Validation of Diagnostic Markers for Streak Virus Disease Resistance in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Genotyping of Early Generation Maize Inbred Lines
2.2. Phenotyping for MSV Response
2.3. Determination of Relative Accumulation of MSV by Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Data Analysis
3. Results
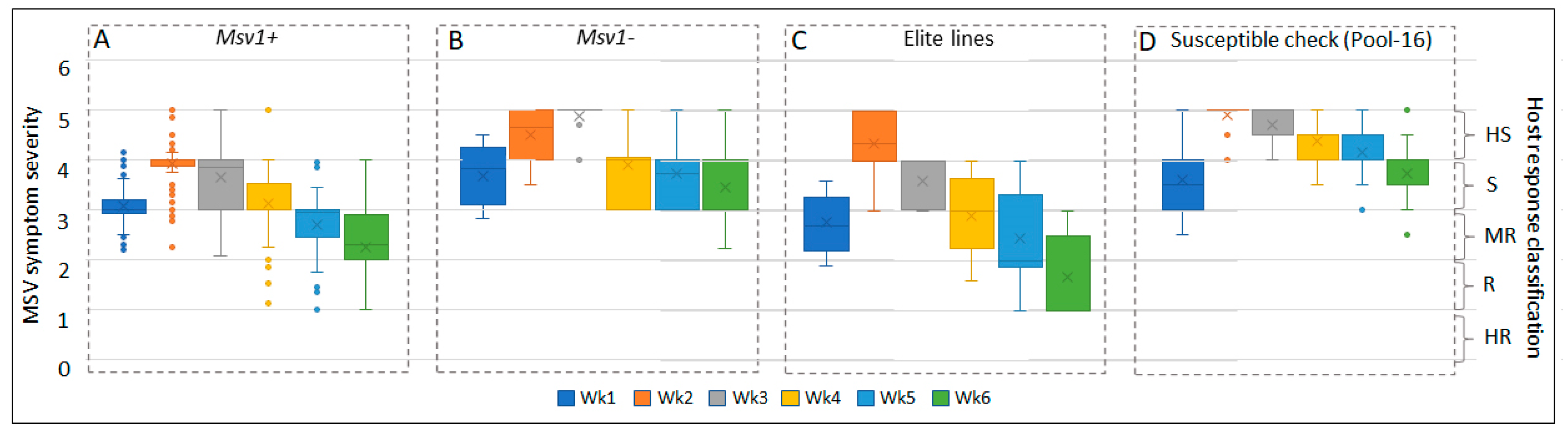
3.1. MSV Symptom Severity Scores and Relationship with Alleles of SNP Markers
3.2. Variation in Recovery Response among Lines Derived from Biparental Crosses
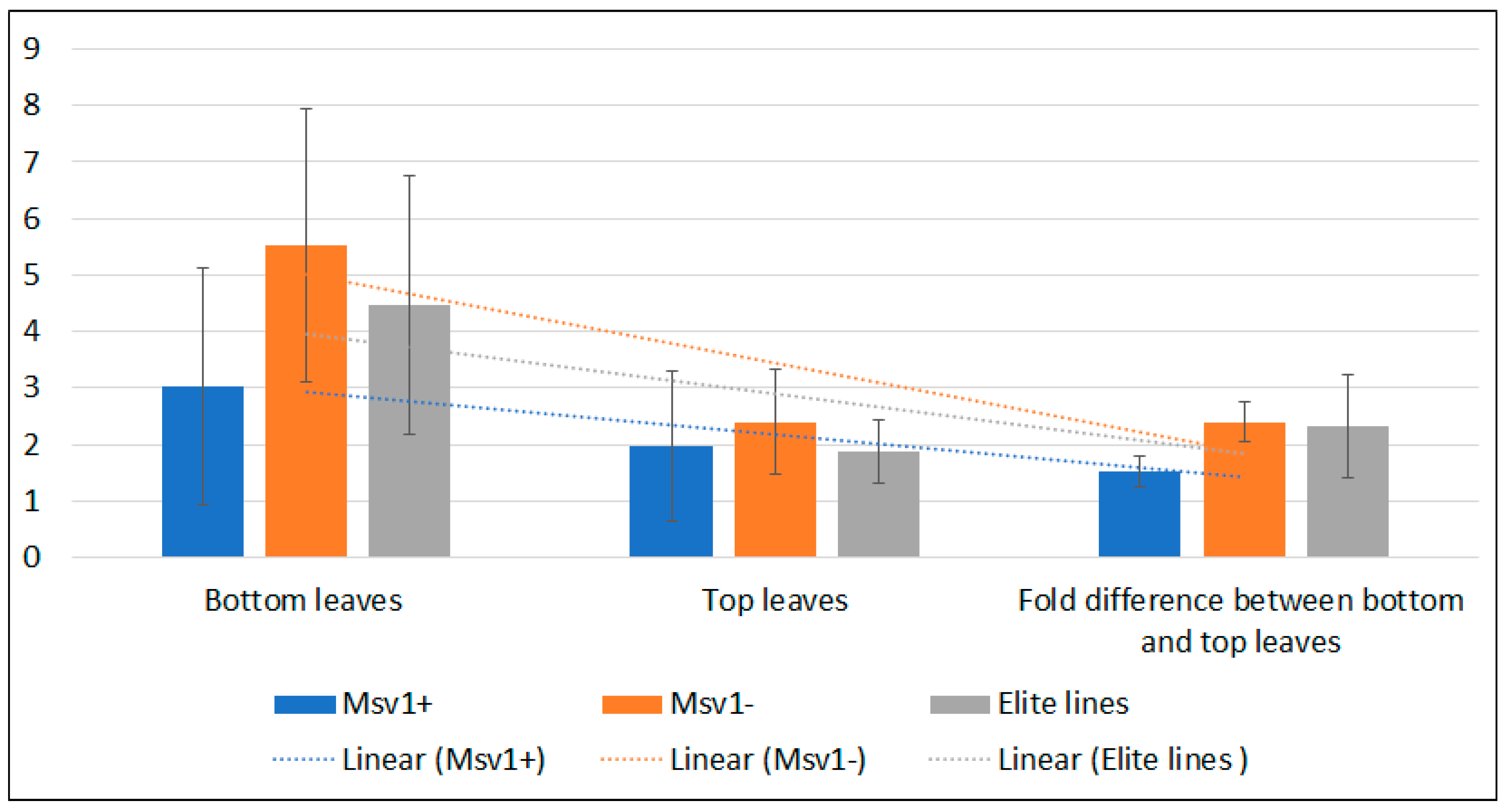
3.3. MSV Detection and Relative Titer Estimation Using ELISA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratt, R.C.; Gordon, S.G. Breeding for resistance to maize foliar pathogens. Plant Breed. Rev. 2006, 27, 119–174. [Google Scholar]
- Thottappilly, G.; Bosque–Perez, N.A.; Rossel, H.W. Viruses and virus diseases of maize in tropical Africa. Plant Pathol. 1993, 42, 494–509. [Google Scholar] [CrossRef]
- Guthrie, E.J. Measurement of yield losses caused by maize streak disease. Plant Dis. 1978, 62, 839–841. [Google Scholar]
- Magenya, O.E.V.; Mueke, J.; Omwega, C. Significance and transmission of maize streak virus disease in Africa and options for management. Afr. J. Biotechnol. 2008, 7, 4897–4910. [Google Scholar]
- Efron, Y.; Kim, S.K.; Fajemisin, J.M.; Mareck, J.H.; Tang, C.Y.; Dabrowski, Z.T.; Rossel, H.W.; Thottappilly, G.; Buddenhagen, I.W. Breeding for resistance to maize streak virus: A multidisciplinary team approach. Plant Breed. 1989, 103, 1–36. [Google Scholar] [CrossRef]
- Shepherd, D.N.; Martin, D.P.; van de Walt, E.; Varsani, K.D.A.; Rybicki, E.P. Maize streak virus: An old and complex ‘emerging’ pathogen. Mol. Plant Pathol. 2010, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.P.; Shepherd, D.M. The epidemiology, economic impact and control of maize streak disease. Food Secur. 2009, 1, 305–315. [Google Scholar] [CrossRef]
- Storey, H.H.; Howland, A.K. Inheritance of resistance in maize to the virus of streak disease in East Africa. Ann. Appl. Biol. 1967, 59, 429–436. [Google Scholar] [CrossRef]
- Soto, P.E.; Buddenhagen, I.W.; Asnani, V.L. Development of streak virus–resistant maize populations through improved and selection methods. Ann. Appl. Biol. 1982, 100, 539–546. [Google Scholar] [CrossRef]
- Bosque–Perez, N.A. Eight decades of maize streak virus research. Virus Res. 2000, 71, 107–121. [Google Scholar]
- Pernet, A.D.; Hoisington, J.; Dintinger, D.; Jewel, C.; Jiang, C.; Khairallah, M.; Letourmy, P.; Marchand, J.L.; Glaszmann, J.C.; de leon, D.G. Genetic mapping of Maize streak virus resistance from the Mascarene source II. Resistance in line CIRAD390 and stability against across germplasm. Theor. Appl. Genet. 1999, 99, 540–553. [Google Scholar] [CrossRef]
- Pernet, A.D.; Hoisington, J.; Franco, M.; Isnard, M.; Jewel, C.; Jiang, C.; Marchand, J.L.; Reynaud, B.; Glaszmann, J.C.; de leon, D.G. Genetic mapping of Maize streak virus resistance from the Mascarene source I. Resistance in line D211 and stability against different virus clones. Theor. Appl. Genet. 1999, 99, 524–539. [Google Scholar] [CrossRef] [PubMed]
- Welz, H.G.; Schechert, A.; Pernet, A.; Pixley, K.V.; Geiger, H.H. A gene for resistance to the maize streak virus in the African CIMMYT maize inbred line CML 202. Mol. Breed. 1998, 4, 147–154. [Google Scholar] [CrossRef]
- Kyetere, D.T.; Ming, R.; McMullen, M.D.; Pratt, R.C.; Brewbaker, J.; Musket, T. Genetic analysis of tolerance to maize streak virus in maize. Genome 1999, 42, 20–26. [Google Scholar] [CrossRef]
- Garcia–Oliveira, A.L.; Menkir, A.; Kumar, P.L.; Azuh, V.; Oyetunji, O.J.; Gedil, M. Mapping of new loci for maize streak virus disease resistance in F2:3 population of tropical maize. Cereal Res. Commun. 2020, 48, 195–202. [Google Scholar] [CrossRef]
- Lagat, M.; Danson, M.; Kimani, M.; Kuria, A. Quantitative trait loci for resistance to maize streak virus in maize lines used in hybrid development. Afr. J. Biotechnol. 2008, 7, 2573–2577. [Google Scholar]
- Nair, S.K.; Raman, B.; Magorokosho, C.; Mahuku, G.; Semagn, K.; Beyene, Y.; Das, B.; Makumbi, D.; Kumar, P.L.; Olsen, M.; et al. Fine mapping of Msv1, a major QTL for resistance to maize streak virus leads to development of production markers for breeding pipelines. Theor. Appl. Genet. 2015, 128, 1839–1854. [Google Scholar] [CrossRef]
- Peterschmitt, M.; Reynaud, B.; Sommermeyer, G.; Baudin, P. Characterization of maize streak virus isolates using monoclonal and polyclonal antibodies and by transmission to a few hosts. Plant Dis. 1991, 75, 27–32. [Google Scholar] [CrossRef]
- Wilcoxson, R.D.; Skovmand, B.; Latif, A.H. Evaluation of wheat cultivars for ability to retard development of stem rust. Ann. Appl. Biol. 1975, 80, 275–281. [Google Scholar] [CrossRef]
- Ladejobi, O.; Salaudeen, M.T.; Kumar, P.L.; Menkir, A.; Adesoye, A.; Atiri, G.; Gedil, M. Mapping of QTLs associated with recovery resistance to streak virus disease in maize. Ann. Agric. Sci. 2018, 63, 115–121. [Google Scholar] [CrossRef]
- Shiferaw, B.; Boddupalli, M.P.; Hellin, J.; Bänziger, M. Crops that feed the world 6 Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Parlevliet, J.E. Durability of resistance against fungal, bacterial and viral pathogens; present situation. Euphytica 2002, 124, 147–156. [Google Scholar] [CrossRef]
- Mesfin, T.; Den Hollander, J.; Markham, P.G. Feeding activities of Cicadulina mbila (Hemiptera: Cicadellidae) on different host–plants. Bull. Entomol. Res. 1995, 85, 387–396. [Google Scholar] [CrossRef]
- Abalo, G.; Edema, R.; Derera, J.; Tongoona, P. A comparative analysis of conventional and marker–assisted selection methods in breeding maize streak virus resistance in maize. Crop Sci. 2009, 49, 509–520. [Google Scholar] [CrossRef]

| SNP ID * | Intertek ID | Target Sequence (5′ → 3′) | MSV Resistance Allele | Susceptible Allele |
|---|---|---|---|---|
| PZE-101093951 | snpZM-0020 | TAACTCTCTGCTGTTGCTTGTCTTCAGGTTGTCATGAGAGATCCTCACAT[A/G]GCAGCAGATGGCTTCACCTACGAAGCTGACGCTCTTAGATACTGGCTCGA | A:A | G:G |
| PZE-0186065237 | snpZM-0021 | ACATCTCCAGTAACAAACAGAAGTCTTTCGAATCGTGATACCATCCCCAA[T/C]CACGCACTGCGKTCGGCCATCCAAGAATACCTCCGGCAGAACGAGCTGCA | C:C | T:T |
| PZE-0186365075 | snpZM-0022 | AGAAGAAAATGGCCTGCCATATATATATCCCGGTTAATCGCTARTGCATT[A/C]TCAGGAATCATTCTCATAGGTCATAAGACGAGCAAGGGATACTCTTCTAC | C:C | A:A |
| Pedigree | No. of Maize Lines | MSV Resistant Allele 1 | MSV Phenotyping | Relative MSV Accumulation Estimated from A405 nm Values | |||||
|---|---|---|---|---|---|---|---|---|---|
| MSV Symptom Severity Score at 6 wpi 2 (Mean) | Reduction in MSV Symptom Severity Score 3 (Mean) | Mean Symptom Recovery 4 (%) | Mean AUDPC | AFV of Bottom Leaves 5 | AFV of Top Leaves 5 | Decrease in MSV Accumulation 6 (%) | |||
| Bi-parental populations | |||||||||
| IITATZI1715/TZISTR1262 | 20 | + | 3.1 (±0.5) | 4.3 → 2.1 | 51.8 (±19.4) | 110.8 (±16.7) | 0.9 (±0.5) | 0.5 (±0.1) | 44.5 |
| IITATZI1716/TZISTR1262 | 12 | + | 2.4 (±0.5) | 3.1 → 1.3 | 52.9 (±18.5) | 99.4 (±24.6) | 0.5 (±0.1) | 0.4 (±0.1) | 20.0 |
| ((KU1409/DE3/KU1409)S2-18-2-BBB/(KU1409/SC55/KU1409)-S2-19-1-BBB-17-B-3-B/TZISTR1262) | 3 | + | 2.9 (±0.5) | 3.5 → 2.1 | 41.3 (±20.3) | 104.7 (±11.8) | 0.5 (±0.02) | 0.4 (±0.02) | 20.0 |
| ((KU1409/SC55/KU1409-4-BBB/(KU1409/DE3/KU1409)S2-18-2-BBB-9-B-1-B/TZISTR1276) | 27 | + | 3.3 (±0.5) | 4.0 → 2.4 | 38.0 (±20.3) | 117.4 (±17.2) | 5.8 (±4.1) | 3.4 (±2.0) | 41.4 |
| (((KU1409/KU1414-SR/A619)-S2-2/9450/KI21-7-2-2-1-1-BB)-28-B*4-B-1/TZISTR1276 | 47 | + | 3.2 (±0.3) | 4.0 → 2.3 | 41 (±14.3) | 115.7 (±10.3) | 3.1 (±2.6) | 1.7 (±1.4) | 45.2 |
| ((KU1409/DE3/KU1409)S2-18-2-BBB/(KU1409/SC55/KU1409)-S2-19-1-BBB-17-B-3-B-B-1/TZISTR1277) | 2 | + | 3.5 (±0.04) | 4.2 → 2.7 | 34.8 (±20.3) | 128.4 (±12.6) | 3.4 (±0.4) | 3.1 (±0.1) | 8.8 |
| ((KU1409/DE3/KU1409)S2-18-2-B/SYN-Y-STR-34-1-1-1-1-2-1-B*7-21-BB-B-1/TZISTR1277) | 22 | + | 3.2 (±0.3) | 3.9 → 2.7 | 34.8 (±20.3) | 128.4 (±12.6) | 4.6 (±1.6) | 2.7 (±0.9) | 41.3 |
| (SW 5 (S) C6-18-2-1-B/TZISTR1248) | 11 | - | 4.2 (±0.3) | 4.9 → 3.6 | 26.6 (±14.9) | 148.8 (±11.7) | 4.4 (±2.1) | 2.4 (±1.4) | 45.5 |
| (SW 5 (S) C6-18-3-1-B/TZISTR1248) | 5 | - | 3.7 (±0.4) | 4.7 → 3.0 | 36.5 (±12.5) | 131.6 (±17.2) | 3.8 (±1.2) | 1.5 (±0.3) | 60.5 |
| KS 27 (S) C3-2-6-2-B-1/TZISTR1262 | 2 | - | 4.0 (±0.7) | 5.0 → 3.6 | 28.3 (±20.3) | 146.6 (±16.6) | 8.3 (±1.1) | 3.3 (±0.0) | 60.2 |
| Elite lines | |||||||||
| TZISTR1090 | 1 | nt | 3.3 | 5.0 → 2.0 | 60.0 | 117.6 | 4.4 | 1.6 | 63.6 |
| TZISTR109 | 1 | nt | 2.4 | 4.0 → 1.0 | 75.0 | 86.5 | 2.6 | 1.4 | 46.2 |
| TZISTR1100 | 1 | nt | 1.9 | 3.0 → 1.0 | 66.7 | 70.4 | 1.6 | 1.5 | 6.3 |
| TZISTR1103 | 1 | nt | 2.8 | 5.0 → 1.0 | 80.0 | 100.5 | 5.2 | 1.4 | 73.1 |
| TZISTR1104 | 1 | nt | 2.6 | 4.2 → 1.0 | 75.9 | 99.2 | 4.1 | 2.5 | 39.0 |
| 1368STR-B-B-B | 1 | nt | 3.4 | 5.0 → 2.0 | 60.0 | 123.2 | 4.9 | 2.0 | 59.2 |
| 9071STR-B-B-B | 1 | nt | 3.7 | 4.0 → 3.0 | 25.0 | 132.5 | 6.6 | 2.6 | 60.6 |
| KU1414-SR | 1 | nt | 2.9 | 4.4 → 1.0 | 77.0 | 110.3 | 2.0 | 1.3 | 35.0 |
| 4001 | 1 | nt | 3.9 | 4.5 → 3.0 | 33.3 | 135.3 | 8.8 | 2.6 | 70.5 |
| Type | Severity Rating | ||||
|---|---|---|---|---|---|
| 1.0–2.0 (R) | 2.1–3.0 (MR) | 3.1–4.0 (S) | 4.1–5.0 (HS) | Total | |
| Early generation lines with three SNPs associated with MSV resistance | 54 | 76 | 3 | 0 | 133 |
| Early generation lines without SNPs for MSV resistance | 0 | 10 | 5 | 3 | 18 |
| Elite maize lines | 7 | 2 | 0 | 0 | 9 |
| Total | 61 | 88 | 8 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sime, S.S.; Menkir, A.; Adetimirin, V.O.; Gedil, M.; Kumar, P.L. Validation of Diagnostic Markers for Streak Virus Disease Resistance in Maize. Agriculture 2021, 11, 130. https://doi.org/10.3390/agriculture11020130
Sime SS, Menkir A, Adetimirin VO, Gedil M, Kumar PL. Validation of Diagnostic Markers for Streak Virus Disease Resistance in Maize. Agriculture. 2021; 11(2):130. https://doi.org/10.3390/agriculture11020130
Chicago/Turabian StyleSime, Solomon Shibeshi, Abebe Menkir, Victor O. Adetimirin, Melaku Gedil, and P. Lava Kumar. 2021. "Validation of Diagnostic Markers for Streak Virus Disease Resistance in Maize" Agriculture 11, no. 2: 130. https://doi.org/10.3390/agriculture11020130
APA StyleSime, S. S., Menkir, A., Adetimirin, V. O., Gedil, M., & Kumar, P. L. (2021). Validation of Diagnostic Markers for Streak Virus Disease Resistance in Maize. Agriculture, 11(2), 130. https://doi.org/10.3390/agriculture11020130







