Alternaria alternata as a Seed-Transmitted Pathogen of Sida hermaphrodita (Malvaceae) and Its Suppression by Aureobasidium pullulans
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of Plant Health
2.2. Mycological Analysis of Experimental Seeds and Estimation of The Share of A. alternata
2.3. Morphological Analysis of Alternaria alternata
2.4. Molecular Identification of Selected A. alternata Isolates
2.5. Isolate Accession Numbers
2.6. Standard Seed Germination Test
2.7. Pathogenicity of Alternaria alternata in The Detached Leaf Bioassay
2.8. Seed Transmission Test and Evaluation of Seedling Health in a Greenhouse Experiment
2.9. Biological Protection of Virginia Petals Seeds In Vitro and in a Greenhouse
2.10. Data Analysis
3. Results
3.1. Disease Symptoms on Leaves and Seed Capsules
3.2. Evaluation of Seed Health
3.3. Molecular Identification of Alternaria alternata
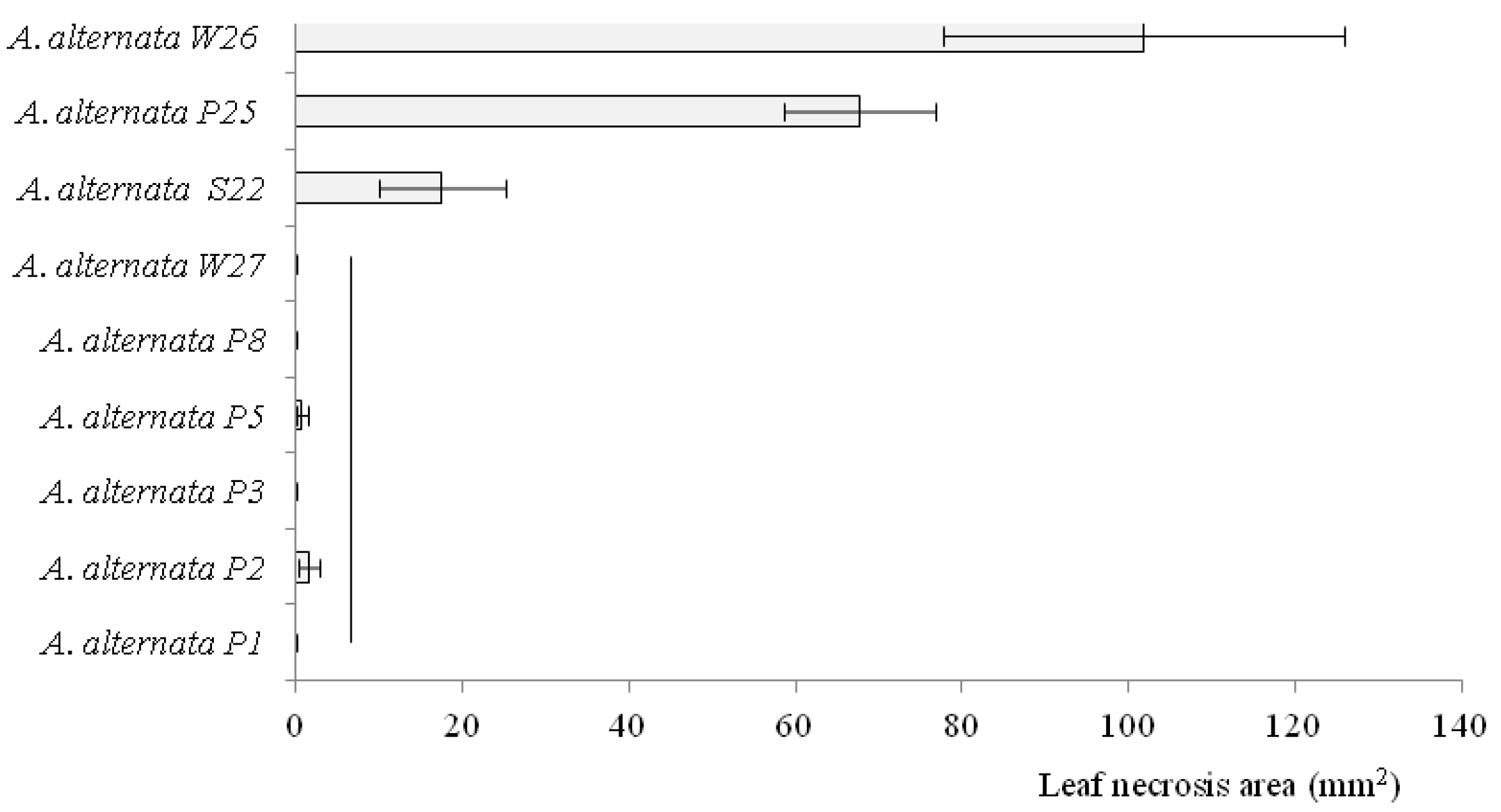
3.4. Pathogenicity of A. alternata in the Detached Leaf Bioassay
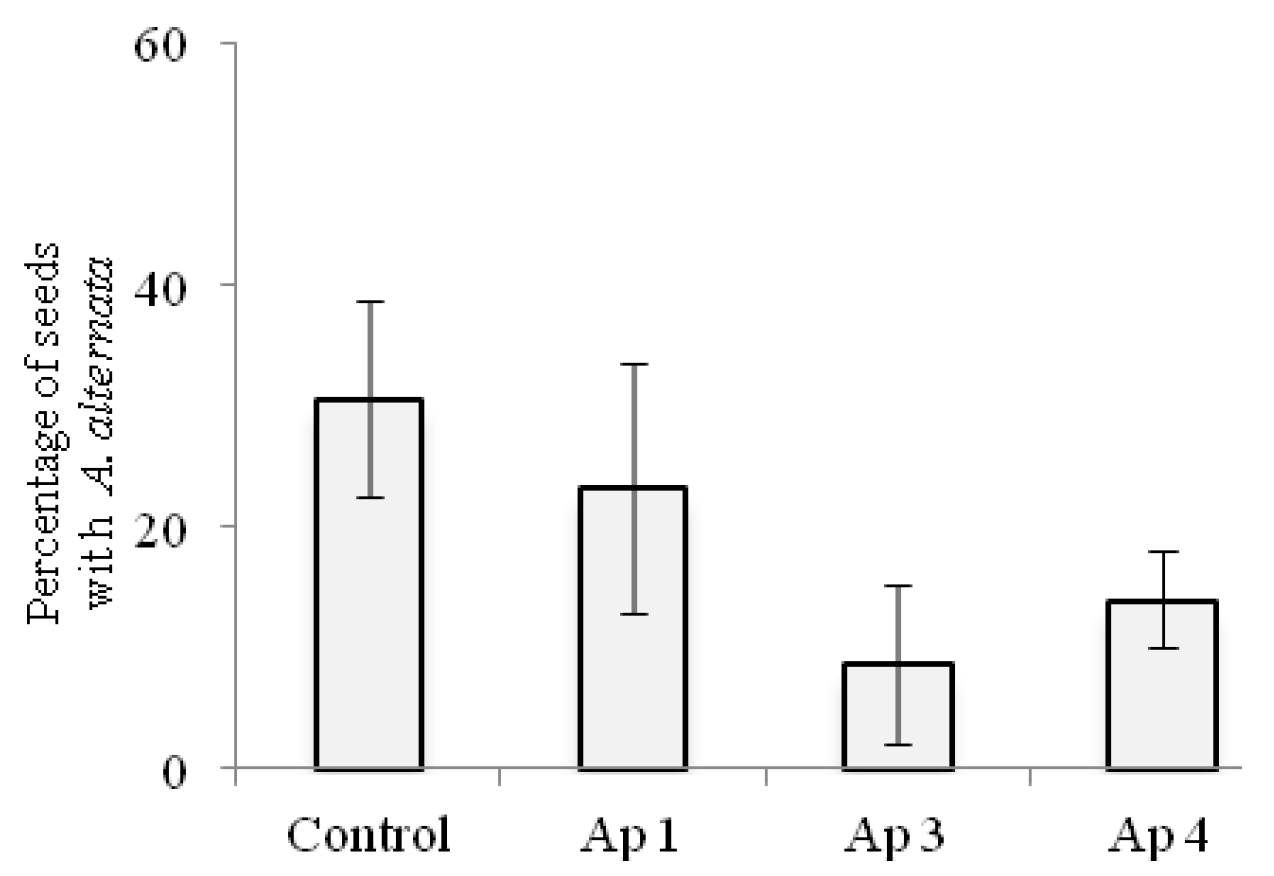
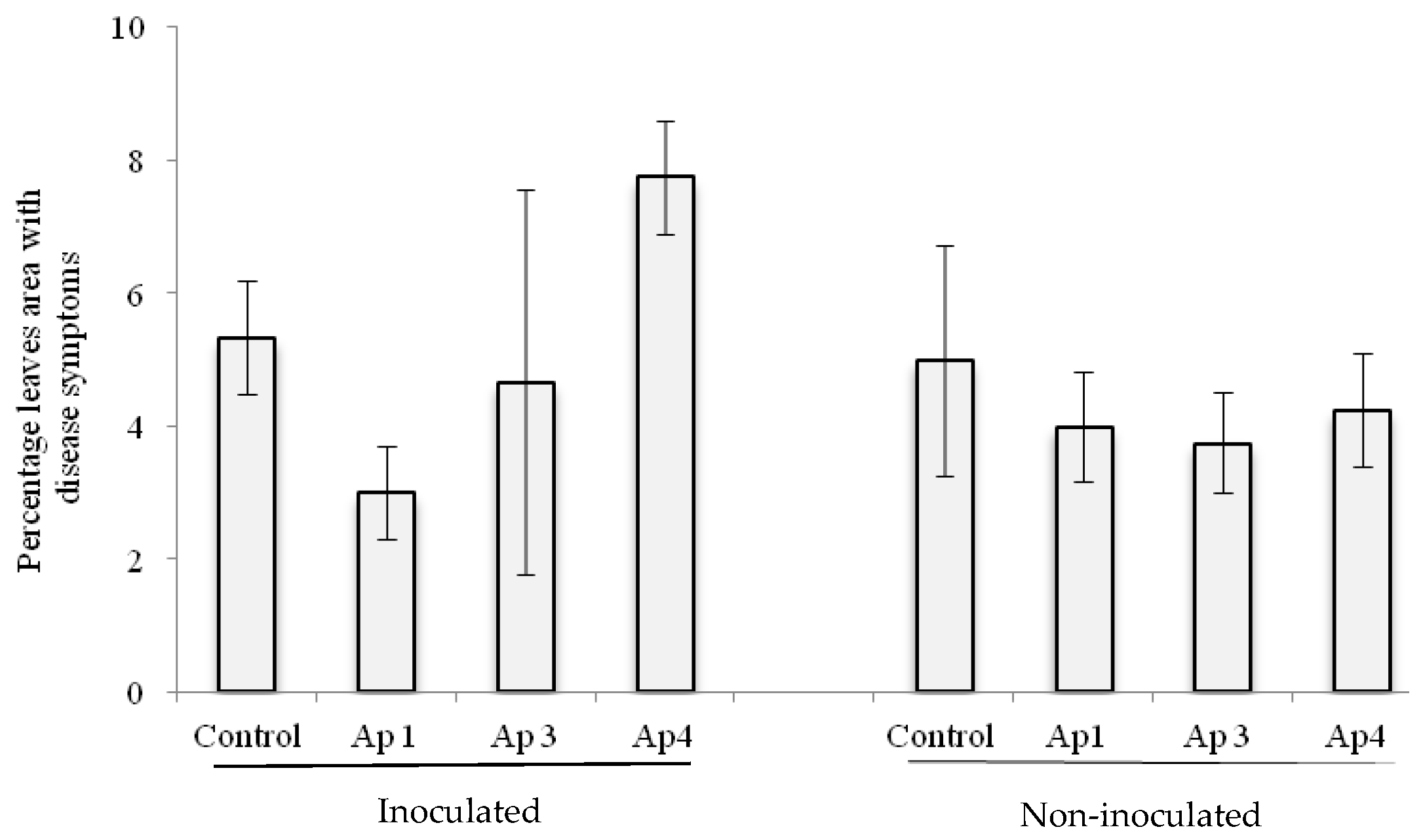
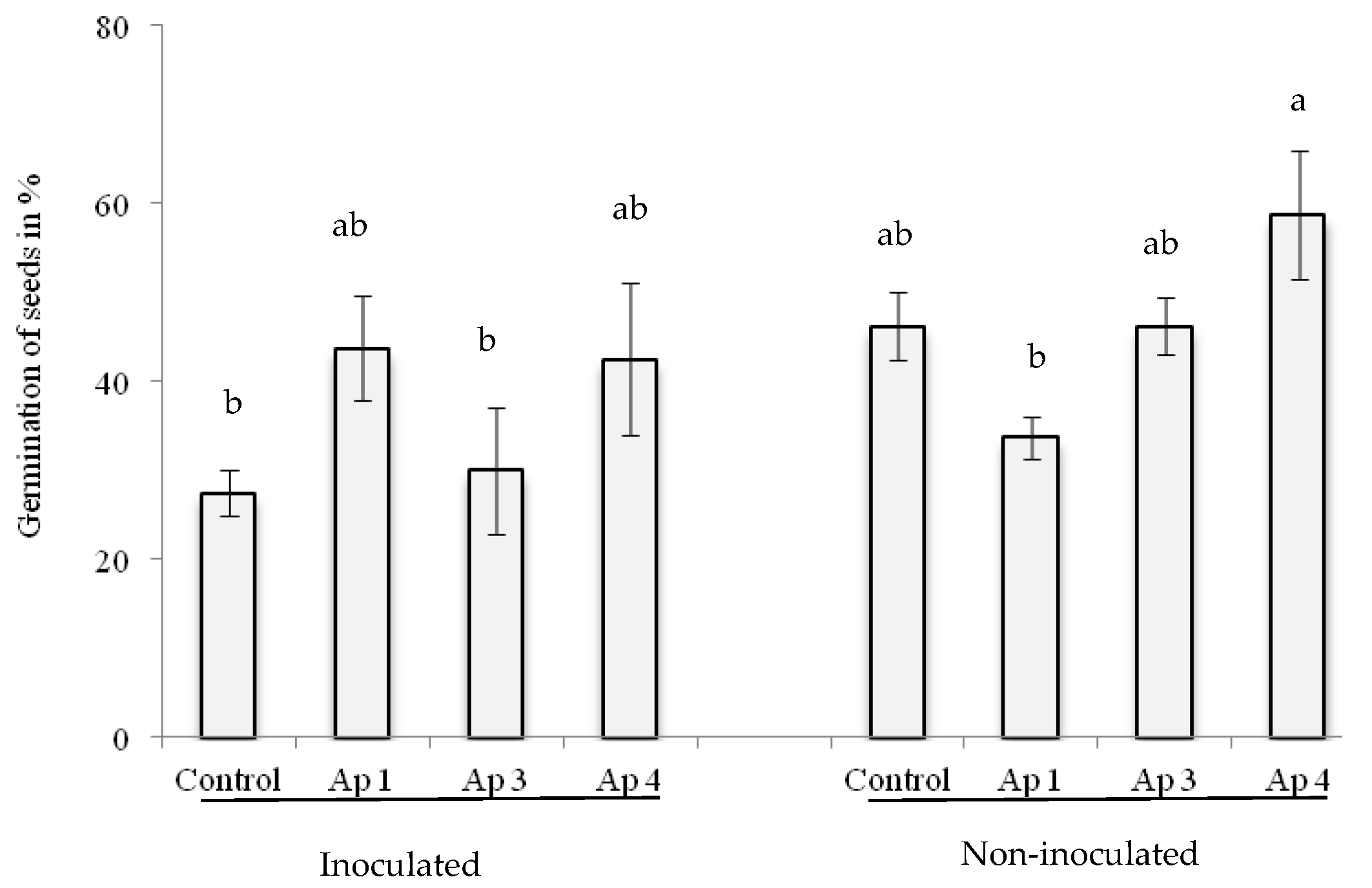
3.5. Seed Transmission Test
3.6. Biological Control In Vitro and in Greenhouse
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spooner, D.M.; Cusick, A.W.; Hall, G.F.; Baskin, J.M. Observations on the distribution and ecology of Sida hermaphrodita (L.) Rusby (Malvaceae). Sida Contrib. Bot. 1985, 11, 215–225. [Google Scholar]
- Stolarski, M.J. Industrial and bioenergy crops for bioeconomy development. Agriculture 2021, 11, 852. [Google Scholar] [CrossRef]
- Borkowska, H.; Molas, R.; Kupczyk, A. Virginia fanpetals (Sida hermaphrodita Rusby) cultivated on light soil; height of yield and biomass productivity. Pol. J. Environ. Stud. 2009, 18, 563–568. [Google Scholar]
- Borkowska, H.; Molas, R. Two extremely different crops, Salix and Sida, as sources of renewable bioenergy. Biomass Bioenergy 2012, 36, 234–240. [Google Scholar] [CrossRef]
- Kocoń, A.; Jurga, B. The evaluation of growth and phytoextraction potential of Miscanthus × giganteus and Sida hermaphrodita on soil contaminated simultaneously with Cd, Cu, Ni, Pb, and Zn. Environ. Sci. Pollut. Res. 2017, 24, 4990–5000. [Google Scholar] [CrossRef]
- Ignatowicz, K. The assessment usability of Virgina mallow Sida hermaphrodita for phytoremediation of soil contaminated with pesticides. Ecol. Eng. 2015, 45, 89–92. [Google Scholar] [CrossRef][Green Version]
- Lewtak, K.; Fiołka, M.J.; Czaplewska, P.; Macur, K.; Kaczyński, Z.; Buchwald, T.; Szczuka, E.; Rzymowska, J. Sida hermaphrodita seeds as the source of anti—Candida albicans activity. Sci. Rep. 2019, 9, 12233. [Google Scholar] [CrossRef]
- Mangwende, E.; Kritzinger, Q.; Truter, M.; Aveling, T.A.S. Alternaria alternata: A new seed-transmitted disease of coriander in South Africa. Eur. J. Plant. Pathol. 2018, 152, 409–416. [Google Scholar] [CrossRef]
- Łacicowa, B.; Kiecana, I. White mold—A dangerous disease affecting Sida hermaphrodita (L.) Rusby. Ochrona Roślin. 1991, 10, 16–17. (In Polish) [Google Scholar]
- Kalembasa, D.; Jaremko, D.; Bik, B.; Karczmarek, J.; Jędryczka, M. Effect of infection of Virginia mallow shoots by fungus Sclerotinia sclerotiorum on the contents and distribution of aluminium, manganese and iron. Acta Sci. Pol. Agric. 2016, 15, 39–47. [Google Scholar]
- Remlein-Starosta, D.; Nijak, K. Virginia mallow—First results on investigation on possibilities of pest and diseases control. Prog. Plant Prot. 2007, 47, 358–362. (In Polish) [Google Scholar]
- Remlein-Starosta, D. Epidemic incidence of Sclerotinia rot on Virginia mallow. Prog. Plant Prot. 2009, 49, 705–709. (In Polish) [Google Scholar]
- European Green Deal. Available online: https://ec.europa.eu›eu-action›european-green-deal_pl (accessed on 18 October 2021).
- Kandula, J.; Kaur, H.; Alizadeh, H.; Hampton, J.G. Disease Control and Plant Growth Promotion of Miscanthus × giganteus with Trichoderma Bio-Inoculants. In Track 2-2-1: Plant Diseases, Insect Pests and Weed Management, International Grassland Congress; Available online: https://uknowledge.uky.edu (accessed on 18 October 2021).
- Remlein-Starosta, D.; Krzymińska, J.; Kowalska, J.; Bocianowski, J. Evaluation of yeast-like fungi to protect Virginia mallow (Sida hermaphrodita) against Sclerotinia sclerotiorum. Can. J. Plant Sci. 2016, 96, 243–251. [Google Scholar] [CrossRef]
- Huang, H.; Tian, C.; Huang, Y.; Huang, H. Biological control of poplar anthracnose caused by Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. Egypt J. Biol. Pest Control 2020, 30, 104. [Google Scholar] [CrossRef]
- Meena, M.; Gupta, S.K.; Swapnil, P.; Zehra, A.; Dubey, M.K.; Upadhyay, R.S. Alternaria toxins: Potential virulence factors and genes related to pathogenesis. Front. Microbiol. 2017, 8, 1451. [Google Scholar] [CrossRef]
- Lanzuise, S.; Cozzolino, A.; Gualtieri, L.; Parrella, G.; Ruocco, M. First report of brown leaf spot caused by Alternaria alternata on cast iron plant (Aspidistra elatior) in Italy. J. Plant Pathol. 2018, 100, 117. [Google Scholar] [CrossRef]
- Alam, M.W.; Rehman, A.; Malik, A.U.; Aslam, S.; Sarwar, M.; Ali, S.; Khan, M.A.; Hameed, A.; Sarfraz, S. First report of Alternaria alternata causing postharvest fruit rot of peach in Pakistan. J. Plant Pathol. 2018, 101, 209. [Google Scholar] [CrossRef]
- Garibaldi, A.; Tabone, G.; Matić, S.; Luongo, I.; Gullino, M.L. First report of Alternaria alternata causing leaf spots on Hibiscus syriacus in Italy. J. Plant Pathol. 2020, 102, 953. [Google Scholar] [CrossRef]
- Dadabhau, P.A. Investigation on Leaf Spot (Alternaria alternata (Fr.) Keissler.) Disease of Okra (Abelmoschus esculentus L.) under South Gujarat Conditions. Master’s Thesis, N.M. College of Agriculture Gujarat Agricultural University, Navsari, India, 2009; p. 155. [Google Scholar]
- Bassimba, D.D.M.; Mira, J.L.; Vicent, A. Inoculum sources, infection periods, and effects of environmental factors on Alternaria brown spot of mandarin in Mediterranean climate conditions. Plant Dis. 2014, 98, 409–417. [Google Scholar] [CrossRef]
- Vu, A.L.; Dee, M.M.; Russell, T.; Zale, J.; Gwinn, K.D.; Ownley, B.H. First report of leaf spot caused by Alternaria alternata on Switchgrass in Tennessee. Plant Dis. 2012, 96, 763. [Google Scholar] [CrossRef] [PubMed]
- Pusz, W.; Urbaniak, J. Foliar diseases of willows (Salix spp.) in selected locations of the Karkonosze Mts. (the Giant Mts). Eur. J. Plant Pathol. 2017, 148, 45–51. [Google Scholar] [CrossRef]
- Szpunar-Krok, E.; Stompor-Chrzan, E.; Grochowska, S.; Bobrecka-Jamro, D. Evaluation of the health of energetic crops in Podkarpacie region. Prog. Plant Protec. 2017, 3, 196–200. [Google Scholar] [CrossRef]
- Salo, P.M.; Arbes, S.J.; Sever, M.; Jaramillo, R.; Cohn, R.D.; London, S.J.; Zeldin, D.C. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J. Allergy Clin. Immunol. 2006, 118, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.F.; Postigo, I.; Tomaz, C.T.; Martínez, J. Alternaria alternata allergens: Markers of exposure, phylogeny and risk of fungi-induced respiratory allergy. Environ. Int. 2016, 89–90, 71–80. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Ellis, M.B. Demataceous Hyphomycetes; The Eastern Press: London, UK, 1971; p. 608. [Google Scholar]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. PCR Protocols: A Guide to Methods and Applications, Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Duba, A.; Goriewa, K.; Wachowska, U.; Wiwart, M. Alternaria alternata (Fr.) Keissl with mutation G143A in the Cyt b gene is the source of a difficult-to-control allergen. Environ. Sci. Pollut. Res. Int. 2018, 25, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Ramires, F.A.; Masiello, M.; Somma, S.; Villani, A.; Susca, A.; Logrieco, A.F.; Luz, C.; Meca, G.; Moretti, A. Phylogeny and mycotoxin characterization of Alternaria species isolated from wheat grown in Tuscany, Italy. Toxins 2018, 10, 472. [Google Scholar] [CrossRef]
- PAS. Available online: www.ibb.waw.pl (accessed on 12 October 2020).
- Genomed. Available online: http://www.genomed.pl (accessed on 2 February 2020).
- BLAST. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 10 January 2021).
- Kgatle, M.G.; Truter, M.; Ramusi, T.M.; Flett, B.; Aveling, T.A.S. Alternaria alternata, the causal agent of leaf blight of sunflower in South Africa. Eur. J. Plant. Pathol. 2018, 151, 677–688. [Google Scholar] [CrossRef]
- ISTA (International Seed Testing Association). International Rules for Seed Testing. Proceedings of the International Seed Testing Association. Available online: https://www.seedtest.org/en/international-rules-for-seed-testing-_content---1--1083.html (accessed on 10 January 2021).
- Rasband, W.S. ImageJ; U.S. National Institute of Health: Bethesda, MD, USA, 2016. Available online: http://imagej.nih.gov/ij/ (accessed on 10 October 2020).
- Eppo, C. European and Mediterranean Plant Protection Organization Guideline for the Efficacy Evaluation of Fungicides. Foliar Dis. Cereals 1998, 28, 279–290. [Google Scholar]
- Wachowska, U.; Irzykowski, W.; Jędryczka, M. Agrochemicals: Effect on genetic resistance in yeasts colonizing winter wheat kernels. Ecotoxicol. Environ. Saf. 2018, 162, 77–84. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil; University of California, College of Agriculture, Agricultural Experiment Station: Upper Darby, PA, USA, 1950; p. 347. [Google Scholar]
- StatSoft Inc. STATISTICA (Data Analysis Software System); Version 12; StatSoft Inc.: Palo Alto, CA, USA; Available online: www.statsoft.com (accessed on 20 September 2021).
- Grzesik, M.; Janas, R.; Romanowska-Duda, Z. Stimulation of growth and metabolic processes in Virginia mallow (Sida hermaphrodita L. Rusby) by seed hydroconditioning. Probl. Inżynierii Rol. 2011, 4, 81–89. [Google Scholar]
- Saletnik, B.; Bajcar, M.; Zakuła, G.; Saletnik, A.; Tarapatskyy, M.; Puchalski, C. Biochar as a stimulator for germination capacity in seeds of Virginia mallow (Sida hermaphrodita (L.) Rusby). Appl. Sci. 2019, 9, 3213. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Argel, P.J.; Paton, C.J. Tropical and Subtropical Species. In Forage Seed Production, Overcoming Legume Hardseedeness; Loch, D.S., Ferguson, J.E., Eds.; CAB International: Wallingford, UK, 1999; Volume 2, pp. 247–259. [Google Scholar]
- Dos Santos, R.R.; de Morais, T.P.; Juliatti, F.C. Detection of Sclerotinia sclerotiorum on soybean and common bean seeds by modified Neon-s test. Biosci. J. Uberlândia 2018, 34, 67–74. [Google Scholar] [CrossRef]
- De Carvalho, C.; Forti, V.A.; Bonfim, M.F.; Moraes, M.H.D.; Menten, J.O.M. Transmission of Sclerotinia sclerotiorum from soybean seed to seedlings. Rev. Agric. 2016, 91, 67–80. [Google Scholar] [CrossRef]
- Thomma, B.P. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Rodríguez, C.; Garcia, M.A. Seed-bank dynamics of the tropical weed Sida rhombifolia (Malvaceae): Incidence of seedling emergence, predators and pathogens. Seed Sci. Res. 2009, 19, 241–248. [Google Scholar] [CrossRef]
- Agrawal, S. Seed-Borne and Post-Harvest Diseases of Okra [Ablemoschus esculentus L. (Monech)]. Ph.D. Thesis, University of Rajasthan, Jaipur, India, 2000; p. 121. [Google Scholar]
- Begum, M.; Lokesh, S.; Ravishankar, R.V.; Shailaja, M.D.; Kumar, T.V.; Shetty, H.S. Evaluation of certain storage conditions for okra (Abelmoschus esculentus (L.) Moench) seeds against potential fungal pathogens. Int. J. Agric. Biol. 2005, 7, 550–554. [Google Scholar]
- Borkowska, H. Seed germination and field emergence ability of Virginia mallow depending on applied seed treatment. Fragm. Agron. 2006, 3, 269–276. [Google Scholar]



| Estimated Percentage Leaf Area with Disease Systems | Description of Disease Severity |
|---|---|
| 0 | No infection |
| 1 | 1–5 leaf lesions (diameter < 3 mm) on leaf surface |
| 5 | 5–10 leaf lesions (diameter 3–5 mm) on leaf surface |
| 10 | >10 leaf lesions (diameter > 5 mm) on leaf surface |
| Seed Lot | Incidence (%) of Fungi * | ||||
|---|---|---|---|---|---|
| Aa | Fus | Pen | Cla | Acr | |
| 2 months of storage | 34.74 a | 0.35 b | 3.16 | 8.07 a | 11.23 b |
| 12 months of storage | 45.09 a | 0 b | 4.21 | 0.53 b | 6.14 b |
| 24 months of storage | 18.24 b | 1.58 a | 2.11 | 0 b | 27.72 a |
| Inoculation | Morphology of Virginia Fanpetals Seeds | Leaves Infected by A. alternata (Mean Score) | ||||
|---|---|---|---|---|---|---|
| Seedling Height (cm) | Root Length (cm) | No. of Leaves | Seedling Weight (g) | Seed Germination Capacity (%) | ||
| Control | 6.31 a | 3.5 | 3.16 a | 0.51 | 45.03 a | 5.01 |
| A. alternaria | 5.07 b | 3.16 | 2.51 b | 0.62 | 27.5 b | 5.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachowska, U.; Kwiatkowska, E.; Pluskota, W. Alternaria alternata as a Seed-Transmitted Pathogen of Sida hermaphrodita (Malvaceae) and Its Suppression by Aureobasidium pullulans. Agriculture 2021, 11, 1264. https://doi.org/10.3390/agriculture11121264
Wachowska U, Kwiatkowska E, Pluskota W. Alternaria alternata as a Seed-Transmitted Pathogen of Sida hermaphrodita (Malvaceae) and Its Suppression by Aureobasidium pullulans. Agriculture. 2021; 11(12):1264. https://doi.org/10.3390/agriculture11121264
Chicago/Turabian StyleWachowska, Urszula, Edyta Kwiatkowska, and Wioletta Pluskota. 2021. "Alternaria alternata as a Seed-Transmitted Pathogen of Sida hermaphrodita (Malvaceae) and Its Suppression by Aureobasidium pullulans" Agriculture 11, no. 12: 1264. https://doi.org/10.3390/agriculture11121264
APA StyleWachowska, U., Kwiatkowska, E., & Pluskota, W. (2021). Alternaria alternata as a Seed-Transmitted Pathogen of Sida hermaphrodita (Malvaceae) and Its Suppression by Aureobasidium pullulans. Agriculture, 11(12), 1264. https://doi.org/10.3390/agriculture11121264






