Abstract
Ectomyelois ceratoniae (Lepidoptera: Pyralidae) is the primary pest of pomegranates in Saudi Arabia and is mostly controlled using broad-spectrum pesticides. Providing environmentally sound choices to limit reliance on chemical management is a major challenge in the control of E. ceratoniae and, as a consequence, in the protection of pomegranate crops from its invasion. Entomopathogenic bacteria (EPB) symbiotically associated with entomopathogenic nematodes (EPNs) are well-known biocontrol agents of soil-dwelling or aerial pests. The bacterium symbiont (EPB) is the real insect-killing biocontrol agent, while the nematode (EPN) serves as a vector. We wondered whether the EPB vector, which is extremely vulnerable to adverse environmental conditions, like drought, high temperatures, and repellent soil microorganisms, could be omitted. We intended to evaluate the biocontrol potential of directly applied EPB cells and cell-free culture media (CFCM) on the larval instar E. ceratoniae. Xenorhabdus budapestensis DSM 16342 (EMA), X. szentirmaii DSM 16338 (EMC), and Photorhabdus luminescens ssp. laumondi (TT01) strains were used. After three days of exposure, the cells of EMA, EMC, and TT01 strains resulted in 100%, 88%, and 79.3% larval mortality rates, respectively. The applied EMA CFCM resulted in 53.7% larval mortality, indicating the presences of (at least) one extremely strong component produced by EMA. We concluded that the direct application of either the EPB cells or the CFCM must be a prospective alternative biocontrol of E. ceratoniae, especially to protect the important fruit (pomegranate, Punica granatum) cultivars. Especially, newly identified local EPB isolates could be applied as bio-pesticides for integrated management practices or organic pomegranate production.
1. Introduction
Pomegranate (Punica granatum) is one of the oldest known fruits, as it is found in the writings and artifacts of many cultures and religions. Pomegranate is one of the Kingdom of Saudi Arabia’s most in-demand fruits. There are several pomegranate varieties grown in Saudi Arabia, but the Taify pomegranate supply remains the most popular. Pomegranate cultivation and its potential yield and quality are affected by many insect pests [1,2]. The carob moth (Ectomyelois ceratoniae; Lepidoptera: Pyralidae) is a polyphagus destructive fruit pest worldwide that can cause major problems for pomegranates. Economic loss caused by carob moths in the pomegranate fields of Iran is 30%–80% [3], while the yearly damage rate caused by E. ceratoniae on pomegranate fruits in Tunisia varies by 29%–72% [4]. The larvae-infested fruits either drop or remain on the trees. As larval activity is hidden, commercial insecticides are not efficient against this pest [5]. Pesticides are considered one of the main causes of the observed loss of biodiversity in agro-ecosystems. When pomegranates are severely treated with pesticides, residues are mainly concentrated in the fruits [6]. Given that these fruits are freshly consumed, contamination with pesticides is undesirable.
To achieve this demand, non-chemical pest control strategies are required, which need to be efficient, environmentally sound, and guarantee similar production yields. Biological pest control, alone or as part of an integrated pest management strategy, provides an alternative to chemical pesticides. Natural products derived from living organisms show tremendous chemical diversity and provide great potential for finding new classes of compounds with novel modes of action. Bacteria are microorganisms characterized by their ability to produce secondary metabolites (SMs) or small-molecule natural products. Many of these SMs exhibit a variety of biological activities and are of interest to agrochemical, food, and pharmaceutical industries.
Entomopathogenic nematodes (EPNs) are extensively operated as biocontrol agents against insect pests. Their efficiency is highly dependent on the balance between parasitic activities and the immunological response of the host [7]. EPNs belonging to the Steinernematidae and Heterorhabditidae (Rhabditida) families have received the most attention because they possess many of the attributes of effective biological control agents against a wide spectrum of insect and nematode pests [8,9,10]. Steinernematids are symbiotically associated with entomopathogenic bacteria (EPB) from the genus Xenorhabdus, and heterorhabditid nematodes are symbiotically associated with EPB from the genus Photorhabdus. These symbiotic bacteria multiply and rapidly kill insects within a day or two. The bacteria then convert the insect into suitable food for the nematodes and generate some antibiotics [11] and anti-feedants that preserve the dead insect from putrefaction, while the nematodes feed and reproduce in it. Satisfactory results on the effect of EPNs in the laboratory and in field conditions have been reported for controlling cherry fruit flies [12,13] and pomegranate aphid, Aphis punicae [14]. Xenorhabdus szentirmaii is a unique source of highly efficient antimicrobial peptides against almost all known plant pathogens [15]. The geography of Taif as a high-altitude region in Saudi Arabia, which includes high mountains, agricultural plates, and valleys, is so remarkable that the EPB fauna is expected to be very rich. Therefore, this study aimed to examine the larvicidal activity of three EPB strains that had not been screened for the control of carob moth, E. ceratoniae, in the laboratory.
2. Materials and Methods
2.1. Bacterial Strains
In this study, three strains of symbiotic bacteria included in the bioassay were provided from the Fodor Laboratory at Pannonia University, in Keszthely, Hungary. The symbiotic bacteria, including two strains of Xenorhabdus, X. budapestensis DSM 16342 (EMA) and X. szentirmaii DSM 16338 (EMC), and Photorhabdus luminescens (TT01), which were isolated from the EPNs Steinernema bicornutum, Steinernema rarum, and Heterorhabditis bacteriophora, respectively, were used for determining oral toxicity against E. ceratoniae larvae. The bacteria were regularly cultivated on LBTA (Luria Bertani agar) indicator plates (10 g of peptone, 5 g of yeast extract, 5 g of sodium chloride, 15 g of agar, 25 mg of bromothymol blue, 40 mg of 2,3,5-triphenyltetrazolium chloride and 1 L of distilled water (pH 6.8)) in the dark at 25 °C (Figure 1A,B). In preparing the bacterial filtrate, as an inoculum for a 100-mL culture, a single dark blue/red Xenorhabdus or dark green Photorhabdus colony was placed separately into test tubes containing 5 mL of LB liquid media. Exactly 100-mL aliquots of culture were shaken overnight at room temperature in 500-mL Erlenmeyer flasks before being transferred to flasks containing 400 mL of the same media and shaken at 200 rpm for five days. To obtain the supernatant and bacterial pellet, the multiplied bacterial culture was centrifuged (13,000 rpm for 30 min) at 4 °C. A 0.22 m Millipore filter was used to filter the supernatant to get a cell-free filtrate, while the pellet was resuspended in sterile distilled water. The filtrate was stored at 4 °C for further diluting with sterile distilled water to provide concentrations of 600, 400, 200, 150, 75, and 25 μL/mL. Adjustment of the bacterial cell suspension at OD600 to 1.0 was performed using a spectrophotometer (SpectroStar Omega, BMC Labtech, Aylesbury, UK). A 10-fold serial dilution spread plate was used, and the bacterial suspension concentration was 108 (CFU/mL). Three dilutions of each bacterial cell suspension were adjusted to 108, 106, and 104 CFU/mL.
Figure 1.
EPB colonies of Xenorhabdus budapestensis (EMA, (A)), and Photorhabdus luminescens ssp. laumondii (TT01, (B)) on LBTA indicator plates.
2.2. Insects
Carob moth (E. ceratoniae) cultures were established from larvae obtained from commercial pomegranate orchards in Taif, Saudi Arabia, during the growth seasons of 2020. Insect larvae were reared on a wheat bran diet (300 g of wheat bran, 50 g of sugar, 120 mL of water, 130 mL of glycerol and 9 g of yeast), and adults were fed with a 10% honey–water solution. The rearing room was set at 25 ± 1 °C, 60% relative humidity and a 16 L:8 D light–dark cycle.
2.3. Bioassay for Larvicidal Activity
In the bioassays, late third and early fourth instar larvae were employed. Xenorhabdus and Photorhabdus suspensions were tested for toxicity against E. ceratoniae larvae. Each bioassay consisted of 25 larvae deposited in five wells of a 12-well plate (five larvae/well) provided with 5 g of pomegranate seeds as food for the larvae. Subsequently, 2 mL from each bacterial suspension or bacterial cell was distributed on the seeds in each well. Equal proportions of distilled water or sterile and filtered culture LB media were used as controls. The mortality rate of the larvae was observed after 12, 24, 48, and 72 h of exposure to bacterial suspensions or cells. On different dates, five replicates of each bioassay were examined. The experiment was repeated twice. When the larvae were teased with a fine sterile toothpick and no movement was detected, the larvae were pronounced dead. The larval mortality of E. ceratoniae after exposure to symbiotic bacteria was corrected following formula [16]:
where MT is mortality in treatment, and MC mortality in control.
The 50% lethal concentration (LC50) and the 50% lethal time (LT50) were determined by Probit analysis [17].
2.4. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
Chemical analysis of the bacterial filtrates was performed using GC-MS (GCMS QP2010 Ultra, Shimadzu, Kyoto, Japan). The GC column used was a Restek RT-2560 capillary column, which had 100% biscyanopropyl polysiloxane, length of 100 m, internal diameter of 0.25 mm ID, film thickness of 0.20 µm, fused silica and a highly polar phase, and was not bonded. The carrier gas was helium at a flow rate of 1 mL/min. The oven temperature was set to 130–145 °C, and the run time was 50 min. The purified samples were identified by comparing the mass spectra of the compounds with the library of the NIST 11 database (version 2.0, U.S. Department of Commerce, Gaithersburg, MD, USA).
2.5. Statistical Analysis
The corrected mortality percentage of the larvae was analysed using a two-way analysis of variance followed by Duncan’s multiple range test. All analyses were conducted using the Costat program. p-values less than 0.05 were considered significantly different. Data of the LC50 and LT50 were analysed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA). The data obtained were expressed as mean ± standard error.
3. Results
3.1. Larvicidal Activity of Bacterial Filtrates on E. ceratoniae
Table 1 shows that the larvae of E. ceratoniae were highly susceptible (p < 0.05) to both species of Xenorhabdus among all concentrations and exposure periods tested, with averaged corrected mortality percentages of 53.7% and 43.9% for EMA and EMC, respectively. TT01 bacteria were less effective, as they induced a larval mortality rate of only 26.7% (p < 0.05). The mortality rate (p < 0.05) of the positive control (pomegranate seeds treated with the media alone) was 0.43%. The percentage mortality was likewise observed to have a direct association with the bacterial supernatant concentration (p < 0.05). Thus, as the concentration of supernatant increased, the mortality percentage increased. The mortality of E. ceratoniae larvae was significantly high (p < 0.05) with value of 57.2% when a bacterial cell-free suspension was used at a concentration of 600 µL mL−1. Exposure time significantly affected the percentage of mortality (p < 0.05), as 38.9% larval mortality was recorded 72 h post-exposure. As shown in Table 1, EMA, EMC, and TT01 bacteria at concentrations of 25–600 µL mL−1 caused larval mortality (Means ± SE) ranging from 8 ± 4.9% to 100 ± 0%, 0 ± 0% to 91.3 ± 5.3%, and 0 ± 0% to 78.3 ± 0%, respectively (p < 0.05). No significant difference was found between the two Xenorhabdus species in larval mortality after a 72-h exposure (p < 0.05), indicating a closer mean mortality of 100 ± 0% for EMA and 91.3 ± 5.3% for EMC. We reported high larval mortality rates for high concentrations of EMA and EMC bacteria for all counting days, as well as considerable mortalities due to TT01. Although EMA at a low concentration (25 µL mL−1) showed remarkable larval mortality for all revised days, no significant difference was found in the mortalities due to EMC, TT01, and control (absence of natural products derived from bacteria) treatments, specifically at a 12-h exposure (0 ± 0%).

Table 1.
Larvicidal activity of three bacterial species filtrates on the carob moth, Ectomyelois ceratoniae under laboratory condition.
3.2. Larvicidal Activity of Bacterial Cells on E. ceratoniae
As shown in Table 2, the same trend was recorded regarding the effect of bacterial cells on the mortality of E. ceratoniae larvae. The data also indicated that larval mortality was strongly dependent on the bacterium species, concentration of bacterium cells, and exposure duration (p < 0.05). According to the results, EMA surpassed EMC and TT01 in inducing mortality in E. ceratoniae among all concentrations and exposure periods tested. EMA induced larval mortality of 81.6% compared with the 67.4% and 42.8% induced by EMC and TT01, respectively. EMA bacterial cells at a higher concentration (108) were more effective than EMC, as they had the ability to induce 84 ± 4%, 88 ± 4.9%, 100 ± 0%, and 100 ± 0% mortalities of the late third instar larvae of E. ceratoniae at 12, 24, 48, and 72 h of application, respectively, compared with the 72 ± 4.9%, 80 ± 0%, 83.7 ± 4.1%, and 88 ± 4.9% mortalities induced by EMC at 12, 24, 48, and 72 h of application, respectively. The results showed that all bacterial species cells at a low concentration (104), except for TT01, induced considerable larval mortality rates for all exposition periods ranging from 56 ± 4% to 87.7 ± 5.1%, 48 ± 4.9% to 71.3 ± 4.6%, and 8 ± 4.9% to 42.3 ± 3.7% for EMA, EMC, and TT01, respectively (Table 2).

Table 2.
Larvicidal activity of three bacterial species cells on the carob moth, Ectomyelois ceratoniae under laboratory condition.
3.3. Comparison of the Effect of the Bacterial Filtrate and Bacterial Cells on the Larval Mortality of E. ceratoniae
Figure 2 shows that the cell-free supernatant and the cells of all tested bacteria had a highly significant effect on the mortality of E. ceratoniae larvae at 72 h of exposure (p < 0.0001). The application of bacterial cells induced a more significant (F = 176.5; p = 0.0001; df = 24) larval mortality of E. ceratoniae, with a mean mortality rate of 77.6%. Conversely, the bacterial cell-free supernatant had a lower virulence, with a mortality rate of 51.7%. Moreover, the results indicated that the mean percentage of larval mortality caused by EMA (77.4%) was significantly (F = 69.03; p = 0.0001; df = 24) higher than that caused by EMC (66.9%) and TT01 (49.6%). The interactive effect of the bacterium species and the application method on larval mortality was not significant (F = 2.439; p = 0.1086; df = 24).
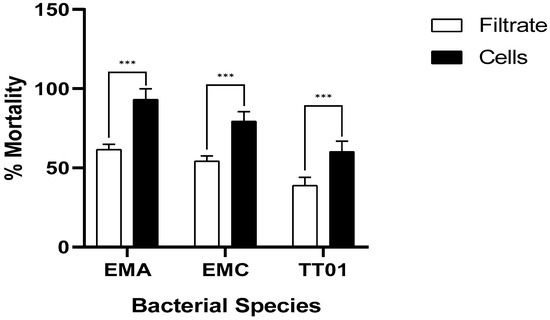
Figure 2.
Insecticidal efficacy of bacterial filtrates in comparison with cells on mortality of E. ceratoniae. Asterisks above bars indicate statistical differences (p < 0.0001, based on Duncan test).
3.4. Lethal Concentration and Time of the Bacterial Filtrate and Cells in the Larval Mortality of E. ceratoniae
As shown in Table 3, the calculated LC50 values in the carob moth larvae under laboratory conditions were 125.9 µL/mL cell-free supernatant and 2.14 × 102 CFU/mL, 204.2 µL/mL and 1.78 × 103 CFU/mL, and 575.4 µL/mL and 8.13 × 106 CFU/mL for the EMA, EMC, and TT01 bacteria, respectively. Moreover, the lethal time of larval mortality was lower in the bacterial cell application than in the bacterial filtrate application. The EMA and EMC cells were more virulent against E. ceratoniae larvae, as they recorded lower LT50 values of 5.25 h and 7.36 h, respectively. The TT01 cells had an LT50 of 44.57 h. The lethal times of the bacterial filtrates were 19.95, 52.48, and 208.93 h for EMA, EMC, and TT01, respectively.

Table 3.
Lethal concentration and lethal time of bacterial species against third instar larvae of E. ceratoniae.
3.5. GC-MS Analysis of the Bacterial Cell-Free Supernatant
Table 4, Table 5 and Table 6 show the chemical compositions of three different samples of bacterial filtrates. The samples were run using GC-MS, which identified different substances in one test sample, and the components of the mixtures were separated and analysed. GC-MS has the capacity to resolve sample extracts containing hundreds of compounds. During the analysis process, samples are ionized and fragmented by the mass spectrometer, and the ions are separated based on their different mass-to-charge (m/z) ratios. GC-MS data acquisition can be performed to cover a wide range of m/z ratios. Using extensive commercially available libraries of mass spectra, unknown compounds and target analytes can then be identified and quantified accurately. Table 4, Table 5 and Table 6 show the retention time, chemical name, and chemical formula of three bacterial filtrate samples. Similarities were found among the three samples. For example, compounds 1, 2, and 3 were the same in the three bacterial species. Moreover, the compounds retained at 12.89–12.92 min and the samples retained at 20.04–20.06 min and at 41.83–41.85 min were the same in the three bacterial filtrates. Some similarities appeared in only two species: compounds 9 and 10 in EMC were the same as compounds 9 and 10 in EMA, compound 14 in EMC was the same as compound 15 in EMA, and compound 20 in EMA was the same as compound 21 in TT01. Some compounds were identified in only one species, such as compound 11 in EMC. Thus, by comparing the retention time and the chemical name, we found that the three bacterial species were similar, especially the EMA and EMC samples, as shown in the results in Table 4 and Table 5.

Table 4.
The chemical composition of Xenorhabdus szentirmaii DSM 16338 (EMC).

Table 5.
The chemical composition Xenorhabdus budapestensis DSM 16342 (EMA).

Table 6.
The chemical composition of Photorhabdus luminescens (TT01).
4. Discussion
In this study, we demonstrated the toxicity of the cell-free supernatant or the cells of three bacterial species in the late third and early fourth instar larvae of the carob moth E. ceratoniae under laboratory conditions. The results revealed that all the bacterial species tested successfully induced mortality in E. ceratoniae larvae. The mortality % was directly related to the exposure period and bacterial concentration if they were applied as the cell-free supernatant or cells. Moreover, the larvae of E. ceratoniae were more susceptible to bacterial cell infestation than the bacterial cell-free supernatant. These results were consistent with those of Yooyangket et al. [18], who supposed that symbiotic bacteria become virulent when ingested by mosquito larvae. The findings also revealed that the two species of Xenorhabdus were more effective than the Photorhabdus species against E. ceratoniae larvae. This higher lethality of Xenorhabdus than Photorhabdus correlated with the better efficacy of Steinernema carpocapsae and Steinernema feltiae compared with H. bacteriophora, which showed low virulence on carob moth larvae in laboratory conditions [19]. Conversely, Elbrense et al. [20] found that Photorhabdus sp. was more effective against Pieris rapae and Pentodon algerinus larvae than Xenorhabdus sp. Salvadori et al. [21] reported that the Photorhabdus species killed 75–96% of Spodoptera frugiperda larvae, while Xenorhabdus bacteria were less active, causing 33%–57% mortality.
Our results revealed that the maximum larval mortality was 100% after 72 h of exposure to 600 µL/mL EMA cell-free supernatant and 108 CFU/mL after 48 and 72 h. Similarly, after three days of exposure, we found a larval mortality rate of over 65% for EMC cell application, but the larval mortality rate did not reach 50% when the cell-free supernatant was applied. These results were consistent with those of Fukruksa et al. [22], who reported that the cell suspensions of Xenorhabdus ehlersii isolated from Steinernema scarabaei were found to be efficient at killing 100% of Ae. aegypti. Conversely, E. ceratoniae larvae had a low mortality rate (8 ± 4.9%) after exposure to P. luminescens (TT01) for 12 h at a concentration of 75 µL/mL cell-free supernatant or 104 CFU/mL. This could be attributed to its failure to produce toxic metabolites to E. ceratoniae. Various symbiotic bacteria strains can produce a variety of secondary metabolites, resulting in varying levels of efficacy in eliminating target organisms. Furthermore, the test of insect mortality rate may be related to the variable number of bacterial cells ingested by each larva. The fact that both Xenorhabdus sp. and Photorhabdus sp. bacteria produce toxin complexes, proteases, lipases, lipopolysaccharides, and other active components may explain their insecticidal activity [23]. These compounds cause caterpillars to become floppy [24], induce apoptosis, inhibit haemocyte motility, and suppress cellular and humoral immunity [25]. Xenorhabdus and Photorhabdus species devote a large portion of their resources to the production of specialized metabolites generated from non-ribosomal peptide synthetase (NRPS) or polyketide synthase (PKS). Both bacteria form a symbiotic relationship with nematodes, followed by an insect pathogenic phase [26]. At the DNA sequence level, both bacteria appear to be almost identical, indicating a close evolutionary relationship. However, high-resolution mass spectrometry investigations show that the two taxa contain a wide range of chemical compounds. The xefoampeptides [26,27] and tilivalline [28] were among the previously unknown metabolite groups discovered through molecular network reconstruction.
At least eight bacterial metabolites are produced by Xenorhabdus nematophila that play central functions in destroying and inhibiting the immune reactions of the targeted insects [29]. Toxin complexes (Tcs), make caterpillars floppy (Mcf), Photorhabdus insect-related proteins, and Photorhabdus virulence cassettes have been reported to be produced by Photorhabdus species [30]. The Tcs damage the insect’s midgut epithelial cells, but the Mcf is active immediately after injection and disturbs the insect’s haemocytes by causing apoptosis [31]. The GC-MS analysis of our EPBs revealed that the Xenorhabdus bacteria possessed 21 main components, while the Photorhabdus bacteria had 23 main components. Six of these compounds (3.beta,17.beta.-dihydroxyestr-4-ene, 1-methylcyclopropanemethanol heptanal, 2-methylpiperazine, 2-methyl, oxirane tetramethyl, nonanal, 2-decenal (Z), and 1-formyl-3-methylaziridine-2- carbonitrile) were commonly found in the three bacterial species. Moreover, the levels in the two species of Xenorhabdus were higher than those in the Photorhabdus species. Similarly, Hasan et al. [32] attributed the virulence of six X. nematophila strains against Spodoptera exigua to active secondary substances, such as benzeneacetic acid, n-decanoic acid, tetradecane, 1-decene, and 3-benzylidene-hexahydro-pyrrolo, which suppress the immune system of insects. Mollah and Kim [33] also detected fatty alcohol, 1-ecosine, heptadecane, octadecanes, and methyl-12-tetradecen-1-ol acetate in different strains of Xenorhabdus and Photorhabdus bacteria. The authors suggested that these compounds inhibited insects’ phospholipase A2, thereby eradicating their immune system.
5. Conclusions
As E. ceratoniae is the most destructive pest in Saudi Arabia, there is an urgent need to provide tools to reduce its propagation, particularly in organic production, where the use of pesticides is strictly limited. To the best of our knowledge, no previous studies have related the direct application of EMA, EMC, and TT01 bacteria to control the same pest. On the other hand, for the first time, we verified the insecticidal activity of two Xenorhabdus and Photorhabdus bacterial cells or cell-free supernatants against E. ceratoniae larvae when ingested orally. Nevertheless, additional studies are required to evaluate the effect of EPB application on E. ceratoniae larvae in the field for a sustainable agro-environment.
Author Contributions
Conceptualization, S.S.A. and A.N.; data curation, H.D., S.A. and A.N.; formal analysis, H.D., A.N., A.M.F. and A.A.-B.; investigation, S.S.A., A.A., A.N., A.F., B.A. and A.B.; methodology, H.D., S.A., A.N., A.M.F. and A.A.-B.; project administration, S.S.A.; resources, S.S.A., H.D., S.A., A.A., A.N. and A.B.; validation, H.D.; visualization, A.F.; writing—original draft, S.S.A. and A.N.; writing—review & editing, S.S.A., S.A., A.A., A.F. and B.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number 1-441-117.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Day, K.R.; Wilkins, E.D. Commercial Pomegranate (Punica granatum L.) Production in California. Acta Hort. 2011, 890, 275–286. [Google Scholar] [CrossRef]
- Braham, M. Insect Larvae Associated with Dropped Pomegranate Fruits in an Organic Orchard in Tunisia. J. Entomol. Nematol. 2015, 7, 5–10. [Google Scholar]
- Hoseini, S.A.; Goldansaz, S.H.; Sadeghhasani, S.; Mousavi, S.G. A Field Screening of 10 High Yield Pomegranate Cultivars for Resistance to the Carob Moth, Ectomyelois ceratoniae, in the Climate Condition of Karaj, Alborz, Iran. In Proceedings of the 21st Iranian Plant Protection Congress, Urmia, Iran, 23–26 August 2014. [Google Scholar]
- Dhouibi, M.H. Lutte Intégrée Pour la Protection du Palmier Dattier en Tunisie; Centre de Publication Universitaire: Tunis, Tunisia, 2000. [Google Scholar]
- Shakeri, M. Carob Moth and Its Control Methods; Yazd Utilization Publishing: Yazd, Iran, 2005. [Google Scholar]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A Critical Review of Plant Protection Tools for Reducing Pesticide Use on Grapevine and New Perspectives for the Implementation of IPM in Viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Brivio, M.F.; Mastore, M. Nematobacterial Complexes and Insect Hosts: Different Weapons for the Same War. Insects 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grewal, P.S.; Ehlers, R.U.; Shapiro-Ilan, D.I. Nematodes as Biological Control Agents. CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Koppenhofer, A.M. Nematodes. In Field Manual of Techniques in Invertebrate Pathology: Application and Evaluation of Pathogens for Control of Insects and Other Invertebrate Pests, 3rd ed.; Lacey, L.A., Kaya, H.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 249–264. [Google Scholar]
- Nour El-Deen, A.H.; Al-Barty, A.F.; Darwesh, H.Y.; Al-Ghamdi, A.S. Eco-Friendly Management of Root-Knot Nematode, Meloidogyne incognita Infecting Pomegranate at Taif Governorate, KSA. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1070–1076. [Google Scholar]
- Akhurst, R.; Bedding, R.A. Natural Occurrence of Insect Pathogenic Nematodes (Steinernematidae and Heterorhabditidae) in Soil in Australia. J. Austr. Entomol. Soc. 1986, 25, 241–244. [Google Scholar] [CrossRef]
- Koppler, K.; Peters, A.; Vogt, H. Initial Results in the Application of Entomopathogenic Nematodes against the European Cherry Fruit Fly Rhagoletis cerasi L. (Diptera: Tephritidae). IOBC/WPRS Bull. 2003, 23, 13–18. [Google Scholar]
- Herz, A.; Koppler, K.; Vogt, H.; Elias, E.; Katz, P.; Peters, A. Biological Control of the Cherry Fruit Fly, Rhagoletis cerasi L. (Diptera, Tephritidae) by Use of Entomopathogenic Nematodes: First Experiences Towards Practical Implementation. In Proceedings of the 12th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit Growing (Eco-fruit), Weinsberg, Germany, 18–20 February 2006; Boos, M., Ed.; Foerdergemeinschaft Oekologischer Obstbau: Weinsberg, Germany; Kernen, Germany; pp. 67–72. [Google Scholar]
- Alghamdi, A.S.; Nour El-Deen, A.H.; Al-Barty, A.F.; Hassan, M.M. Toxicity of Some Plant Extracts and Entomopathogenic Nematode against Pomegranate Aphid, Aphis punicae under Laboratory Condition. Annu. Res. Rev. Biol. 2017, 21, 1–6. [Google Scholar] [CrossRef]
- Fuchs, S.W.; Grundmann, F.; Kurz, M.; Kaiser, M.; Bode, H.B. Fabclavines: Bioactive Peptide–Polyketide-Polyamino Hybrids from Xenorhabdus. Chembiochem 2014, 15, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A Method of Computing the Effectiveness Insecticides. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; University Press: Cambridge, UK, 1971. [Google Scholar]
- Yooyangket, T.; Muangpat, P.; Polseela, R.; Tandhavanant, S.; Thanwisai, A.; Vitta, A. Identification of Entomopathogenic Nematodes and Symbiotic Bacteria from Nam Nao National Park in Thailand and Larvicidal Activity of Symbiotic Bacteria against Aedes aegypti and Aedes albopictus. PLoS ONE 2018, 13, e0195681. [Google Scholar] [CrossRef]
- Memari, Z.; Javad, K.; Shokoofeh, K.; Seyed, H.G.; Mojtaba, H. Are Entomopathogenic Nematodes Effective Biological Control Agents against the Carob Moth, Ectomyelois ceratoniae? J. Nematol. 2016, 48, 261–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbrense, H.; Elmasry, A.M.; Seleiman, M.F.; AL-Harbi, M.S.; Abd El-Raheem, A.M. Can Symbiotic Bacteria (Xenorhabdus and Photorhabdus) Be More Efficient than Their Entomopathogenic Nematodes against Pieris rapae and Pentodon algerinus Larvae? Biology 2021, 10, 999. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, J.D.; Defferrari, M.S.; Ligabue-Braun, R.; Lau, E.Y.; Salvadori, J.R.; Carlini, C.R. Characterization of Entomopathogenic Nematodes and Symbiotic Bacteria Active Against Spodoptera frugiperda (Lepidoptera: Noctuidae) and Contribution of Bacterial Urease to the Insecticidal Effect. Biol. Control 2012, 63, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Fukruksa, C.; Yimthin, T.; Suwannaroj, M.; Muangpat, P.; Tandhavanant, S.; Thanwisai, A.; Vitta, A. Isolation and Identification of Xenorhabdus and Photorhabdus Bacteria Associated with Entomopathogenic Nematodes and Their Larvicidal Activity against Aedes aegypti. Parasit Vectors 2017, 10, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillman, A.R.; Guillermin, M.L.; Lee, J.H.; Kim, B.; Sternberg, P.W.; Hallem, E.A. Olfaction Shapes Host-Parasite Interactions in Parasitic Nematodes. Proc. Natl. Acad. Sci. USA 2012, 109, E2324–E2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daborn, P.J.; Waterfield, N.; Silva, C.P.; Au, C.P.Y.; Sharma, S.; Ffrench-Constant, R.H. A Single Photorhabdus Gene, Makes Caterpillars Floppy (mcf), Allows Escherichia coli to Persist within and Kill Insects. Proc. Natl. Acad. Sci. USA 2002, 99, 10742–10747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowling, A.J.; Waterfield, N.R.; Hares, M.C.; Le Goff, G.; Streuli, C.H.; Ffrench-Constant, R.H. The Mcf1 Toxin Induces Apoptosis via the Mitochondrial Pathway and Apoptosis is Attenuated by Mutation of the BH3-like Domain. Cell. Microbiol. 2007, 9, 2470–2484. [Google Scholar] [CrossRef] [PubMed]
- Tobias, N.J.; Wolff, H.; Djahanschiri, B.; Grundmann, F.; Kronenwerth, M.; Shi, Y.M.; Simonyi, S.; Grün, P.; Shapiro-Ilan, D.; Pidot, S.J.; et al. Natural Product Diversity Associated with the Nematode Symbionts Photorhabdus and Xenorhabdus. Nat Microbiol. 2017, 2, 1676–1685. [Google Scholar] [CrossRef]
- Kegler, C.; Bode, H.B. Artificial Splitting of a Non-Ribosomal Peptide Synthetase by Inserting Natural Docking Domains. Angew. Chem. 2020, 59, 13463–13467. [Google Scholar] [CrossRef]
- Wolff, H.; Bode, H.B. The Benzodiazepine-like Natural Product Tilivalline is Produced by the Entomopathogenic Bacterium Xenorhabdus eapokensis. PLoS ONE 2018, 13, e0194297. [Google Scholar]
- Eom, S.; Park, Y.; Kim, Y. Sequential Immunosuppressive Activities of Bacterial Secondary Metabolites From the Entomopahogenic Bacterium Xenorhabdus nematophila. J. Microbiol. 2014, 529, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Rodou, A.; Ankrah, D.O.; Stathopoulos, C. Toxin and Secretion Systems of Photorhabdus luminescens. Toxins 2010, 2, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, W.; Zouari, N.; Jaoua, S. Involvement of Oxidative Stress and Growth at High Cell Density in the Viable but Nonculturable State of Photorhabdus temperata ssp. temperata Strain K122. Process Biochem. 2010, 45, 706–713. [Google Scholar] [CrossRef]
- Hasana, A.; Ahmeda, S.; Mollaha, M.M.; Leeb, D.; Kima, Y. Variation in Pathogenicity of Different Strains of Xenorhabdus nematophila: Differential Immunosuppressive Activities and Secondary Metabolite Production. J. Invertebr. Pathol. 2019, 166, 107221. [Google Scholar] [CrossRef] [PubMed]
- Mollah, M.I.; Kim, Y. Virulent Secondary Metabolites of Entomopathogenic Bacteria Genera, Xenorhabdus and Photorhabdus, Inhibit Phospholipase A2 to Suppress Host Insect Immunity. BMC Microbiol. 2020, 20, 359. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).