Effects of Inorganic, Organic and Bio-Organic Fertilizer on Growth, Rhizosphere Soil Microflora and Soil Function Sustainability in Chrysanthemum Monoculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Plant Material
2.2. Preparation of IPN, OF and BOF
2.3. Experimental Design
2.4. Chrysanthemum Growth Study and Disease Incidence
2.5. Soil Sampling of Chrysanthemum Monoculture
2.6. DNA Extraction and PCR Amplification
2.7. Illumina HiSeq Sequencing and Data Analysis
2.8. Statistical Analyses and Data Accessibility
3. Results
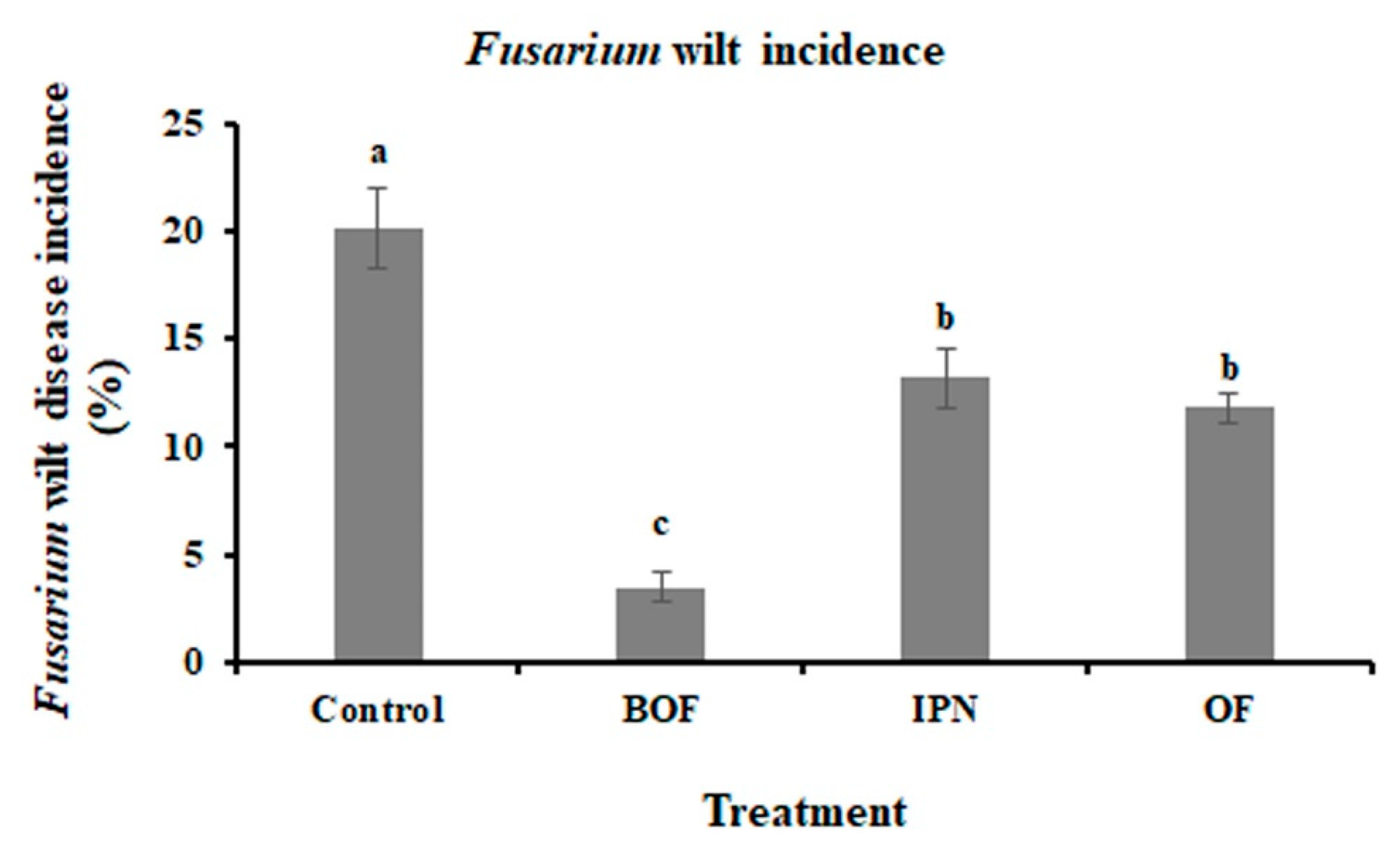
3.1. Disease Incidence and Growth Indexes of Chrysanthemum
3.2. α-Diversity of Soil Microbiomes
3.3. β-Diversity of Soil Microbiomes
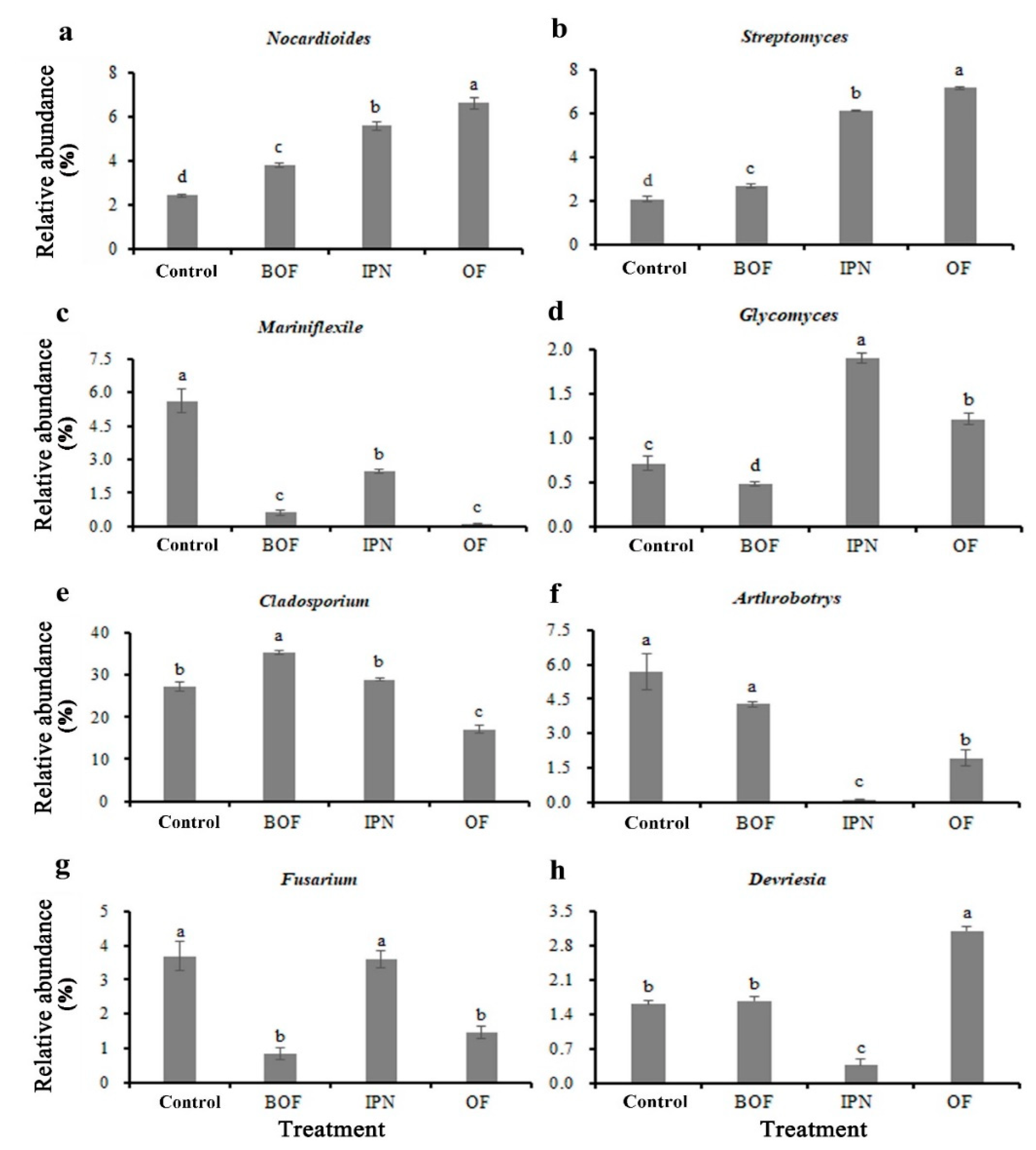
3.4. Taxonomic Composition of Soil Microbiome
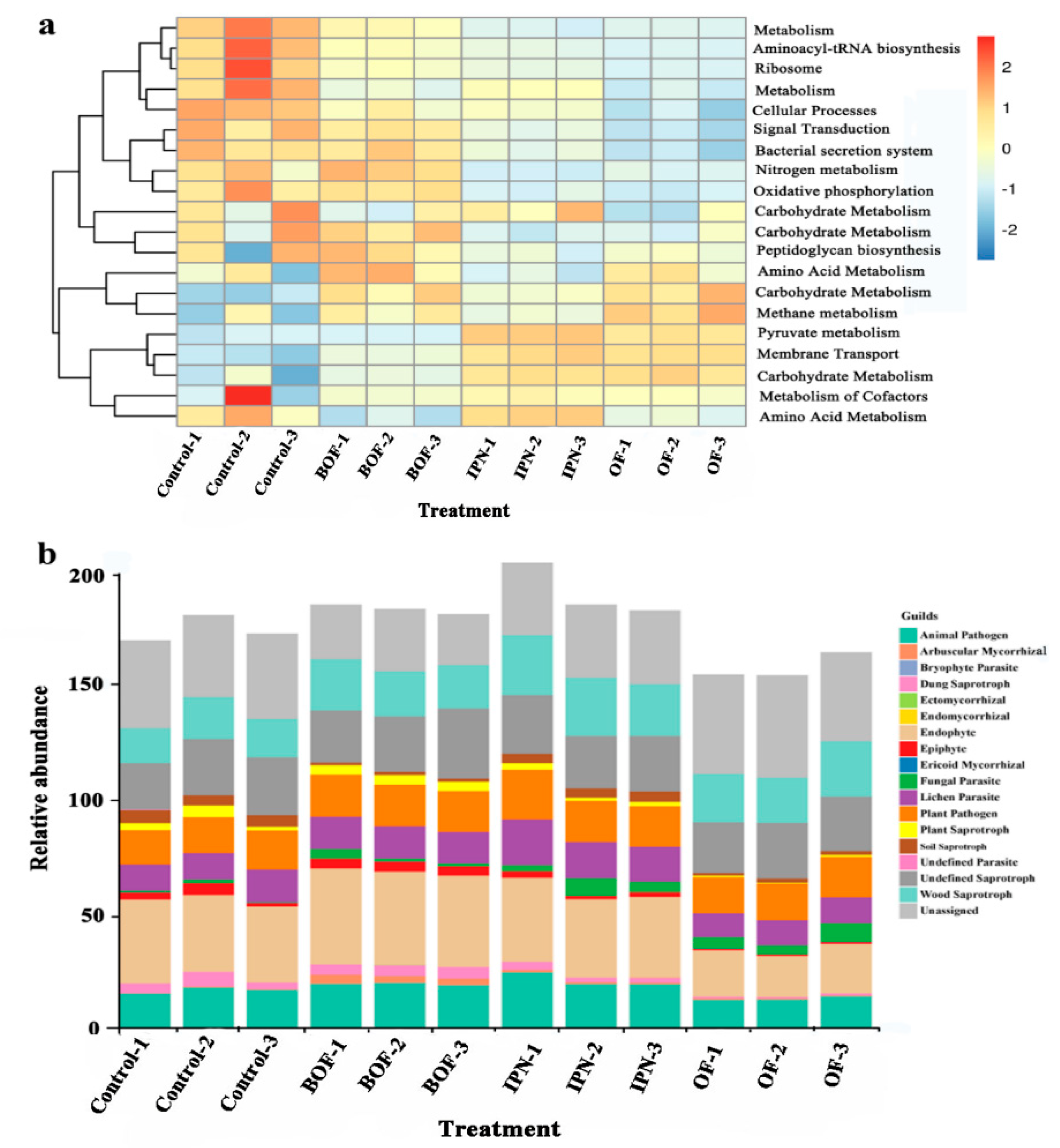
3.5. Functional Predictions of Microbial Community Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasaki, K.; Mitsuda, N.; Nashima, K.; Kishimoto, K.; Katayose, Y.; Kanamori, H.; Ohmiya, A. Generation of expressed sequence tags for discovery of genes responsible for floral traits of Chrysanthemum morifolium by next-generation sequencing technology. BMC Genom. 2017, 18, 683. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Li, X.; Ma, Z.; Hu, S.; Tu, C.; Xing, S. Bio-organic Fertilizer Promotes Plant Growth and Yield and Improves Soil Microbial Community in Continuous Monoculture System of Chrysanthemum morifolium cv. Chuju. Int. J. Agric. Biol. 2017, 19, 563–568. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, X.; Deng, S.; Dong, X.; Song, A.; Yao, J.; Fang, W.; Chen, F. The Effects of Fungicide, Soil Fumigant, Bio-Organic Fertilizer and Their Combined Application on Chrysanthemum Fusarium Wilt Controlling, Soil Enzyme Activities and Microbial Properties. Molecules 2016, 21, 526. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, S.; Zhao, J.; Zhang, K.; Jiang, J.; Guan, Z.; Chen, S.; Chen, F.; Fang, W. Deep tillage combined with biofertilizer following soil fumigation improved chrysanthemum growth by regulating the soil microbiome. Microbiologyopen 2020, 9, e1045. [Google Scholar] [CrossRef]
- Adediran, J.A.; Taiwo, L.B.; Akande, M.O.; Sobulo, R.A.; Idowu, O.J. Application of Organic and Inorganic Fertilizer for Sustainable Maize and Cowpea Yields in Nigeria. J. Plant Nutr. 2005, 27, 1163–1181. [Google Scholar] [CrossRef]
- Haling, R.E.; Simpson, R.J.; Delhaize, E.; Hocking, P.J.; Richardson, A.E. Effect of lime on root growth, morphology and the rhizosheath of cereal seedlings growing in an acid soil. Plant Soil 2010, 327, 199–212. [Google Scholar] [CrossRef]
- Samy, T.N.; Mishra, M.; Sahu, R.K.; Padhy, R.N. Growth, yield and metabolism of rice (Oryza sativa L.) during repeated applications of fly-ash on soil. Adv. Food Sci. 2010, 32, 110–117. [Google Scholar]
- Tulin, A. Improvement of the growth, yield, and tuber quality of purple yam through macro and micronutrient fertilization. Ann. Trop. Res. 2009, 31, 95–119. [Google Scholar] [CrossRef]
- Tan, Q.; Cheng, Y.; Zhao, X.; Liu, E. Different dosages of silicon calcium potassium magnesium fertilizer affect soil nutrients and tobacco quality. Chin. Agric. Sci. Bull. 2019, 35, 25–29. [Google Scholar]
- Muktamar, Z.; Putri, D.; Setyowati, N. Reduction of Synthetic Fertilizer for Sustainable Agriculture: Influence of Organic and Nitrogen Fertilizer Combination on Growth and Yield of Green Mustard. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 361–364. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Valadez-Blanco, R.; Salas-Coronado, R.; Sustaita-Rivera, F.; Hernández-Carlos, B.; García-Ortega, S.; Santos-Sánchez, N.F. Effect of nitrogen fertilization and Bacillus licheniformis biofertilizer addition on the antioxidants compounds and antioxidant activity of greenhouse cultivated tomato fruits (Solanum lycopersicum L. var. Sheva). Sci. Hortic. 2016, 201, 338–345. [Google Scholar] [CrossRef]
- Huang, N.; Wang, W.; Yao, Y.; Zhu, F.; Wang, W.; Chang, X. The influence of different concentrations of bio-organic fertilizer on cucumber Fusarium wilt and soil microflora alterations. PLoS ONE 2017, 12, e0171490. [Google Scholar] [CrossRef]
- Bhowmik, A.; Fortuna, A.-M.; Cihacek, L.J.; Bary, A.I.; Carr, P.M.; Cogger, C.G. Potential carbon sequestration and nitrogen cycling in long-term organic management systems. Renew. Agric. Food Syst. 2017, 32, 498–510. [Google Scholar] [CrossRef]
- Zulueta-Rodriguez, R.; Cordoba-Matson, M.V.; Hernandez-Montiel, L.G.; Murillo-Amador, B.; Rueda-Puente, E.; Lara, L. Effect of Pseudomonas putidaon Growth and Anthocyanin Pigment in Two Poinsettia (Euphorbia pulcherrima) Cultivars. Sci. World J. 2014, 2014, 810192. [Google Scholar] [CrossRef]
- Akkopru, A.; Demir, S. Biological Control of Fusarium Wilt in Tomato Caused by Fusarium oxysporum f. sp. lycopersici by AMF Glomus intraradices and some Rhizobacteria. J. Phytopathol. 2005, 153, 544–550. [Google Scholar] [CrossRef]
- Manikandan, R.; Saravanakumar, D.; Rajendran, L.; Raguchander, T.; Samiyappan, R. Standardization of liquid formulation of Pseudomonas fluorescens Pf1 for its efficacy against Fusarium wilt of tomato. Biol. Control. 2010, 54, 83–89. [Google Scholar] [CrossRef]
- Maung, C.E.H.; Choi, T.G.; Nam, H.H.; Kim, K.Y. Role of Bacillus amyloliquefaciens Y1 in the control of Fusarium wilt disease and growth promotion of tomato. Biocontrol Sci. Technol. 2017, 27, 1400–1415. [Google Scholar] [CrossRef]
- Shi, L.; Du, N.; Shu, S.; Sun, J.; Li, S.; Guo, S. Paenibacillus polymyxa NSY50 suppresses Fusarium wilt in cucumbers by regulating the rhizospheric microbial community. Sci. Rep. 2017, 7, srep41234. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Xu, X.; Hu, J.; Sun, X.; Xiao, Y.; Zhang, Y. Trichoderma improves the growth of Leymus chinensis. Biol. Fertil. Soils 2018, 54, 685–696. [Google Scholar] [CrossRef]
- Chaudhary, I.J.; Singh, R.P. Studies on Growth, Mobilization of nutrients and yield of wheat (Triticum aestivum L. PBW-343) applied with organic matrix based slow release bio fertilizers. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3221–3238. [Google Scholar]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Factories 2014, 13, 66. [Google Scholar] [CrossRef]
- Zhen, L.; Gu, J.; Hu, T.; Chen, Z. Effects of compost containing oxytetracycline on enzyme activities and microbial communities in maize rhizosphere soil. Environ. Sci. Pollut. Res. 2018, 25, 29459–29467. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes Vertical Distribution of Soil Bacterial and Fungal Communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Xiong, W.; Guo, S.; Jousset, A.; Zhao, Q.; Wu, H.; Li, R.; Kowalchuk, G.A.; Shen, Q. Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol. Biochem. 2017, 114, 238–247. [Google Scholar] [CrossRef]
- Legay, N.; Lavorel, S.; Personeni, E.; Bataillé, M.P.; Robson, T.M.; Clément, J.C. Temporal variation in the nitrogen uptake competition between plant community and soil microbial community. In Proceedings of the EGU General Assembly Conference, Vienna, Austria, 22–27 April 2012. [Google Scholar]
- Wu, L.H.; Zhao, J.; Hui, M.; Shao, Y.Q. The Quantitative Characters of Soil Microbes under Different Vegetations in an Eutrophic Lake Wetland. Appl. Mech. Mater. 2013, 295–298, 178–183. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, D.; Sharma, P. Effect of organics, biofertilizers and crop residue application on soil microbial activity in rice—Wheat and rice-wheat mungbean cropping systems in the Indo-Gangetic plains. Cogent Geosci. 2015, 1, 1085296. [Google Scholar] [CrossRef]
- Lang, J.; Hu, J.; Ran, W.; Xu, Y.; Shen, Q. Control of cotton Verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biol. Fertil. Soils 2012, 48, 191–203. [Google Scholar] [CrossRef]
- Fu, L.; Penton, C.R.; Ruan, Y.; Shen, Z.; Xue, C.; Li, R.; Shen, Q. Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol. Biochem. 2017, 104, 39–48. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Che, Y.; Deng, S.; Xiao, Y. Effects of different fertilizers on growth and nutrient uptake of Lolium multiflorum grown in Cd-contaminated soils. Environ. Sci. Pollut. Res. 2017, 24, 23363–23370. [Google Scholar] [CrossRef]
- Jelin, J.; Dhanarajan, M.S.; Mariappan, V. Assessment of compost as a bio-fertilizer for the growth of paddy. J. Environ. Biol. 2013, 34, 975. [Google Scholar] [PubMed]
- Zhang, F.; Huo, Y.; Cobb, A.B.; Luo, G.; Zhou, J.; Yang, G.; Wilson, G.W.T.; Zhang, Y. Trichoderma Biofertilizer Links to Altered Soil Chemistry, Altered Microbial Communities, and Improved Grassland Biomass. Front. Microbiol. 2018, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Bartelt-Ryser, J.; Joshi, J.; Schmid, B.; Brandl, H.; Balser, T. Soil feedbacks of plant diversity on soil microbial communities and subsequent plant growth. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 27–49. [Google Scholar] [CrossRef]
- Garbeva, P.; Postma, J.; van Veen, J.A.; van Elsas, J.D. Effect of above-ground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ. Microbiol. 2006, 8, 233–246. [Google Scholar] [CrossRef]
- Soni, R.; Rawal, K.; Keharia, H. Genomics assisted functional characterization of Paenibacillus polymyxa HK4 as a biocontrol and plant growth promoting bacterium. Microbiol. Res. 2021, 248, 126734. [Google Scholar] [CrossRef]
- Li, B.; Wu, S.; Zhou, S.; Wang, T.; Chen, H. Quantifying and mapping threats to soil biodiversity in Nanjing, China. Eur. J. Soil Biol. 2017, 82, 72–80. [Google Scholar] [CrossRef]
- Schmidt, J.; Bowles, T.; Gaudin, A.C.M. Using Ancient Traits to Convert Soil Health into Crop Yield: Impact of Selection on Maize Root and Rhizosphere Function. Front. Plant Sci. 2016, 7, 373. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Osanai, Y.; Lai, K.; Anderson, I.C.; Bange, M.; Tissue, D.; Singh, B.K. Responses of the soil microbial community to nitrogen fertilizer regimes and historical exposure to extreme weather events: Flooding or prolonged-drought. Soil Biol. Biochem. 2018, 118, 227–236. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.; Liang, H.; Huang, J.; Chen, Z.; Nie, Y.; Wang, C.; Wang, Y. Manipulation of the rhizosphere microbial community through application of a new bio-organic fertilizer improves watermelon quality and health. PLoS ONE 2018, 13, e0192967. [Google Scholar] [CrossRef]
- Hu, Y.; Pang, S.; Yang, J.; Zhao, X.; Cao, J. Changes in soil microbial community structure following amendment of biosolids for seven years. Environ. Pollut. Bioavailab. 2019, 31, 24–31. [Google Scholar] [CrossRef]
- Yu, Z.; Hu, X.; Wei, D.; Liu, J.; Zhou, B.; Jin, J.; Liu, X.; Wang, G. Long-term inorganic fertilizer use influences bacterial communities in Mollisols of Northeast China based on high-throughput sequencing and network analyses. Arch. Agron. Soil Sci. 2019, 65, 1331–1340. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Chao, X.; Zhang, J.; Li, R.; Shen, Q. Rhizosphere microbial community manipulated by 2 years of consecutive biofertilizer application associated with banana Fusarium wilt disease suppression. Biol. Fertil. Soils 2015, 51, 553–562. [Google Scholar] [CrossRef]
- Battini, F.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiol. Res. 2016, 183, 68–79. [Google Scholar] [CrossRef]
- Qin, S.; Feng, W.-W.; Wang, T.-T.; Ding, P.; Xing, K.; Jiang, J.-H. Plant growth-promoting effect and genomic analysis of the beneficial endophyte Streptomyces sp. KLBMP 5084 isolated from halophyte Limonium sinense. Plant Soil 2017, 416, 117–132. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, S.; Zhang, K.; Zhao, J.; Jiang, J.; Chen, F.; Fang, W. Evaluation of Soil-Applied Chemical Fungicide and Biofungicide for Control of the Fusarium Wilt of Chrysanthemum and Their Effects on Rhizosphere Soil Microbiota. Agriculture 2018, 8, 184. [Google Scholar] [CrossRef]
- Hamedi, J.; Mohammadipanah, F. Biotechnological application and taxonomical distribution of plant growth promoting actinobacteria. J. Ind. Microbiol. Biotechnol. 2015, 42, 157–171. [Google Scholar] [CrossRef]
- Hamayun, M.; Khan, S.A.; Ahmad, N.; Tang, D.-S.; Kang, S.-M.; Na, C.-I.; Sohn, E.-Y.; Hwang, Y.-H.; Shin, D.-H.; Lee, B.-H.; et al. Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J. Microbiol. Biotechnol. 2009, 25, 627–632. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, K.P. Assessment of Predacity and Efficacy of Arthrobotrys dactyloides for Biological Control of Root Knot Disease of Tomato. J. Phytopathol. 2006, 154, 1–5. [Google Scholar] [CrossRef]
- Wang, B.; Brubaker, C.L.; Burdon, J.J. Fusarium species and Fusarium wilt pathogens associated with native Gossypium populations in Australia. Mycol. Res. 2004, 108, 35–44. [Google Scholar] [CrossRef]
- Jayakumar, A.; Krishna, A.; Mohan, M.; Nair, I.C.; Radhakrishnan, E.K. Plant Growth Enhancement, Disease Resistance, and Elemental Modulatory Effects of Plant Probiotic Endophytic Bacillus sp. Fcl1. Probiotics Antimicrob. Proteins 2019, 11, 526–534. [Google Scholar] [CrossRef]
- Han, K.; Chen, Y.; Tiejun, H.U.; Zhang, F.; Zhou, F.; Cheng, J.; Wu, L. Effects of silicon, calcium, potassium and magnesium fertilizer on acid paddy soil improvement in Zhejiang Province. Acta Agric. Zhejiangensis 2018, 30, 117–122. [Google Scholar]
- Sharma, P.; Pandey, R. Biological control of root-knot nematode; Meloidogyne incognita in the medicinal plant; Withania somnifera and the effect of biocontrol agents on plant growth. Afr. J. Agric. Res. 2009, 4, 564–567. [Google Scholar]
- Shimizu, M. Endophytic Actinomycetes: Biocontrol Agents and Growth Promoters. In Bacteria in Agrobiology: Plant Growth Responses; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Mahey, R.; Katz, S. A non-specific Ca2+ (or Mg2+)-stimulated ATPase in rat heart sarcoplasmic reticulum. Mol. Cell. Biochem. 1990, 96, 175–182. [Google Scholar] [CrossRef]




| Treatment | Shoot | Leaf | Root | Flower | |||||
|---|---|---|---|---|---|---|---|---|---|
| Height | Diameter | Fresh Wt | Dry Wt | Width | Length | Fresh Wt | Dry Wt | Ray Floret | |
| (cm) | (mm) | (g/Plant) | (g/Plant) | (cm) | (cm) | (g/Plant) | (g/Plant) | Number (No.) | |
| Control | 80.08 ± 2.17 d | 4.15 ± 0.04 b | 75.70 ± 2.54 c | 22.67 ± 0.64 c | 1.47 ± 0.06 b | 2.64 ± 0.02 b | 2.81 ± 0.02 b | 0.55 ± 0.06 c | 162.00 ± 2.65 d |
| OF | 99.04 ± 1.23 b | 4.69 ± 0.05 a | 94.26 ± 2.04 a | 26.20 ± 0.51 b | 1.63 ± 0.04 b | 3.14 ± 0.02 a | 2.85 ± 0.04 b | 0.88 ± 0.00 a | 172.36 ± 2.37 c |
| IPN | 91.54 ± 2.42 c | 4.59 ± 0.12 a | 84.70 ± 1.72 b | 24.09 ± 0.98 bc | 1.60 ± 0.03 b | 2.88 ± 0.10 b | 2.68 ± 0.03 c | 0.67 ± 0.02 b | 184.33 ± 1.15 b |
| BOF | 105.21 ± 0.59 a | 4.74 ± 0.04 a | 98.63 ± 0.80 a | 30.18 ± 0.28 a | 1.96 ± 0.07 a | 3.32 ± 0.10 a | 3.43 ± 0.07 a | 0.86 ± 0.02 a | 204.33 ± 4.01 a |
| Microbe | Treatment | Chao1 | ACE | Shannon–Weaver | Simpson |
|---|---|---|---|---|---|
| Bacteria | Control | 5715.72 ± 19.34 b | 5714.33 ± 23.56 b | 9.51 ± 0.02 b | 0.994 ± 0.001 b |
| OF | 6048.60 ± 29.30 ab | 6042.41 ± 31.05 ab | 9.51 ± 0.01 b | 0.993 ± 0.000 b | |
| IPN | 5643.82 ± 65.03 b | 5672.93 ± 10.39 b | 9.07 ± 0.06 c | 0.992 ± 0.001 c | |
| BOF | 6441.25 ± 42.48 a | 6420.13 ± 34.76 a | 10.06 ± 0.02 a | 0.997 ± 0.000 a | |
| Fungi | Control | 279.82 ± 0.97 bc | 298.55 ± 1.25 bc | 4.36 ± 0.06 c | 0.898 ± 0.006 b |
| OF | 315.26 ± 6.06 a | 332.25 ± 4.89 a | 4.49 ± 0.04 b | 0.918 ± 0.001 a | |
| IPN | 270.61 ± 13.16 c | 271.82 ± 13.84 c | 4.21 ± 0.02 d | 0.901 ± 0.001 b | |
| BOF | 308.74 ± 10.41 ab | 316.33 ± 7.36 ab | 4.65 ± 0.04 a | 0.913 ± 0.001 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Zhao, J.; Jiang, J.; Zhao, Z.; Guan, Z.; Chen, S.; Chen, F.; Fang, W.; Zhao, S. Effects of Inorganic, Organic and Bio-Organic Fertilizer on Growth, Rhizosphere Soil Microflora and Soil Function Sustainability in Chrysanthemum Monoculture. Agriculture 2021, 11, 1214. https://doi.org/10.3390/agriculture11121214
Chen H, Zhao J, Jiang J, Zhao Z, Guan Z, Chen S, Chen F, Fang W, Zhao S. Effects of Inorganic, Organic and Bio-Organic Fertilizer on Growth, Rhizosphere Soil Microflora and Soil Function Sustainability in Chrysanthemum Monoculture. Agriculture. 2021; 11(12):1214. https://doi.org/10.3390/agriculture11121214
Chicago/Turabian StyleChen, Huijie, Jiamiao Zhao, Jing Jiang, Zhiguo Zhao, Zhiyong Guan, Sumei Chen, Fadi Chen, Weimin Fang, and Shuang Zhao. 2021. "Effects of Inorganic, Organic and Bio-Organic Fertilizer on Growth, Rhizosphere Soil Microflora and Soil Function Sustainability in Chrysanthemum Monoculture" Agriculture 11, no. 12: 1214. https://doi.org/10.3390/agriculture11121214
APA StyleChen, H., Zhao, J., Jiang, J., Zhao, Z., Guan, Z., Chen, S., Chen, F., Fang, W., & Zhao, S. (2021). Effects of Inorganic, Organic and Bio-Organic Fertilizer on Growth, Rhizosphere Soil Microflora and Soil Function Sustainability in Chrysanthemum Monoculture. Agriculture, 11(12), 1214. https://doi.org/10.3390/agriculture11121214








