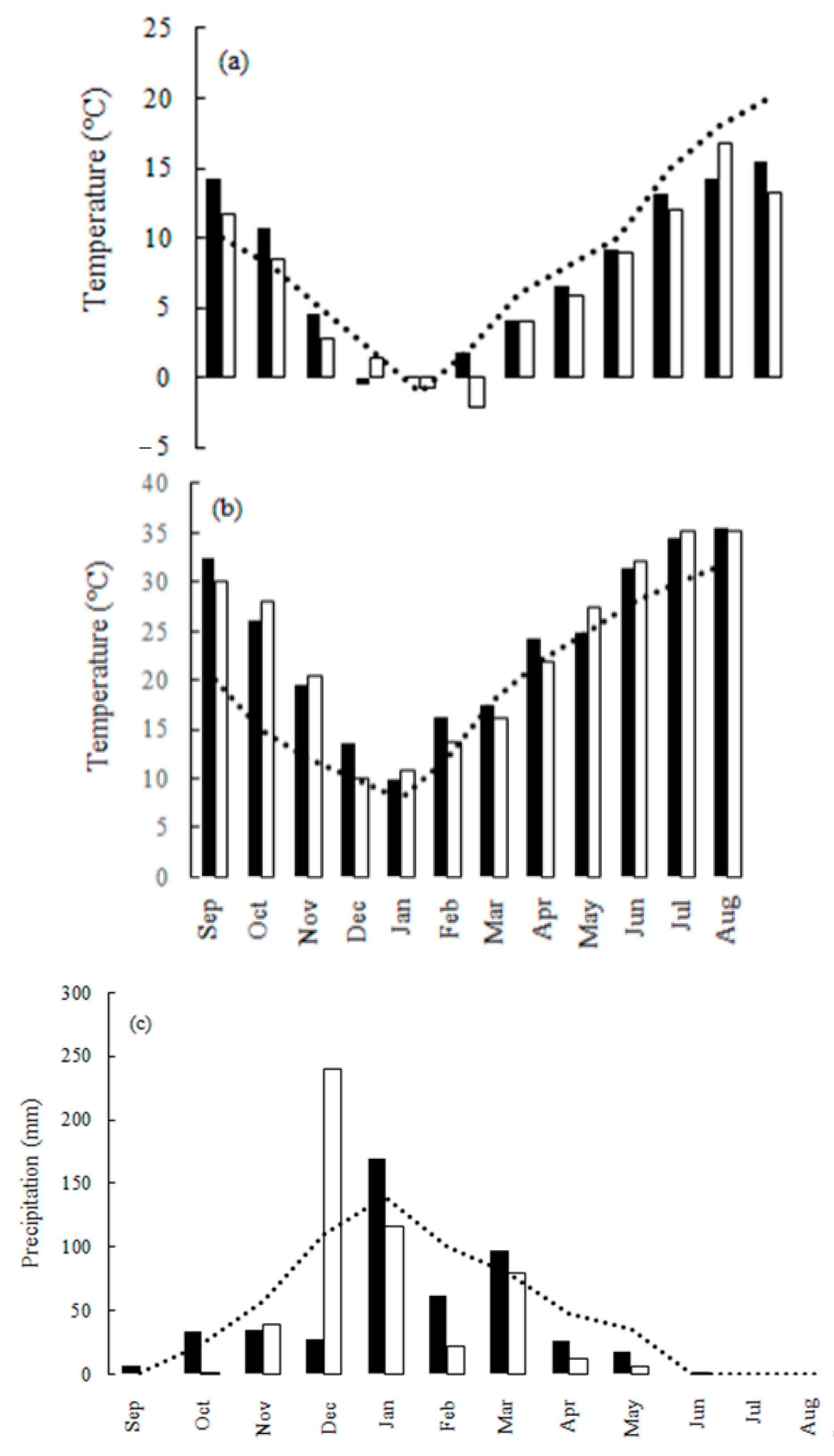
| Wheat Genotypes | Line/Pedigree Source |
|---|---|
| SHAM 8 | KAUZ = JUP/BJY/URES/CM67458 (ICARDA, 2015) |
| TAL AMARA 2 | Belikh/Gediz/Bit/ACS-D-7284-22 IZ-17-IZ-4 IZ-OIZ (ACSAD, 2015) |
| KATILA | KAUZ/ATTILA/CMSS93Y0066S-5AP-2AP-6AP-0APS-0AP (ICARDA, 2015) |
| ACSAD 1133 | Snb›s›//shi4414/crow›s›/3/Mon›s›/crows›s›/ACS-W-9678 (2001)-23IZ-2IZ-0IZ (ACSAD, 2015) |
| Genotype | TKW (g) | Protein Content (3) | Starch Content (3) | Prolamin | UPP Content (4) | ||
|---|---|---|---|---|---|---|---|
| Glutenin Content (3) | Gliadin Content (3) | Gliadin/Glutenin | |||||
| SHAM 8 | 21.42 a (1) | 16.96 a | 57.86 b | 30.69 a | 55.18 b | 1.82 a | 46.70 a |
| TAL AMARA 2 | 25.85 b | 17.58 b | 54.34 a | 36.51 b | 50.19 a | 1.38 b | 41.35 b |
| KATILA | 24.85 b | 17.46 b | 54.31 a | 29.92 a | 55.42 b | 1.89 a | 44.74 a |
| ACSAD 1133 | 25.96 b | 18.14 c | 54.13 a | 31.25 a | 55.47 b | 1.79 a | 46.40 a |
| Mean (2) | 24.52 | 17.53 | 55.16 | 32.09 | 54.06 | 1.72 | 45.00 |
| CV (%) | 8.67 | 2.76 | 3.27 | 9.33 | 4.79 | 13.46 | 5.48 |
| 2015 mean | 25.69 | 20.29 | 53.37 | 32.54 | 53.73 | 1.70 | 46.50 |
| 2016 mean | 23.35 | 14.78 | 56.75 | 31.64 | 54.40 | 1.73 | 43.10 |
| Genotype | Mn (1) (×106 g/mol) | Mw (1) (×106 g/mol) | Ip (1) | Rz (nm) (2) | Rh (nm) (2) |
|---|---|---|---|---|---|
| SHAM 8 | 0.55 c (3) | 5.13 a | 9.41 a | 94.16 a | 8.06 a |
| TAL AMARA 2 | 0.58 b | 3.92 c | 6.96 b | 87.74 b | 8.21 a |
| KATILA | 0.61 a | 4.47 b | 7.40 b | 88.18 b | 8.23 a |
| ACSAD 1133 | 0.56 bc | 4.17 bc | 7.30 b | 85.70 b | 8.01 a |
| Mean (4) | 0.58 | 4.42 | 7.77 | 92.95 | 8.13 |
| CV (%) | 4.60 | 11.81 | 14.27 | 4.09 | 1.32 |
| Genotype | HMW/LMW | HMW-GS (%) (1) | |||||
|---|---|---|---|---|---|---|---|
| 9 | 10 | 7 | 1 | 2* | 5 | ||
| SHAM 8 | 0.25 a (2) | 13.77 a | 16.95 ab | 29.24 a | - | 12.41 b | 27.63 a |
| TAL AMARA 2 | 0.23 a | 13.71 a | 17.30 ab | 29.38 a | - | 12.41 b | 27.03 a |
| KATILA | 0.25 a | 11.92 a | 18.87 b | 31.25 a | 13.62 | - | 24.33 a |
| ACSAD 1133 | 0.26 a | 18.67 b | 15.84 a | 31.05 a | - | 4.59 a | 27.54 a |
| Mean (3) | 0.25 | 14.52 | 17.24 | 30.23 | - | 9.86 | 26.63 |
| CV (%) | 4.11 | 19.95 | 7.27 | 3.53 | - | 46.31 | 5.85 |
| Genotype | Amylopectin (AMP) | Amylose (AML) | Starch Granules | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn (1) | Mw (1) | Rz (1) | V(1) | AMP Content (%) | Mn (1) | Mw (1) | Rz (1) | AML Content (%) | A-Type (%) | B-Type (%) | C-Type (%) | |
| SHAM 8 | 12.7 a (2) | 55.3 a | 134.4 a | 0.33 b | 66.0 a | 0.61 a | 0.33 b | 69.3 b | 34.2 a | 72.8 a | 22.8 c | 3.9 b |
| TALAMARA 2 | 24.0 b | 66.2 b | 139.3 a | 0.29 a | 66.6 a | 0.80 b | 0.37 c | 72.7 b | 33.4 a | 76.5 b | 17.3 a | 4.7 c |
| KATILA | 14.8 a | 59.5 a | 140.3 a | 0.32 ab | 65.0 a | 0.61 a | 0.31 ab | 70.5 b | 34.5 a | 79.2 c | 16.8 a | 3.3 a |
| ACSAD 1133 | 13.0 a | 54.3 a | 140.6 a | 0.33 b | 65.3 a | 0.60 a | 0.29 a | 58.9 a | 34.7 a | 76.7 b | 18.4 b | 4.2 b |
| Mean (3) | 16.1 | 58.8 | 138.6 | 0.32 | 65.7 | 0.66 | 0.33 | 67.8 | 34.2 | 76.3 | 18.8 | 4.0 |
| CV (%) | 33.0 | 9.2 | 2.1 | 6.7 | 1.1 | 14.4 | 10.4 | 9.0 | 1.6 | 3.5 | 14.6 | 13.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakr, N.; Rhazi, L.; Aussenac, T. Bread Wheat Quality under Limiting Environmental Conditions: I-Molecular Properties of Storage Proteins and Starch Constituents in Mature Grains. Agriculture 2021, 11, 289. https://doi.org/10.3390/agriculture11040289
Sakr N, Rhazi L, Aussenac T. Bread Wheat Quality under Limiting Environmental Conditions: I-Molecular Properties of Storage Proteins and Starch Constituents in Mature Grains. Agriculture. 2021; 11(4):289. https://doi.org/10.3390/agriculture11040289
Chicago/Turabian StyleSakr, Nada, Larbi Rhazi, and Thierry Aussenac. 2021. "Bread Wheat Quality under Limiting Environmental Conditions: I-Molecular Properties of Storage Proteins and Starch Constituents in Mature Grains" Agriculture 11, no. 4: 289. https://doi.org/10.3390/agriculture11040289
APA StyleSakr, N., Rhazi, L., & Aussenac, T. (2021). Bread Wheat Quality under Limiting Environmental Conditions: I-Molecular Properties of Storage Proteins and Starch Constituents in Mature Grains. Agriculture, 11(4), 289. https://doi.org/10.3390/agriculture11040289








