Abstract
The large amount of biomass waste generated by vineyard pruning causes many environmental concerns. The production of briquettes represents an alternative to obtaining a value-added product. The transformation of vineyard wastes into briquettes could produce a densified product having high energy potential. The study investigates the production and chemical, structural, and thermal characterization of briquettes. The thermogravimetric analysis (TGA) shows that the briquettes have different stages of decomposition depending on temperature, such as drying, heating, devolatilization, and char aggregation. All the briquettes are decomposed around 600 °C. The analysis by X-ray diffraction (XRD) shows the crystallinity of briquettes. The pollutant emissions resulted from briquettes burning were measured as 444.7 mg N−1m−1 nitrogen oxide (NOX), 157.0 mg N−1m−1 sulphur dioxide (SO2) and 2165.0 mg N−1m−1 carbon monoxide (CO). The flue gases are below the admitted limits, with the exception of carbon monoxide content due to the incomplete combustion and high lignin content. Therefore, it can be concluded that briquettes produced from vineyard wastes have similar properties to briquettes produced from wood. This study demonstrates the potential of the obtained briquettes to replace the wood or charcoal through a desulphurization method.
1. Introduction
The use of renewable raw materials, especially waste and by-products, has become increasingly important. Viticulture is one of the most important agricultural activities in Romania. Vineyard pruning produces a large quantity of wastes which usually are left in the field. The valorification of agricultural residues is extremely important in energy production and utilization of biomass as alternative energy source [1,2]. The interest for the conversion of vineyard biomass into thermal/electrical energy comes not only from the fact that it represents a valuable and renewable source, but also because it offers various advantages in terms of development and environmental protection. The use of renewable sources is a necessity of the present and future civilizations, biomass being an important raw material to ensure the necessary energy. The category of lignocellulosic raw materials includes the vine resulting from the dry cuttings of vine plantations, having a high calorific value. Biomass requirements have steadily increased in the heating, energy, and transportation sectors, with the evolution of the densified solid biofuel production sector. Currently, the interest in fuel briquetting increased due to opportunities to use the agricultural wastes and their transformation into solid fuel [3,4].
Presently, briquettes are produced from different agricultural residues like straw, husks, bagasse, peels, stalks, shells, stems, and leaves [5,6,7,8]. The biomass preprocessing includes cleaning, drying, size reduction, binder addition, and biomass densification [5,9]. The densification processes involve the use of pressure to press the biomass in order to reduce its volume and to agglomerate the material in a compressed state. The non-wood briquettes are a densified product made by compressing ground biomass, with or without additive, with a cubic, prismatic, or cylindrical shape, with a diameter greater than 25 mm. Briquettes can be obtained from biomass by two methods: (i) the biomass is pressed with a piston at high pressure using a hydraulic or mechanical press, with intermittent release, and (ii) the biomass is pressed with a screw, with continuous release. The majority of briquettes are produced in the presence of a binder, such as xanthan gum or guar gum [10,11,12]. Lignin can act as internal binder at high briquetting pressure. The use of vineyard briquettes can create a market opportunity for agriculture but can also reduce the dependence on coal and the greenhouse emissions [13].
In the literature, there are some studies regarding the production of briquettes from various waste types, such as cashew nut shells and areca nuts [14,15], sawdust admixture [16], sawdust, rice and coconut husks [17], cassava waste [18], banana peel, corn husk and their combination [19], and maize cobs [20,21]. Bot et al. [22] investigated the production process of briquettes from coconut shells, banana peels, sugarcane bagasse, and rattan waste and concluded that from the analyzed waste types, coconut husks had reduced mass loss and good combustion properties. Lubwama et al. [23] demonstrated that rice and coffee husks briquettes had superior thermal efficiency compared to groundnut shells. Briquettes from coffee husks were also studied by Setter et al. [24], revealing the effect of particle size on the mechanical and energy parameters of briquettes and by Tesfaye et al. [25], presenting the benefits of coffee husk briquettes compared to firewood.
Our previous publication reports the methods used for briquettes production from vineyard wastes, but intensive studies about chemical structural and combustion products are needed [26]. The most important quality parameters are durability and stability. The properties of solid biofuels are affected by many characteristics of the biomass, such as the great diversity of species, various habitats, the type, and the nature of the culture. The problems of the agricultural solid biofuels are the sulphur and ash content [27].
The emission studies are very important for the evaluation of pollutants emission and particulate matter, but the majority of the studies reported only on the technology of briquettes obtained from various biomass types, while the combustion experiments are missing. The present paper presents a solution for producing high-quality briquettes from vine shoot waste. The results regarding vineyard pruning valorization were already published, presenting bioethanol production from cellulose extracted from vineyard waste after autohydrolysis and delignification pretreatment [28], pellets production [29], and a preliminary study of briquettes production from vine tendrils [26]. In this regard, this study aims to continue the chemical investigation, structural characterization, and combustion experiments of briquettes obtained from several Romanian vineyard varieties.
2. Materials and Methods
2.1. Chemical and Reagents
All used chemicals were of analytical reagent grade. Acetic acid, dichloromethane, sulphuric acid (98%), sodium hydroxide, methanol, hydrochloric acid (37%), nitric acid (65%), sulphuric acid (98%), ethanol, toluene, acetone, and hydrogen peroxide (30%) were purchased from Merck (Darmstadt, Germany). Sodium chlorite (80%) was purchased from Alfa Aesar GmbH & Co (Karlsruhe, Germany). All solutions were prepared by using ultrapure water (18.2 MΩ cm−1 at 20 °C) obtained from a Direct-Q3 UV Water Purification System (Millipore, France).
2.2. Sample Description
A total of eight Romanian varieties biomass samples were selected from the following varieties: Pinot Noir (PN), Muscat Ottonel (MO), Feteasca Neagra (FN), Feteasca Alba (FA), Feteasca Regala (FR), Cabernet Sauvignon (CS), Sauvignon Blanc (SB), and Busuioaca de Bohotin (BB).
2.3. Production of Briquettes
The initial study regarding the production of briquettes from vineyard waste was initiated and the first results were published in our recent paper [26]. The applied technology contains the following steps: collecting the tendrils, storage, natural drying, coarse and fine grinding, sieving and separation of the particles between 3.15 and 8 mm, and briquetting. The obtained briquettes were characterized and the information about moisture, heating value, and physical characteristics were reported. The obtained Pini Kay briquettes have a moisture content around 10%, a diameter of 58 mm, a length of around 200 mm, a bulk density of around 650 kg m−3, a unit density of 1300 kg m−3, and a mechanical durability of 95% (Figure 1).

Figure 1.
Briquettes obtained from vine shoot waste varieties.
2.4. Chemical Characterization of Briquetted Obtained from Vineyard Waste
2.4.1. Carbohydrate and Lignin Analysis
The content of carbohydrates from the briquettes was determined as holocellulose (mixtures between cellulose and hemicelluloses). The content of holocellulose was determined according to the method presented in detail in our publication [28] as residues insoluble in NaOH. The lignin content was determined as solid weight after the reaction with H2SO4 (72%).
2.4.2. Elemental Analysis and Ash of Briquettes
The elemental composition of the obtained briquettes, expressed as percentage content on dry basis of carbon, hydrogen, nitrogen, oxygen, and sulphur, was determined by Flash EA 2000 CHNS/O analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The ash was determined by drying in the oven at 600 °C for 5 h a quantity of briquettes, and the remaining solids were quantified.
2.4.3. Metals Determination
Metals concentrations were determined according to the 16968:2015 standard [30], by the digestion of samples with a mixture of 65% nitric acid and 30% hydrogen peroxide in a closed polytetrafluoroethylene (PTFE) vessel, using a microwave digestion system (Speedwave MWS-3+, Berghof, Eningen, Germany), followed by the metals determination using an ICP-OES Optima 5300 DV (Perkin Elmer, Woodbridge, ON, USA).
2.4.4. Mercury and Chlorine Determination
The measurements of mercury from briquettes samples were carried out using an Automated Direct Hg Analyzer Hydra-C (Teledyne Instruments, Leeman Labs, Mason, OH, USA), based on thermal desorption atomic absorption spectrometry.
The Hydra-C Hg Analyzer includes a furnace module where the sample is dried and decomposed. The final decomposition products pass through a mercury amalgamator which collects Hg. By heating of amalgamation tube, mercury is released and carried to the atomic absorption spectrometer. The absorbance signal for Hg was identified at a wavelength of 253.65 nm. The chlorine determination was performed according to ISO 16994 (2016) [31].
2.4.5. Determination of the Energy Consumption for Vineyard Waste Briquetting
For determining the energy consumption of each technological step used in the briquetting technology, a Voltcraft Energy-Logger 4000 consumption meter (Voltcraft) was used. Through these counters all devices were fed: coarse and fine chopping of the vineyard, sieving and separation, and briquetting steps. The equipment measured the energy consumption, and the results were transferred to a PC.
2.5. Structural Characterization of Briquettes
2.5.1. TGA/DTG Analysis
The thermal decomposition and derivative thermogravimetric (DTG) analysis of briquettes was determined by using a TA Instruments SDT O 600 (TA Instruments, New Castle, DE, USA) with temperature ranging from 30 to 1000 °C at 10 °C per minute. A mass of 7.65 ± 0.3 mg of the dried samples (SB variety) was used for test, and the experiment was conducted under a nitrogen atmosphere. The experiments were repeatable with a standard deviation in peak temperature value.
2.5.2. X-ray Diffraction (XRD)
The X-ray diffraction (XRD) patterns were performed using a D8 Advance diffractometer (Bruker, Karlsruhe, Germany), operating at 40 kV and 35 mA, with CuKα radiation (λ = 1.5406 Å), at room temperature. The crystallinity index (CrI) was calculated using Equation (1).
where Ic is the maximum intensity of the (002) diffraction peak at a 20 angle close to 22°, and Iam is the intensity at a 20 angle close to 15°, representing the amorphous part of the sample. The degree of crystallinity (DC) was calculated as the relationship between the crystalline and amorphous regions using Equation (2).
where DC is the degree of crystallinity, Fc and Fa are the areas of the crystalline and amorphous regions [32,33].
2.6. Fuel Indexes Estimation
The fuel indexes were estimated based on the metal contents according to the Sommersacher method [34]. The fuel indices estimated were: (i) NOx emissions, calculated on the basis of the nitrogen content of the briquettes; (ii) aerosol emissions, calculated from the sum of the concentrations of K, Na, Zn and Pb; (iii) the risk of corrosion at high temperatures, calculated from the 2S Cl−1 molar ratio; (iv) problems caused by melting ash were calculated from the Si (Ca + Mg)−1 molar ratio; (v) HCl and SO2 emissions were calculated from the molar ratio of (K + Na) [x·(2S + Cl)]−1.
2.7. Combustion Experiments
Classical heat storage was used to test the gaseous emissions. The flue gases were measured by using a flue gas analyzer (Testo, 350 XL, Titisee-Neustadt, Germany) according to the EN 15259 (2007) standard [35] to measure O2 (oxygen), CO (carbon monoxide), CO2 (carbon dioxide), NO (nitrogen oxide), NOX (nitrogen oxides), and SO2 (sulphur dioxide) contents. All tests were carried out in a fixed bed stove [29].
2.8. Statistics
For the statistical processing of the data, the XLStat Microsoft Excel (software BASIC+, 2019.3.2) plug-in (Addinsoft, Paris, France) was used. Tukey method for varieties comparison was used to determine the difference between them. Significance was declared at p < 0.05 for all statistical analyses. The a, b, c, d, e, f, and g letters indicate statistically significant difference at p < 0.05.
3. Results and Discussion
3.1. Energy Consumption for Briquetting of Vineyard Waste
The vineyard wastes were collected from north-west of Romania, a region with a high production of wine. The total energy consumption, briquetting time and quantity of briquetting were considered for the determination of the energy consumption and the capacity of the briquetting equipment. The moisture of vineyard wastes was adjusted to 10%. The energy consumption is crucial for analyzing how much energy was consumed in each technological step. Table 1 presents the results obtained for energy consumption in the briquetting process.

Table 1.
Specific energy consumption and working capacity of the briquetting machine fed with vineyard wastes. (Data represents means ± standard deviations, n = 3 parallel measurement).
The experimental data shows that the maximum energy consumption was obtained for the PN variety (0.340 MJ kg−1), and the minimum for the FA variety (0.313 MJ kg−1). According to the study of Srivastava et al. [36], the energy required for obtaining briquettes from the vegetable market waste was in the range of 17–20 kWh t−1 and the working capacity of the briquetting machine was in the range of 200–310 kg h−1. The working capacity of the briquetting machine of the wastes was of approximately 230 kg h−1. Additionally, Cesprini et al. [37] reported an energy consumption of 34–77 kW ton−1 for briquettes made from glued wood waste. Table 2 presents the results obtained for energy potential of each vineyard varieties used in this study.

Table 2.
Quantity of briquettes produced from vineyard wastes and energy potential of each variety. (Data represents means ± standard deviations, n = 3 parallel measurement).
The vineyard briquettes’ energy potential confirms their great potential to be used as solid fuel. The statistical analysis for quantity used for briquettes and briquettes produced shows no significant difference between samples (confidence level 95%, p-value < 0.05).
3.2. Analysis of the Briquettes Obtained from Vineyard Waste
The content of cellulose, hemicelluloses, and lignin are presented in Figure 2. This information on the major components will help to understand results. Lignin content in briquettes ranges between 22.4 and 36.5 %, depending on the variety. The amount of lignin was critical for the briquetting process because the lignin acts as a binding agent and determines the quality of the final product. According to the study of Afra et al. [38], the content of 9% lignin can substantially improve the calorific value of the biomass. The content of cellulose was between 30.2 and 36.2 % and the content of hemicellulose was of 16.5–24.3%. The content of cellulose and lignin are the most important components that affect the combustion process. According to Gani et al. [39], a high content of cellulose in briquettes accelerates the burning and, conversely, the content of lignin has the opposite effect [39]. The present study demonstrates that the use of natural lignin binder leads to obtaining more economical and cheap biobriquettes. The effect of cellulose and lignin content from briquettes will be evaluated in the combustion process presented below.
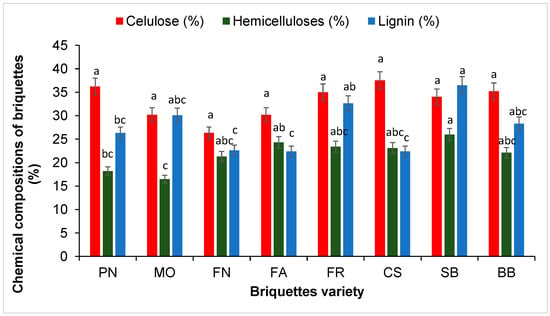
Figure 2.
Chemical compositions of briquettes. Each point is the mean ± standard deviation (n = 3). The means with different letters (a–c) above the bars indicate significant differences based on Tukey’s test (p < 0.05).
Table 3 shows the proximate analysis of the obtained briquettes. The content of N, S, Cl, and ash were compared with the limits imposed by the existing standards. All briquettes’ samples respect the limit imposed by ISO 17225-7 and ISO 17225-3 standards [40,41]. The calorific value of the obtained briquettes was already presented in our recent paper [26] and all the samples have a gross calorific value of around 19 MJ kg−1 and a net calorific value of around 17 MJ kg−1. The high calorific value is important for longer burning time and to release more heat [42].

Table 3.
Chemical composition of briquettes (mean ± standard deviation, n = 3).
The chemical composition of biomass has an important role for its energy value because it consists of organic mass (C, H, N, S, O), inorganic mass (the chemical elements in the ash that influences the energy characteristics), and the moisture. The higher C and H content is correlated with the higher the calorific value. On the other hand, the content of O must be as low as possible. Besides the elements that have a direct effect on the level of harmful emissions resulting from the combustion of solid biofuels, i.e., S, N and Cl, the ash content should be considered [43]. In all the samples, the S (<0.04%) and N (<1.5%) contents fitted into the standard requirement of briquettes made from wood. The low ash content showed that the obtained briquettes are suitable for combustion. The Tukey test for N and ash content shows a strong significance between samples (p-value < 0.05). The major (Al, Ca, Fe, K, Mg, Na, P, and Si) and the minor (As, Ba, Cd, Co, Cr, Cu, Hg, Mn, Mo, Ni, Sb, Ti, and Zn) elements form the ash components [44]. These elements are important for the ash melting, leading to the formation of deposits, aerosols emissions and corrosion of boilers. The high Ca and Mg content increases the melting point of ash, while Cl- and alkali metals significantly decreases it, leading to the formation of slag in the combustion chamber.
Large amounts of Ca, K, and Mg were found in all the samples. The obtained results were compared with those from ISO 17225-7 [40] standard for non-woody briquettes and with the ISO 17225-3 [41] standard for wood briquettes. The existing standard imposed the limit for the content of Ni, Cr, Cu, Zn, Cd, Pb, As, and Hg. High quantities of Ca, K, and Na were found in all the samples. The contents of metals varied in the following ranges (mg kg−1): 61.3–86.2 (Si) > 58.03–412.5 (Fe) > 40.55–54.81 (Al) > 39.75–59.08 (Na) > 18.96–41.83 (Mn) > 22.34–25.34 (Cu) > 24.42–27.54 (B) > 9.63–23.55 (Zn) > 12.11–27.03 (Ba) > 11.45–12.27 (Cr) > 10.57–10.70 (Co) > 9.73–10.05 (Ni) > 9.80–11.78 (Pb) > 2.78–2.89 (Cd) > 0.09–0.16 (As) (Table 4). The Cu content respected the standard for non-wood briquettes, but not the ISO 17225-3 for woody briquettes. The combustion process was affected by inorganic elements, like K, Na, Si, Mg, and Al. According to Moreno et al. [45], the presence of Ca is beneficial for combustion, but the presence of Si decreases the melting point of ash and leads to fouling issues for combustion.

Table 4.
Content of metals (mg kg−1) from briquettes obtained from vineyard residues (Data represents means ± standard deviations, n = 3 parallel measurement).
3.3. Estimation of Fuel Indexes of Briquettes
Some metals present in briquettes, like K, Na, Zn, Pb, Ca, and Mg could offer information about the combustion process, suggesting problems like slagging, corrosion, etc. In Table 5 are presented the results of fuel indices obtained.

Table 5.
Estimation of fuel indices calculated based on the metal content determined from the obtained briquettes.
The estimation of fuel indices offers the following information: due to the N content from briquettes, NOX emissions are expected in all samples. The high content of K found in all the samples can lead to the formation of a huge quantity of aerosols. The corrosion risk calculated from the molar ratio 2S Cl−1 was higher than 1 mol mol−1 in all briquette samples; high corrosion risk of boilers is expected. The molar ratio between (K + Na)(2S + Cl)−1 is approximately 3 mol mol−1 in the briquette samples, which indicates a risk of high SOX emissions. The molar ration Si K−1 is low due to the high potassium content, which shows a moderate risk of corrosion at high temperatures.
3.4. Structural Characterization of Briquettes
3.4.1. X-ray Diffraction Analyses of the Briquettes
The X-ray diffraction patterns of the fuel briquettes made from vineyard waste are presented in Figure 3. The peak intensities and peak broadening of the diffraction peaks did not significantly differ from one species to another. Any biomass is mainly composed by cellulose and hemicellulose, both embedded in lignin [46]. Hemicellulose and lignin are amorphous, while cellulose has both amorphous and crystalline components. The XRD technique offers information on the crystalline and the amorphous parts of cellulose, but not related to the presence of hemicellulose and lignin. The degree of crystallinity is one of the most important structure parameters, considering that the rigidity of cellulose fibers increases with the ratio of crystalline to amorphous regions. Two typical diffraction peaks determining the crystallinity of the samples were remarked at 20 around 15° and 22°, corresponding to the minimum (101) and highest (002) peaks [47]. All the diffraction patterns displayed a well-defined main peak around 20 = 22° indicating a type I cellulose as the main form of cellulose of the investigated samples. The crystalline index (CrI) values calculated based on the peak height method varied in the range of 19.3–24.0%, while the degree of crystallinity (DC) assessed based on the area of crystalline and amorphous region ranged between 68.1–70.6%.
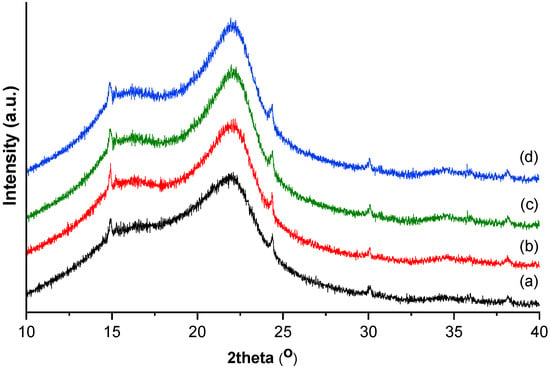
Figure 3.
X-ray diffraction patterns of fuel briquettes made from vineyard waste: (a) BB, (b) FA, (c) MO, and (d) SB.
3.4.2. TGA of Briquettes
The TGA/DTG curves of the obtained briquettes are presented in Figure 4. The briquettes were heated up from room temperature to 1000 °C in N2 atmosphere at a rate of 10 °C min−1. In the first stage (bellow 200 °C), the mass loss was of 4.85 ± 0.23%, being attributed to the evaporation of water. The second stage (200–700 °C) is the most important stage of briquettes heating and presents two characteristic peaks. The first peak appeared at 319.33 °C and was attributed to the heating process and hemicellulose and crystalline cellulose elimination. Additionally, some dehydrogenation and decarbonization reaction can take place. The second peak at 418.63 °C and 40.45 ± 3.40% from the biomass was lost due to the devolatilization phase and to the thermal degradation when carbon and volatiles were released. In the SB variety, the hemicellulose, cellulose, and lignin contents were 26%, 34%, and 36.5%, respectively. The decomposition of hemicellulose was faster than of cellulose and began at the temperature of 220 °C with physical loss of water, dehydration, and cleavage of the chain between heteropolymers. According to literature studies [48,49], hemicellulose is degraded first, then cellulose, and finally lignin, which are directly related to their chemical properties. The hemicellulose was decomposed within the temperature range of 220–315 °C. The decomposition of cellulose occurred in the temperature range of 315–400 °C. Lignin was the most difficult to decompose and generally was decomposed slowly under a wide range of temperatures until 900 °C. At 550 °C, the linkage of the oxygen and hydrogen containing compounds are released from the lignin. According to Koskela et al. [50], the study of lignin pyrolysis shows that at temperatures ranging between 500 to 900 °C, the benzene bonds from lignin are broken and lead to the formation of new aromatic clusters and favors char formation. The appearance of a small peak at 600 °C was attributed to the formation of biochar. In the last stage, the almost complete degradation (98.08%) of briquettes occurred, corresponding to the char aggregation. The thermal decomposition of lignin results in the formation of quaiacyl, syringyl and p-hydroxylphenyl compounds which occur in several stages: melting of lignin, volatilization, and the breaking of β-O-4 linkages from phenolic compounds. The complex structure of lignin requires higher temperature for decomposition than hemicellulose. The thermal decompositions of vineyard briquettes are in accordance with the reported literature for briquettes obtained from another lignocellulosic biomass [49]. The results presented suggest that a temperature higher than 1000 °C is recommended for the complete degradation of briquettes.
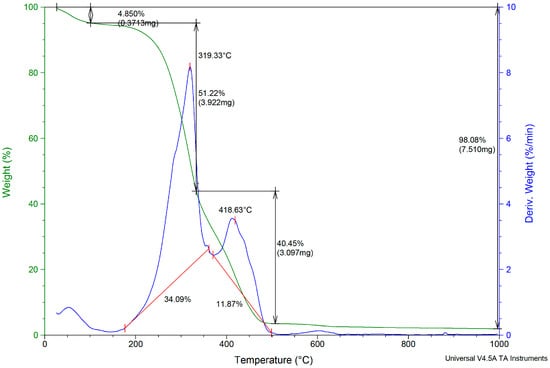
Figure 4.
The TGA/DTG curves of the obtained briquettes for SB variety.
3.5. Analysis of Gaseous Emissions from the Combustion of Briquettes
NOX and SO2 emissions are the main gaseous pollutants. Due to presence of sulphur in the briquette composition, SO2 will be found in gaseous emissions. The emissions of NOX, SO2, and CO are presented in Table 6. The NOX emissions were 444.7 mg N−1m−3 and respect the limit of 500 mg N−1m−3 required by the Romanian Law 462/1993 [51]. NOX content was measured as the sum of nitrogen monoxide (NO) and nitrogen dioxide (NO2) contents. The SO2 emissions were of 157.0 mg N−1m−3, lower than the limit, and its presence was in accordance with the predictable fuel indices. Cong et al. [52] reported the following emissions of charcoal-based briquettes prepared using bio-tar as a binder: 113.2–225.6 mg N−1m−3 (NOX) and 22.4–33.1 mg N−1m−3 (SO2). The CO emissions were of 2165 mg N−1m−3, exceeding the limit imposed by law, due to incomplete burning and no excess of air. Roy and Corscadden [53] reported CO emissions for switched grass briquettes of 1718 mg N−1m−3. Briquettes made from vineyards contain combustible elements such as carbon, hydrogen and sulphur and non-combustible elements such as nitrogen, oxygen, and ash. Sulphur has harmful effects on the combustion because it leads to the formation of sulphur oxides (SO2 and SO3) which produce acids that attack the metal parts of the plant. It is also responsible for the phenomenon of acid rain. The sulphur content of the briquettes was low, which has led to a low concentration of SO2 (157 mg N−1m−3), a value that falls within the limits imposed by the standard. In order to eliminate the suphur before the burning of briquettes, experiments for desulphurization methods (reaction with lime) will be performed and the results will be the step for the following experiments.

Table 6.
The gaseous emissions results from burning of obtained briquettes in a cogeneration plant.
Incomplete combustion of carbon leads to the formation of CO. The high CO content (2165 mg N−1m−3) results from insufficient oxygen during combustion. Carbon burns in two stages: in the first stage there is an incomplete combustion which leads to the formation of CO, and in the second stage there is an oxidation of CO to CO2. If there is not enough oxygen, only the first stage of the carbon combustion takes place, and this results in a high CO content when burning the lighters. The high content of CO can be attributed to a high content of lignin from the briquette composition and to the slow burning rate. The largest variations in the flue gas appear in the first measurements (15–20 min) after ignition, then the concentrations become relatively stable.
4. Conclusions
The conducted study shows that vineyard wastes can be converted into good quality briquettes. Solid briquettes from vineyard wastes represent a cleaner energy alternative, considering the large amount of waste produced annually and the possibility to mitigate the reduction of CO2 emissions and promote its production. Lignin from vineyard waste’s structure was used as a binder in the briquetting process, without using any other binding compound. The composition of briquettes was chemically and structurally characterized.
The TGA-DGT analysis was used to evaluate the thermal decomposition behaviors of briquettes components. Different mass losses were obtained depending on the decomposition of hemicellulose, cellulose, and lignin components, and the following processes occurred as the temperature increased: drying, heating, devolatilization, and char aggregation. The high content of lignin helps the briquetting production but has a negative effect on burning. The content of inorganic matter influenced the flue gases and predicted the pollutants emission. The XRD analysis of briquettes offered information about the crystalline and amorphous parts of cellulose in the briquettes, but it did not provide information about hemicellulose and lignin. The identified emissions pollutants were carbon monoxide, sulphur dioxide, and nitrogen oxide. The concentration of CO exceeded the maximum allowed by Romanian law due to an incomplete combustion. The incomplete combustion is predicted from the TGA analysis that shows that the char formation occurred around 600 °C due to the smoldering effect.
Author Contributions
Conceptualization, L.S.; methodology, I.T. and P.C.; formal analysis, D.A.S., E.K., M.R. and O.C.; writing—reviewing and editing, L.S., E.K. and M.S.; visualization, D.E.D.; supervision, C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Competitiveness Operational Programme of the Ministry of European Funds, code MY SMIS 107874, and the article processing charge (APC) was funded by the Ministry of Research, Innovation and Digitization through Program 1—Development of the national research & development system, Subprogram 1.2—Institutional performance—Projects that finance the RDI excellence, Contract no. 18PFE/30 December 2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Avcioğlu, A.O.; Dayioğlu, M.A.; Tütker, U. Assessment of the energy potential of agricultural biomass residues in Turkey. Renew. Energy 2019, 138, 610–619. [Google Scholar] [CrossRef]
- Nour, M.; Amer, M.; Elwardany, A.; Attia, A.; Li, X.; Nada, S. Pyrolysis, kinetics, and structural analyses of agricultural residues in Egypt: For future assessment of their energy potential. Clean. Eng. Technol. 2021, 2, 100080. [Google Scholar] [CrossRef]
- Asamoah, B.; Josiane, N.; Gebrezgabher, S.; Odonkor, E.; Njenga, M. Fuel briquettes: Making business sense. Urban Agric. Mag. 2017, 32, 42–43. [Google Scholar]
- Manandhar, A.; Mousavi-Avval, S.H.; Tatum, J.; Shrestha, E.; Nazemi, P.; Shah, A. Chapter Eighteen—Solid biofuels. In Biomass, Biofuels, Biochemicals, Green-Economy, Systems Analysis for Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–370. [Google Scholar]
- Kpalo, S.Y.; Zainuddin, M.F.; Manaf, L.A.; Roslan, A.M. A review of technical and economic aspects of biomass briquetting. Sustainability 2020, 12, 4609. [Google Scholar] [CrossRef]
- Brunerova, A.; Roubik, H.; Brozek, M.; Dung, D.V.; Phung, L.D.; Hasanudin, U.; Iryani, D.A.; Herak, D. Briquetting of sugarcane bagasse as a proper waste management technology in Vietnam. Waste Manag. Res. 2020, 38, 1239–1250. [Google Scholar] [CrossRef]
- Velusamy, S.; Subbaiyan, A.; Kandasamy, S.; Shanmugamoorthi, M.; Thirumoorthy, P. Combustion characteristics of biomass fuel briquettes from onion peels and tamarind shells. Arch. Environ. Occup. Health 2021, 1–12. [Google Scholar] [CrossRef]
- Bot, B.V.; Tamba, J.G.; Sosso, O.T. Assessment of biomass briquette energy potential from agricultural residues in Cameroon. Biomass Convers. Biorefin. 2022, 1–13. [Google Scholar] [CrossRef]
- Kizito, S.; Jjagwe, J.; Ssewaya, B.; Nekesa, L.; Tumutegyereize, P.; Zziwa, A.; Komakech, A.J. Biofuel characteristics of non-charred briquettes from dried fecal sludge blended with food market waste: Suggesting a waste-to-biofuel enterprise as a win-win strategy to solve energy and sanitation problems in slums settlements. Waste Manag. 2021, 140, 173–182. [Google Scholar] [CrossRef]
- Fehse, F.; Kummich, J.; Schröder, H.-W. Influence of pre-treatment and variation of briquetting parameters on the mechanical refinement of spent coffee grounds. Biomass Bioenergy 2021, 152, 106201. [Google Scholar] [CrossRef]
- Magnago, R.F.; Costa, S.C.; Ezirio, M.J.A.; Saciloto, V.G.; Parma, G.O.C.; Gasporotto, E.S.; Goncalves, A.C., Jr.; Tutida, A.Y.; Barcelos, R.L. Briquettes of citrus peel and rice husk. J. Clean. Prod. 2020, 276, 123820. [Google Scholar] [CrossRef]
- Setter, C.; Costa, K.L.S.; Oliveira, T.J.P.; Mendes, R.F. The effect of kraft lignin on the physicochemical quality of briquettes produced with sugarcane bagasse and on the characteristics of the bio-oil obtained via slow pyrolysis. Fuel Process. Technol. 2020, 210, 106561. [Google Scholar] [CrossRef]
- Petkovic, D. Technology for producing briquettes from wet biomass. In Encyclopedia of Renewable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 901–907. [Google Scholar]
- Ifa, L.; Yani, S.; Nurjannah, N.; Darnengsih, D.; Rusnaenah, A.; Mel, M.; Mahfud, M.; Kusuma, H.S. Techno-economic analysis of bio-briquette from cashew nut shell waste. Heliyon 2020, 6, e05009. [Google Scholar] [CrossRef] [PubMed]
- Chungcharoen, T.; Srisang, N. Preparation and characterization of fuel briquettes made from dual agricultural waste: Cashew nut shells and areca nuts. J. Clean. Prod. 2020, 256, 120434. [Google Scholar] [CrossRef]
- Bello, R.S.; Onilude, M.A. Effects of critical extrusion factors on quality of high density briquettes produced from sawdust admixture. Mater. Today Proc. 2021, 38, 949–957. [Google Scholar] [CrossRef]
- Akolgo, G.A.; Awafo, E.A.; Essandoh, E.O.; Owusu, P.A.; Uba, F.; Adu-Poku, K.A. Assessment of the potential of charred briquettes of sawdust, rice and coconut husks: Using water boiling and user acceptability tests. Sci. Afr. 2021, 12, e00789. [Google Scholar] [CrossRef]
- Granado, M.P.P.; Suhogusoff, Y.V.M.; Santos, L.R.O.; Yamaji, F.M.; Conti, A.C.D. Effects of pressure densification on strength and properties of cassava waste briquettes. Renew. Energy 2021, 167, 306–312. [Google Scholar] [CrossRef]
- Kapen, P.T.; Tenkeu, M.N.; Yadjie, E.; Tchuen, G. Production and characterization of environmentally friendly charcoal briquettes obtained from agriculture waste: Case of Cameroon. Int. J. Environ. Sci. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Sunardi, S.; Djuanda, D.; Mandra, M.A.S. Characteristics of charcoal briquettes from agricultural waste with compaction pressure and particle size variation as alternative fuel. Int. Energy J. 2019, 19, 139–148. [Google Scholar]
- Oliy, G.B.; Muleta, D.T. Characterization and determination of briquette fuel prepared from five variety of corn cob. Int. J. Sustain. Energy 2020, 3, 59–64. [Google Scholar]
- Bot, B.V.; Sosso, O.T.; Tamba, J.G.; Lekane, E.; Bikai, J.; Ndame, M.K. Preparation and characterization of biomass briquettes made from banana peels, sugarcane bagasse, coconut shells and rattan waste. Biomass Convers. Biorefin. 2021, 1–10. [Google Scholar] [CrossRef]
- Lubwama, M.; Yiga, V.A.; Muhairwe, F.; Kihedu, J. Physical and combustion properties of agricultural residue bio-char bio-composite briquettes as sustainable domestic energy sources. Renew. Energy 2020, 148, 1002–1016. [Google Scholar] [CrossRef]
- Setter, C.; Ataide, C.H.; Mendes, R.F.; de Oliveira, T.J.P. Influence of particle size on the physico-mechanical and energy properties of briquettes produced with coffee husks. Environ. Sci. Pollut. Res. 2021, 28, 8215–8223. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.; Workie, F.; Kumar, V.S. Production and characterization of coffee husk fuel briquettes as an alternative energy source. Adv. Mater. Sci. Eng. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Tenu, I.; Roman, C.; Senila, L.; Rosca, R.; Carlescu, I.; Baetu, M.; Arsenoaia, V.; Dumitrachi, E.P.; Corduneanu, O.R. Valorization of vine tendrils resulted from pruning as densified solid biomass fuel (Briquettes). Processes 2021, 9, 1409. [Google Scholar] [CrossRef]
- Nagarajan, J.; Prakash, L. Preparation and characterization of biomass briquettes using sugarcane bagasse, corncob and rice husk. Mater. Today Proc. 2021, 47, 4194–4198. [Google Scholar] [CrossRef]
- Senila, L.; Kovacs, E.; Scurtu, D.A.; Cadar, O.; Becze, A.; Senila, M.; Levei, E.A.; Dumitras, D.E.; Tenu, I.; Roman, C. Bioethanol production from vineyard waste by autohydrolysis pretreatment and chlorite delignification via simultaneous saccharification and fermentation. Molecules 2020, 25, 2606. [Google Scholar] [CrossRef]
- Senila, L.; Tenu, I.; Carlescu, I.; Corduneanu, O.R.; Dumitrachi, E.P.; Kovacs, E.; Scurtu, D.A.; Cadar, O.; Becze, A.; Senila, M.; et al. Sustainable biomass pellets production using vineyard wastes. Agriculture 2020, 10, 501. [Google Scholar] [CrossRef]
- ISO 16968:2015; Solid Biofuels. Determination of Minor Elements. ISO: Geneva, Switzerland, 2015.
- ISO 16994:2016; Solid Biofuel. Determination of Total Content of Sulphur and Chlorine. ISO: Geneva, Switzerland, 2016.
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. Am empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Azizan, A.; Azmi, I.S.; Darim, R.A.; Jusri, N.A.A.; Jalil, R.; Rahman, M.F.A.; Salleh, R.M.; Ibrahim, N.; Salihon, J. Lignocellulosic ionic liquid pretreated biomaterials/biomass. Mater. Today Proc. 2021, 46, 1688–1692. [Google Scholar] [CrossRef]
- Sommersacher, P.; Brunner, T.; Obernberger, I. Fuel indexes: A novel method for the evaluation of relevant combustion properties of new biomass fuels. Energy Fuels 2012, 26, 380–390. [Google Scholar] [CrossRef]
- EN 15259:2007; Air Quality. Measurement of Stationary Source Emissions. Requirements for Measurement Sections and Site and for the Measurement Objective, Plan and Report. BSi: London, UK, 2007.
- Srivastava, N.S.L.; Narnaware, S.L.; Makwana, J.P.; Singh, S.N.; Vahora, S. Investigating the energy use of vegetable market waste by briquetting. Renew. Energy 2014, 68, 270–275. [Google Scholar] [CrossRef]
- Cesprini, E.; Greco, R.; Causin, V.; Urso, T.; Cavalli, R.; Cavalli, R.; Zanetti, M. Quality assessment of pellets and briquettes made from glue wood waste. Eur. J. Wood Wood Prod. 2021, 79, 1153–1162. [Google Scholar] [CrossRef]
- Afra, E.; Abyaz, A.; Saraeyan, A. The production of bagasse biofuel briquettes and the evaluation of natural binders (LNFC, NFC, and lignin) effects on their technical parameters. J. Clean. Prod. 2021, 278, 123543. [Google Scholar] [CrossRef]
- Gani, A.; Naruse, I. Effect of cellulose and lignin content on pyrolysis and combustion characteristic for several types of biomass. Renew. Energy 2007, 32, 649–661. [Google Scholar] [CrossRef]
- ISO 17225-7; Solid Biofuels. Fuel Specifications and Classes—Part 7: Graded Non-Woody Briquettes. ISO: Geneva, Switzerland, 2014.
- ISO 17225-3; Solid Biofuels. Fuel Specifications and Classes—Part 3: Graded Wood Briquettes. ISO: Geneva, Switzerland, 2021.
- Bisen, K.S.; Sharma, P.; Gupta, B.; Baredar, P. Development and experimental characterization of energy efficient poultry litter & plant weeds based briquettes (PLPWBB) by comparing with rice husk briquettes. Mater. Today Proc. 2021, 4, 5428–5432. [Google Scholar]
- Nyakuma, B.B.; Johari, A.; Ahmad, A.; Abdullah, T.A.T. Thermogravimetric analysis of the fuel properties of empty fruit bunch briquettes. J. Teknol. 2014, 67, 79–82. [Google Scholar] [CrossRef][Green Version]
- Viczek, S.A.; Aldrian, A.; Pomberger, R.; Sarc, R. Origins of major and minor ash constituents of solid recovery fuel for co-processing in the cement industry. Waste Manag. 2021, 126, 423–432. [Google Scholar] [CrossRef]
- Moreno, A.I.; Font, R.; Conesa, J.A. Physical and chemical evaluation of furniture waste briquettes. Waste Manag. 2016, 49, 245–252. [Google Scholar] [CrossRef]
- Taherzadeh, M.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Dlangamandla, N.; Ntwampe, S.K.O.; Angadam, J.O.; Itoba-Tombo, E.F.; Chidi, B.S.; Mekuto, L. Integrated hydrolysis of mixed agro-waste for a second generation biorefinery using Nepenthes mirabilis pod digestive fluids. Processes 2019, 72, 64. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Volli, V.; Gollakota, A.R.K.; Shu, C.-M. Comparative studies on thermochemical behavior and kinetics of lignocellulosic biomass residues using TG-FTIR and Py-GC/MS. Sci. Total Environ. 2021, 792, 148392. [Google Scholar] [CrossRef] [PubMed]
- Kskela, A.; Heikkilä, A.; Bergna, D.; Salminen, J.; Fabritius, T. Effects of briquetting and high pyrolysis temperature and hydrolysis lignin char properties and reactivity in CO-CO2-N2 conditions. Minerals 2021, 11, 187. [Google Scholar] [CrossRef]
- Law No. 462/1993 on Technical Requirements for Air Protection and Limits on Polluting Emissions from Stationary Sources. Available online: http://legislatie.just.ro/Public/DetaliiDocument/3538 (accessed on 10 January 2022).
- Cong, H.; Yao, Z.; Mašek, O.; Meng, H.; Sheng, C.; Wu, Y.; Zhao, L. Co-combustion, co-densification, and pollutant emission characteristics of charcoal-based briquettes prepared using bio-tar as a binder. Fuel 2021, 287, 119512. [Google Scholar] [CrossRef]
- Roy, M.M.; Corscadden, K.W. An experimental study of combustion and emissions of biomass briquettes in a domestic wood stove. Appl. Energy 2012, 99, 206–212. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).