In-Line Technologies for the Analysis of Important Milk Parameters during the Milking Process: A Review
Abstract
1. Introduction
2. Materials and Methods
- Identifying publications in scientific databases by keywords: “milking technology,” “in-line technologies,” “milk parameters,” and “milk analysis.”
- Analyzing the results and selection of relevant publications of journals focused on agriculture and technology using the “Analyze Results” tool (WoS).
- Downloading all selected relevant publications in the analyzed period and extracting their bibliometric data (authors, title, year of issue, keywords, additional keywords, publishing house) using the “Export to Excel” (WoS) and “Extract” (SD) tools.
- Processing bibliometric data using the spreadsheet software MS Excel 2019 (sorting according to required criteria, identification of articles from the same authors, keywords analysis for further search).
- Detailed qualitative analysis of the content of selected publications in terms of the following:
- investigated problem/topic,
- area of application,
- used type of method/algorithm,
- achieved results and their relevance to the solution of the investigated problem.
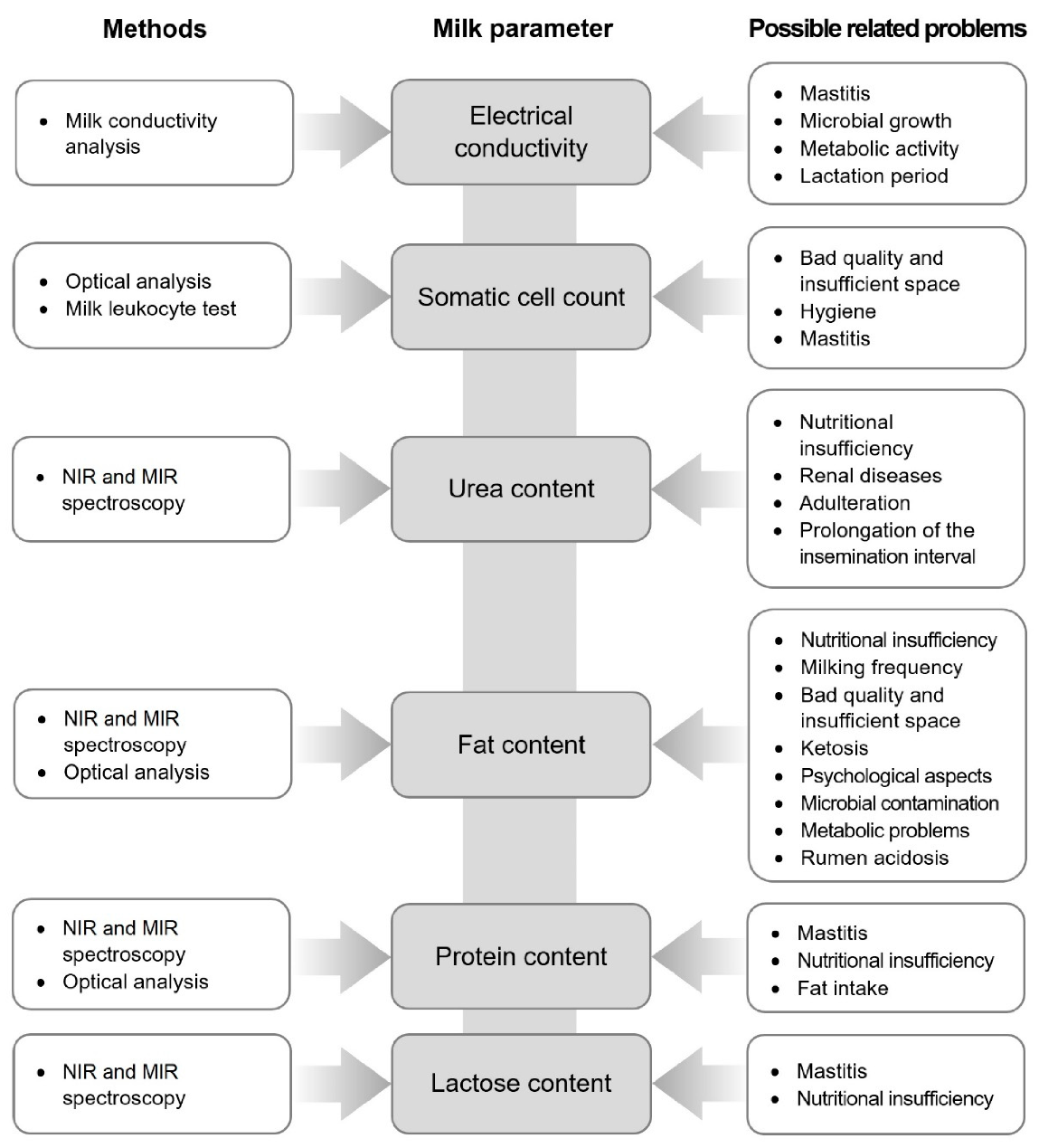
3. Important Milk Parameters Detectable by In-Line Analytical Methods
3.1. Fat Content
3.2. Protein Content
3.3. Lactose Content
3.4. Urea Content
3.5. Somatic Cell Count (SCC)
3.6. Electrical Conductivity (EC)
3.7. Mycotoxin Content
4. Analytical Methods Used in In-Line Milk Production
4.1. Near-Infrared and Mid-Infrared Spectroscopy
4.2. Milk Conductivity Analysis
4.3. Optical Analysis
4.4. Milk Leukocyte Differential Test (MLD)
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kawasaki, M.; Kawamura, S.; Tsukahara, M.; Morita, S.; Komiya, M.; Natsuga, M. Near-infrared spectroscopic sensing system for on-line milk quality assessment in a milking robot. Comput. Electron. Agric. 2008, 63, 22–27. [Google Scholar] [CrossRef]
- Svennersten-Sjaunja, K.; Sjaunja, L.O.; Bertilsson, J.; Wiktorsson, H. Use of regular milking records versus daily records for nutrition and other kinds of management. Livest. Prod. Sci. 1997, 48, 167–174. [Google Scholar] [CrossRef]
- Auldist, M. Effect on processing characteristics. In Encyclopedia of Dairy Science; Academic Press: London, UK, 2002; pp. 2002–2006. [Google Scholar]
- Hamann, J.; Zecconi, A. Evaluation of the electrical conductivity of milk as a mastitis indicator. Bull. Int. Dairy Fed. 1998, 334, 5–22. [Google Scholar]
- Leitner, G.; Lavi, Y.; Merin, U.; Lemberskiy-Kuzin, L.; Katz, G. Online evaluation of milk quality according to coagulation properties for its optimal distribution for industrial applications. J. Dairy Sci. 2011, 94, 2923–2932. [Google Scholar] [CrossRef] [PubMed]
- Brandt, M.; Haeussermann, A.; Hartung, E. Invited review: Technical solutions for analysis of milk constituents and abnormal milk. J. Dairy Sci. 2010, 93, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Borecki, M.; Szmidt, M.; Pawlowski, M.K.; Bebłowska, M.; Niemiec, T.; Wrzostek, P. A method of testing the quality of milk using optical capillaries. Photonics Lett. Pol. 2009, 1, 37–39. [Google Scholar]
- Sifuentes Dos Santos, J.; Schwanz, T.G.; Coelho, A.N.; Heck-Marques, M.C.; Mexia, M.M.; Emanuelli, T.; Costabeber, I. Estimated daily intake of organochlorine pesticides from dairy products in Brazil. Food Control 2015, 53, 23–28. [Google Scholar] [CrossRef]
- Gasull, M.; Porta, M.; Pumarega, J.; Vioque, J.; de Basea, M.B.; Puigdomènech, E.; Morales, E.; Grimalt, J.O.; Malats, N. The relative influence of diet and serum concentrations of organochlorine compounds on K-ras mutations in exocrine pancreatic cancer. Chemosphere 2010, 79, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Gebremichael, S.; Birhanu, T.; Tessema, D.A. Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia. Chemosphere 2013, 90, 1652–1657. [Google Scholar] [CrossRef]
- Kampire, E.; Kiremire, B.T.; Nyanzi, S.A.; Kishimba, M. Organochlorine pesticide in fresh and pasteurized cow’s milk from Kampala markets. Chemosphere 2011, 84, 923–927. [Google Scholar] [CrossRef]
- Fischer, W.J.; Schilter, B.; Tritscher, A.M.; Stadler, R.H. Contaminants of milk and dairy products: Contamination Resulting from Farm and Dairy Practices. Encycl. Dairy Sci. 2011, 2, 887–897. [Google Scholar]
- Castilla-Pinedo, Y.; Alvis-Estrada, L.; Alvis-Guzmán, N. Exposición a órganoclorados por ingesta de leche pasteurizada comercializada em Cartagena. Colombia’s Revista de Salud Publica 2010, 12, 14–26. [Google Scholar] [CrossRef]
- Toft, G.; Hagmar, L.; Giwercman, A.; Bonde, J.P. Epidemiological evidence on reproductive effects of persistent organochlorines in humans. Reprod. Toxicol. 2004, 19, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Linn, J. Factors Affecting the Composition of Milk from Dairy Cows. In Animal Product Options in the Marketplace; National Academy Press (US): Washington, DC, USA, 1998. [Google Scholar]
- Driehuis, F.; Vissers, M. On-Farm Hygienic Milk Production. In Milk Processing and Quality Management; John Wiley: West Sussex, UK, 2009. [Google Scholar]
- Hanuš, O.; Vegricht, J.; Frelich, J.; Macek, A.; Bjelka, M.; Louda, F.; Janů, L. Analysis of raw cow milk quality according to free fatty acid contents in the Czech Republic. Czech J. Anim. Sci. 2008, 53, 17–30. [Google Scholar] [CrossRef]
- Vanbergue, E.; Peyraud, J.L.; Ferlay, A.; Miranda, G.; Martin, P.; Hurtaud, C. Effects of feeding level, type of forage and milking time on milk lipolytic system in dairy cows. Livest. Sci. 2018, 217, 116–126. [Google Scholar] [CrossRef]
- Silanikove, N.; Merin, U.; Shapiro, F.; Leitner, G. Milk metabolites as indicators of mammary gland functions and milk quality. J. Dairy Res. 2014, 81, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Krawczel, P.; Grant, R. Effects of cow comfort on milk quality, productivity and behaviour. In Proceedings of the 48th Annual meeting, New Praque, MN, USA, 1 January 2009; pp. 15–24. [Google Scholar]
- Scholnik, T.; Arazi, A.; Nir, O. How to monitor energy balance using in-line milk testing. Progress. Dairy 2014, 2014, 48–51. [Google Scholar]
- Parr, K. Ketosis: Early detection can prevent costly losses. Cattle Production Guide. 2015, p. 3. Available online: https://www.afimilk.com/app/uploads/ketosis_article_cattle_production_guide_winter_2015.pdf (accessed on 7 January 2021).
- Kelly, A.; Bach Larsen, L. Milk Biochemistry. In Improving the Safety and Quality of Milk; Woodhead Publishing Limited: Cambridge, UK, 2010. [Google Scholar]
- Hamann, J.; Fehlings, K. Leitllinien zur Bekämpfung der Mastitis des Rindes als Bestandsproblem, 4th ed.; Verlag der Deutschen Veterinärmedizinischen Gesellschaft e.V.: Giessen, Germany, 2002. [Google Scholar]
- Smith, K.L.; Hogan, J.S. Environmental Mastitis. Vet. Clin. N. Am. Food A 1993, 9, 489–498. [Google Scholar] [CrossRef]
- Bogdanovičová, K.; Vyletělová-Klimešová, M.; Babák, V.; Kalhotka, L.; Koláčková, I.; Karpíšková, R. Microbiological quality of raw milk in the Czech Republic. Czech J. Food Sci. 2016, 34, 189–196. [Google Scholar]
- Wu, S.; Jin, Y.; Yang, N.; Xu, X.; Xie, Z. Determination of fat content in UHT milk by electroanalytical method. Food Chem. 2019, 270, 538–545. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.S.; Lopetcharat, K.; Drake, M.A. Milk fat threshold determination and the effect of milk fat content on consumer preference for fluid milk. J. Dairy Sci. 2017, 100, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Salfer, I.J.; Dechow, C.D.; Harvatine, K.J. Annual rhythms of milk and milk fat and protein production in dairy cattle in the United States. J. Dairy Sci. 2019, 102, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Rossow, N.; Richardt, W. Využití výsledků kontroly užitkovosti pro kontrolu výživy a látkové výměny. In Sano Encyklopedie; Sano—Moderní výživa zvířat: Domažlice, Czech Republic, 2007. [Google Scholar]
- Hanuš, O.; Manga, I.; Vyletělová, M.; Genčurová, V.; Kopecký, J.; Jadelská, R. Význam sledování minoritních složek mléka pro zdraví zvířat a analytické možnosti jejich monitoringu. Mlékařské Listy 2011, 127, 14–18. [Google Scholar]
- ČSN 57 0529: Raw Cow Milk for Dairy Factory Treatment and Processing; Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 1993.
- Larson, B.L.; Heary, H.L.; Devery, J.E. Immunoglobulin Production and Transport by the Mammary Gland. J. Dairy Sci. 1980, 63, 665–671. [Google Scholar] [CrossRef]
- Steinshamn, H.; Harstad, O. Cow’s diet and milk composition. In Improving the Safety and Quality of Milk; Woodhead Publishing: Cambridge, UK, 2010. [Google Scholar]
- Wolfschoon-Pombo, A.; Klostermeyer, H. Urea in the NPN-fraction of cows milk determination, content and influence on it. Milchwissenschaft 1981, 36, 462–466. [Google Scholar]
- DePeters, E.J.; Ferguson, J.D. Nonprotein Nitrogen and Protein Distribution in the Milk of Cows. J. Dairy Sci. 1992, 75, 3192–3209. [Google Scholar] [CrossRef]
- Heinrichs, J.; Jones, C.; Bailey, K. Milk components: Understanding the causes and importance of milk fat and protein variation in your dairy herd. Pa. State Univ. Dep. Dairy Anim. Sci. 2005, 5, 1–8. [Google Scholar]
- Pechová, A. Kontrola produkce a složení mléka. In Nemoci Skotu; Noviko: Brno, Czech Republic, 2009. [Google Scholar]
- Navrátilová, P.; Králová, M.; Janštová, B.; Přidalová, H.; Cupáková, Š.; Vorlová, L. Hygiena Produkce Mléka; Veterinární a Farmaceutická Univerzita Brno: Brno, Czech Republic, 2012. [Google Scholar]
- Richardt, W. Milk composition as an indicator of nutrition and health. Breeding 2004, 11, 26–27. [Google Scholar]
- Blanco, A.; Blanco, G. Medical Biochemistry; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Gille, D.; Walther, B.; Badertscher, R.; Bosshart, A.; Brügger, C.; Brühlhart, M.; Gauch, R.; Noth, P.; Vergères, G.; Egger, L. Detection of lactose in products with low lactose content. Int. Dairy J. 2018, 83, 17–19. [Google Scholar] [CrossRef]
- Huppertz, T.; Kelly, A. Properties and Constituents of Cow’s Milk. In Milk Processing and Quality Management; John Wiley: West Sussex, UK, 2009. [Google Scholar]
- Mattar, R.; de Campos Mazo, D.F.; Carrilho, F.J. Lactose intolerance: Diagnosis, genetic, and clinical factors. Clin. Exp. Gastroenterol. 2012, 5, 113–121. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Dairy Chemistry and Biochemistry, 1st ed.; Springer International Publishing: Basel, Switzerland, 2015; ISBN 978-3-319-14892-2. [Google Scholar]
- Haile-Mariam, M.; Pryce, J.E. Genetic parameters for lactose and its correlation with other milk production traits and fitness traits in pasture-based production systems. J. Dairy Sci. 2017, 100, 3754–3766. [Google Scholar] [CrossRef]
- Schaafsma, G. Lactose and lactose derivatives as bioactive ingredients in human nutrition. Int. Dairy J. 2008, 18, 458–465. [Google Scholar] [CrossRef]
- Costa, A.; Visentin, G.; De Marchi, M.; Cassandro, M.; Penasa, M. Genetic relationships of lactose and freezing point with minerals and coagulation traits predicted from milk mid-infrared spectra in Holstein cows. J. Dairy Sci. 2019, 102, 7217–7225. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Wang, N.; Xu, P.; Zheng, J. Determination of urea in milk based on N -bromosuccinimide–dichlorofluorescein postchemiluminescence method. J. Food Drug. Anal. 2017, 25, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Q.; Yu, K.X.; Gong, Y.X. Rapid and quantitative determination of urea in milk by reaction headspace gas chromatography. Microchem. J. 2019, 147, 838–841. [Google Scholar] [CrossRef]
- Jha, S.N.; Jaiswal, P.; Borah, A.; Gautam, A.K.; Srivastava, N. Detection and Quantification of Urea in Milk Using Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy. Food Bioprocess Technol. 2015, 8, 926–933. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Y.; Zhang, Q. Dielectric behavior of adulterated milk with urea and water. J. Mol. Liq. 2019, 273, 37–44. [Google Scholar] [CrossRef]
- Jonker, J.S.; Kohn, R.A.; Erdman, R.A. Using milk urea nitrogen to predict nitrogen excretion and utilization efficiency in lactating dairy cows. J. Dairy Sci. 1998, 81, 2681–2692. [Google Scholar] [CrossRef]
- Food Safety and Standards Authority of India. Manual of Analysis of Methods of Foods, Milk and Milk Products; Food Safety and Standards Authority of India: New Delhi, India, 2012. [Google Scholar]
- Franzoi, M.; Manuelian, C.L.; Penasa, M.; De Marchi, M. Effects of somatic cell score on milk yield and mid-infrared predicted composition and technological traits of Brown Swiss, Holstein Friesian, and Simmental cattle breeds. J. Dairy Sci. 2020, 103, 791–804. [Google Scholar] [CrossRef]
- Visentin, G.; Penasa, M.; Niero, G.; Cassandro, M.; De Marchi, M. Phenotypic characterisation of major mineral composition predicted by mid-infrared spectroscopy in cow milk. Ital. J. Anim. Sci. 2018, 17, 549–556. [Google Scholar] [CrossRef]
- Klei, L.; Yun, J.; Sapru, A.; Lynch, J.; Barbano, D.; Sears, P.; Galton, D. Effects of Milk Somatic Cell Count on Cottage Cheese Yield and Quality. J. Dairy Sci. 1998, 81, 1205–1213. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Wilson, D.J.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R.N. Monitoring udder health and milk quality using somatic cell counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Nørstebø, H.; Rachah, A.; Dalen, G.; Østerås, O.; Whist, A.C.; Nødtvedt, A.; Reksen, O. Large-scale cross-sectional study of relationships between somatic cell count and milking-time test results in different milking systems. Prev. Vet. Med. 2019, 165, 44–51. [Google Scholar] [CrossRef]
- Juozaitiene, V.; Juozaitis, A.; Micikeviciene, R. Relationship Between Somatic Cell Count and Milk Production or Morphological Traits of Udder in Black-and-White Cows. J. Vet. Anim. Sci. 2006, 30, 47–51. [Google Scholar]
- Dürr, J.W.; Cue, R.I.; Monardes, H.G.; Moro-Méndez, J.; Wade, K.M. Milk losses associated with somatic cell counts per breed, parity and stage of lactation in Canadian dairy cattle. Livest. Sci. 2008, 117, 225–232. [Google Scholar] [CrossRef]
- Halasa, T.; Huijps, K.; Østerås, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Mucchetti, G.; Gatti, M.; Neviani, E. Electrical Conductivity Changes in Milk Caused by Acidification: Determining Factors. J. Dairy Sci. 1994, 77, 940–944. [Google Scholar] [CrossRef]
- Lien, C.C.; Wan, Y.N.; Ting, C.H. Online detection of dairy cow subclinical mastitis using electrical conductivity indices of milk. Engin. Agr. Envir. Food 2016, 9, 201–207. [Google Scholar] [CrossRef]
- Bauer, R. Ermittlung geeigneter Parameter für eine rechnergestützte Früherkennung von Eutererkrankungen und Stoffwechselstörungen bei Milchkühen. Bundesforschungsanstalt für Landwirtschaft Braunschweig Völkenrode 1990, 112, 195. [Google Scholar]
- Oshima, M. Detection of abnormal quarter milk by difference of the electrical conductivity and its theoretical basis. Jpn. Agric. Res. Q. 1977, 11, 239–245. [Google Scholar]
- Peaker, M. The Electrical Conductivity of Milk for the Detection of Subclinical Mastitis in Cows: Comparison of Various Methods of Handling Conductivity Data with the Use of Cell Counts and Bacteriological Examination. Brit. Vet. J. 1978, 134, 308–314. [Google Scholar] [CrossRef]
- Sheldrake, R.F.; Hoare, R.J.T.; McGregor, G.D. Lactation Stage, Parity, and Infection Affecting Somatic Cells, Electrical Conductivity, and Serum Albumin in Milk. J. Dairy Sci. 1983, 66, 542–547. [Google Scholar] [CrossRef]
- Rossing, W.E.; Benders, E.; Hogewerf, P.H.; Hopster, H.; Maatje, K. Practical experiences with real-time measurements of milk conductivity for detecting mastitis. In Proceedings of the 3rd Symposium Automation in Dairying, Wageningen, The Netherlands, 9–11 September 1987; pp. 138–146. [Google Scholar]
- Maatje, K.; Rossing, W.; Garssen, G.J.; Pluygers, H.G. Automation of electrical conductivity measurements during milking. In Proceedings of the Symposium “Automation in Dairying”, IMAG (Hrsg.), Wageningen, The Netherlands, 20–22 April 1983; pp. 89–100. [Google Scholar]
- Barth, K.; Graupner, M. Experimentelle Untersuchungen zu Eutergesundheits- und Milchqualitätskontrolle auf der Basis der Leitfähigkeitsmessung während des Melkens. Milchwissenschaft 1999, 54, 66–69. [Google Scholar]
- Fernando, R.S.; Rindsig, R.B.; Spahr, S.L. Effect of Length of Milking Interval and Fat Content on Milk Conductivity and Its Use for Detecting Mastitis. J. Dairy Sci. 1981, 64, 678–682. [Google Scholar] [CrossRef]
- McKane, J.; Kandel, J. Microbiology Essentials and Applications, 2nd ed.; McGraw-Hill Companies: Monterey, CA, USA, 1996; ISBN 978-0070451544. [Google Scholar]
- Belakova, S.; Havelka, Z.; Bohata, A.; Hartman, I.; Kabelova, H.; Kriz, P.; Dienstbier, M.; Bartos, P.; Spatenka, P. The effect of treatment of barley grain and malt with low-temperature plasma discharge on the malt gushing potential. KVASNÝ PRŮMYSL 2018, 64, 314–317. [Google Scholar] [CrossRef]
- Peterson, S.W.; Ito, Y.; Horn, B.W.; Goto, T. Aspergillus bombycis, a new aflatoxigenic species and genetic variation in its sibling species, A. nomius. Mycologia 2001, 93, 689–703. [Google Scholar] [CrossRef]
- Acute hepatotoxicity of aflatoxins. In The Toxicology of Aflatoxins Human Health, Veterinary, and Agricultural Significance; Elsevier: Amsterdam, The Netherlands, 1994; pp. 3–26. ISBN 978-0-12-228255-3.
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; World Health Organization: Geneva, Switzerland, 1993; Volume 56.
- Kushiro, M.; Hatabayashi, H.; Nakagawa, H.; Yabe, K. Improvement of mobile phase in thin-layer chromatography for aflatoxins and analysis of the effect of dichlorvos in aflatoxigenic fungi. Mycotoxins 2017, 67, 5–6. [Google Scholar] [CrossRef][Green Version]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 119, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, F.N.; de Azevedo Silva, D.; von Sperling de Souza, R.; de Oliveira, M.S.; Pereira, D.A.A.; de Souza, S.V.C. A Rapid Single-Extraction Method for the Simultaneous Determination of Aflatoxins B1, B2, G1, G2, Fumonisin B1, and Zearalenone in Corn Meal by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2017, 10, 1631–1644. [Google Scholar] [CrossRef]
- Jaiswal, P.; Jha, S.N.; Kaur, J.; Borah, A.; Ramya, H.G. Detection of aflatoxin M1 in milk using spectroscopy and multivariate analyses. Food Chem. 2018, 238, 209–214. [Google Scholar] [CrossRef]
- Wu, X.; Gao, S.; Wang, J.-S.; Wang, H.; Huang, Y.-W.; Zhao, Y. The surface-enhanced Raman spectra of aflatoxins: Spectral analysis, density functional theory calculation, detection and differentiation. Analyst 2012, 137, 4226–4234. [Google Scholar] [CrossRef]
- Stepurska, K.; Dzyadevych, S.; Gridin, S. Potentiometric enzyme biosensor for aflatoxin B1 detection—Kinetic simulation. Sens. Actuators B Chem. 2018, 259, 580–586. [Google Scholar] [CrossRef]
- Santos, V.O.; Pelegrini, P.B.; Mulinari, F.; Lacerda, A.F.; Moura, R.S.; Cardoso, L.P.V.; Bührer-Sékula, S.; Miller, R.N.G.; Grossi-de-Sa, M.F. Development and validation of a novel lateral flow immunoassay device for detection of aflatoxins in soy-based foods. Anal. Methods 2017, 9, 2715–2722. [Google Scholar] [CrossRef]
- Xie, G.; Zhu, M.; Liu, Z.; Zhang, B.; Shi, M.; Wang, S. Development and evaluation of the magnetic particle-based chemiluminescence immunoassay for rapid and quantitative detection of Aflatoxin B1 in foodstuff. Food Agric. Immunol. 2018, 29, 564–576. [Google Scholar] [CrossRef]
- Rhemrev, R.; Pazdanska, M.; Marley, E.; Biselli, S.; Staiger, S. Automated Aflatoxin Analysis Using Inline Reusable Immunoaffinity Column Cleanup and LC-Fluorescence Detection. J. AOAC Int. 2015, 98, 1585–1590. [Google Scholar] [CrossRef]
- Mlček, J.; Šustová, K.; Simeonovová, J. Application of FT NIR spectroscopy in the determination of basic chemical composition of pork and beef. Czech J. Anim. Sci. 2011, 51, 361–368. [Google Scholar] [CrossRef]
- De Marchi, M.; Toffanin, V.; Cassandro, M.; Penasa, M. Invited review: Mid-infrared spectroscopy as phenotyping tool for milk traits. J. Dairy Sci. 2014, 97, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Parra, H.A.; Pustjens, A.; Hettinga, K.; Mongondry, P.; van Ruth, S.M. Evaluation of portable near-infrared spectroscopy for organic milk authentication. Talanta 2018, 184, 128–135. [Google Scholar] [CrossRef]
- De Marchi, M.; Toffanin, V.; Cassandro, M.; Penasa, M. Prediction of coagulating and noncoagulating milk samples using mid-infrared spectroscopy. J. Dairy Sci. 2013, 96, 4707–4715. [Google Scholar] [CrossRef] [PubMed]
- McParland, S.; Banos, G.; McCarthy, B.; Lewis, E.; Coffey, M.P.; O’Neill, B.; O’Donovan, M.; Wall, E.; Berry, D.P. Validation of mid-infrared spectrometry in milk for predicting body energy status in Holstein-Friesian cows. J. Dairy Sci. 2012, 95, 7225–7235. [Google Scholar] [CrossRef]
- Dos Santos, C.; Lopo, M.; Páscoa, R.; Lopez, J. A Review on the Applications of Portable Near-Infrared Spectrometers in the Agro-Food Industry. Appl. Spectrosc. 2013, 67, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- De la Roza-Delgado, B.; Garrido-Varo, A.; Soldado, A.; González Arrojo, A.; Cuevas Valdés, M.; Maroto, F.; Pérez-Marín, D. Matching portable NIRS instruments for in situ monitoring indicators of milk composition. Food Control 2017, 76, 74–81. [Google Scholar] [CrossRef]
- Prasanth, P.; Viswan, G.; Bennaceur, K. Development of a low-cost portable spectrophotometer for milk quality analysis. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Norberg, E.; Hogeveen, H.; Korsgaard, I.R.; Friggens, N.C.; Sloth, K.H.M.N.; Løvendahl, P. Electrical Conductivity of Milk: Ability to Predict Mastitis Status. J. Dairy Sci. 2004, 87, 1099–1107. [Google Scholar] [CrossRef]
- Romero, G.; Roca, A.; Alejandro, M.; Muelas, R.; Díaz, J.R. Relationship of mammary gland health status and other noninfectious factors with electrical conductivity of milk in Manchega ewes. J. Dairy Sci. 2017, 100, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.R.; Schofield, C.P.; Beaulah, S.A.; Mottram, T.T.; Lines, J.A.; Wathes, C.M. A review of livestock monitoring and the need for integrated systems. Comput. Electron. Agric. 1997, 17, 139–159. [Google Scholar] [CrossRef]
- De Mol, R.M.; Keen, A.; Kroeze, G.H.; Achten, J.M.F.H. Description of a detection model for oestrus and diseases in dairy cattle based on time series analysis combined with a Kalman filter. Comput. Electron. Agric. 1999, 22, 171–185. [Google Scholar] [CrossRef]
- Zaninelli, M.; Tangorra, F.; Costa, A.; Rossi, L.; Dell’Orto, V.; Savoini, G. Improved Fuzzy Logic System to Evaluate Milk Electrical Conductivity Signals from On-Line Sensors to Monitor Dairy Goat Mastitis. Sensors 2016, 16, 1079. [Google Scholar] [CrossRef]
- Gowri, A.; Rajamani, A.S.; Ramakrishna, B.; Sai, V.V.R. U-bent plastic optical fiber probes as refractive index based fat sensor for milk quality monitoring. Opt. Fiber Technol. 2019, 47, 15–20. [Google Scholar] [CrossRef]
- Taitt, C.R.; North, S.H. Flow cytometry and pathogen screening in foods. In High Throughput Screening for Food Safety Assessment: Biosensor Technologies, Hyperspectral Imaging and Practical Applications; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Boulaaba, A.; Grabowski, N.; Klein, G. Differential cell count of caprine milk by flow cytometry and microscopy. Small Rumin. Res. 2011, 97, 117–123. [Google Scholar] [CrossRef]
- Brehm-Stecher, B. Flow Cytometry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Yang, B.; Huang, X.; Yan, X.; Zhu, X.; Guo, W. A cost-effective on-site milk analyzer based on multispectral sensor. Comput. Electron. Agric. 2020, 179, 105823. [Google Scholar] [CrossRef]
- Godden, S.M.; Royster, E.; Timmerman, J.; Rapnicki, P.; Green, H. Evaluation of an automated milk leukocyte differential test and the California Mastitis Test for detecting intramammary infection in early- and late-lactation quarters and cows. J. Dairy Sci. 2017, 100, 6527–6544. [Google Scholar] [CrossRef]


| Concentration (mmol·kg−1) | Reference | |
|---|---|---|
| Normal concentration | 5–12 | [19,31] |
| Limit concentrationli (churning method) | 13 | [32] |
| Limit concentration (extraction method) | 32 | [32] |
| Breed | F/P Ratio |
|---|---|
| Holstein | 1.19 |
| Brown Swiss | 1.20 |
| Ayrshire | 1.21 |
| Guernsey | 1.34 |
| Jersey | 1.28 |
| Urea Content (mg·L−1) | Reference | |
|---|---|---|
| Normal concentration | 180–400 | [53] |
| 150–350 | [31] | |
| Limit concentration | >700 | [54] |
| Parameter | SCC in 1 mL of Milk |
|---|---|
| Healthy quarter | 70,000–250,000 |
| Infected quarter/limit for processing | >400,000 |
| Factor | Indicator | Reference |
|---|---|---|
| Number of lactations | EC increases with the number of lactations. | [67,68] |
| Lactation status | EC increases with the number of days of lactation. | [69] |
| Fraction of milk | The highest EC values are at the beginning of milking. They are continuously declining during milking. | [70] |
| Individuality of animals | EC is very different for each breed. | [69] |
| Content of milk fat | EC is decreasing with increasing content of fat. | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunes, R.; Bartos, P.; Iwasaka, G.K.; Lang, A.; Hankovec, T.; Smutny, L.; Cerny, P.; Poborska, A.; Smetana, P.; Kriz, P.; et al. In-Line Technologies for the Analysis of Important Milk Parameters during the Milking Process: A Review. Agriculture 2021, 11, 239. https://doi.org/10.3390/agriculture11030239
Kunes R, Bartos P, Iwasaka GK, Lang A, Hankovec T, Smutny L, Cerny P, Poborska A, Smetana P, Kriz P, et al. In-Line Technologies for the Analysis of Important Milk Parameters during the Milking Process: A Review. Agriculture. 2021; 11(3):239. https://doi.org/10.3390/agriculture11030239
Chicago/Turabian StyleKunes, Radim, Petr Bartos, Gustavo Kenji Iwasaka, Ales Lang, Tomas Hankovec, Lubos Smutny, Pavel Cerny, Anna Poborska, Pavel Smetana, Pavel Kriz, and et al. 2021. "In-Line Technologies for the Analysis of Important Milk Parameters during the Milking Process: A Review" Agriculture 11, no. 3: 239. https://doi.org/10.3390/agriculture11030239
APA StyleKunes, R., Bartos, P., Iwasaka, G. K., Lang, A., Hankovec, T., Smutny, L., Cerny, P., Poborska, A., Smetana, P., Kriz, P., & Kernerova, N. (2021). In-Line Technologies for the Analysis of Important Milk Parameters during the Milking Process: A Review. Agriculture, 11(3), 239. https://doi.org/10.3390/agriculture11030239









