Quality Evaluation of Wild and Cultivated Asparagus: A Comparison between Raw and Steamed Spears
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Plant Material
2.3. Sample Preparation and Steaming
2.4. Physico-Chemical Methods
2.5. Biochemical Methods
2.5.1. Chlorophyll and Carotenoid Content
2.5.2. Sugars
2.5.3. Phenols and Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
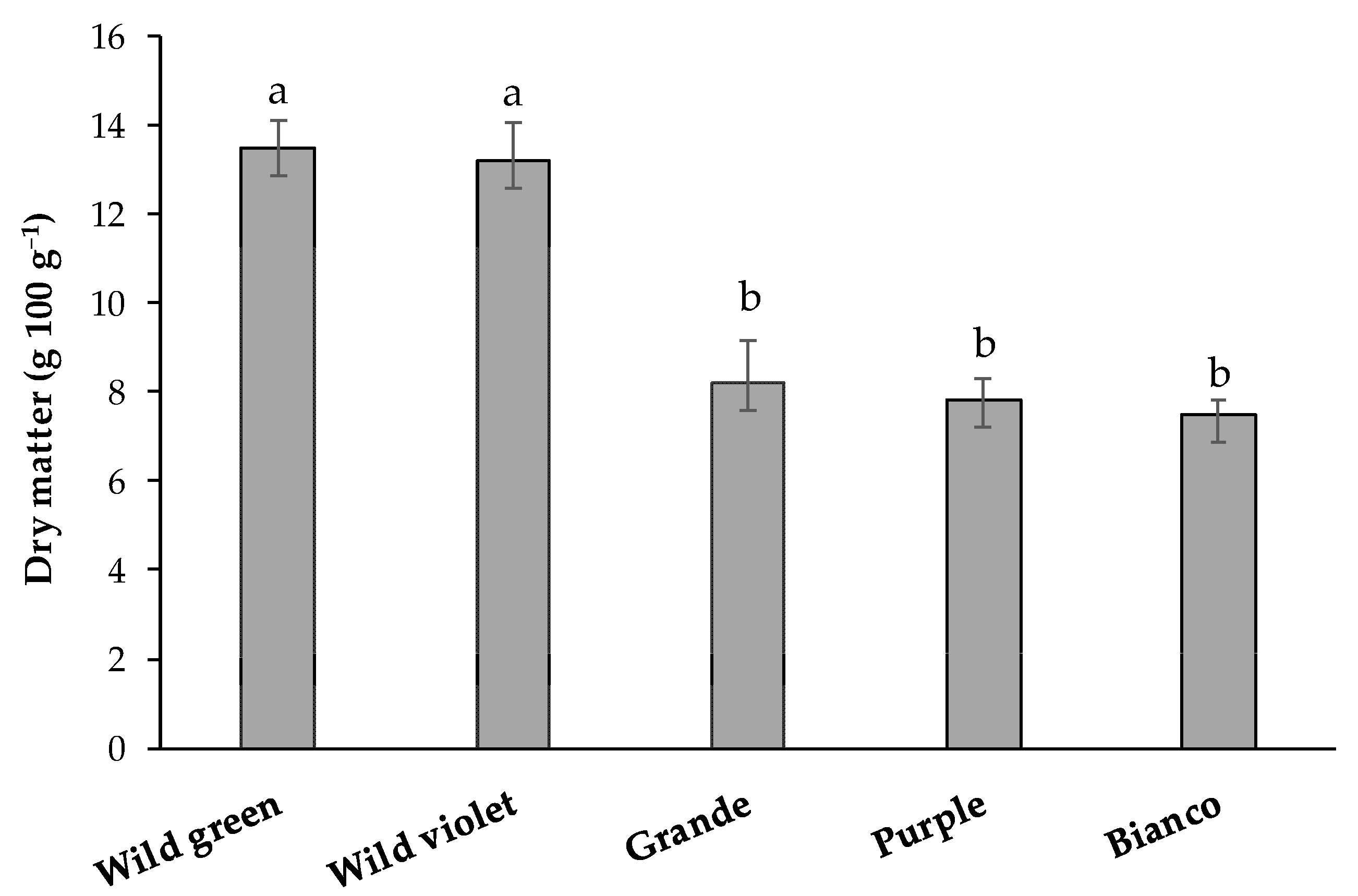
3.1. Dry Matter of Raw Asparagus and Weight Change after Steaming
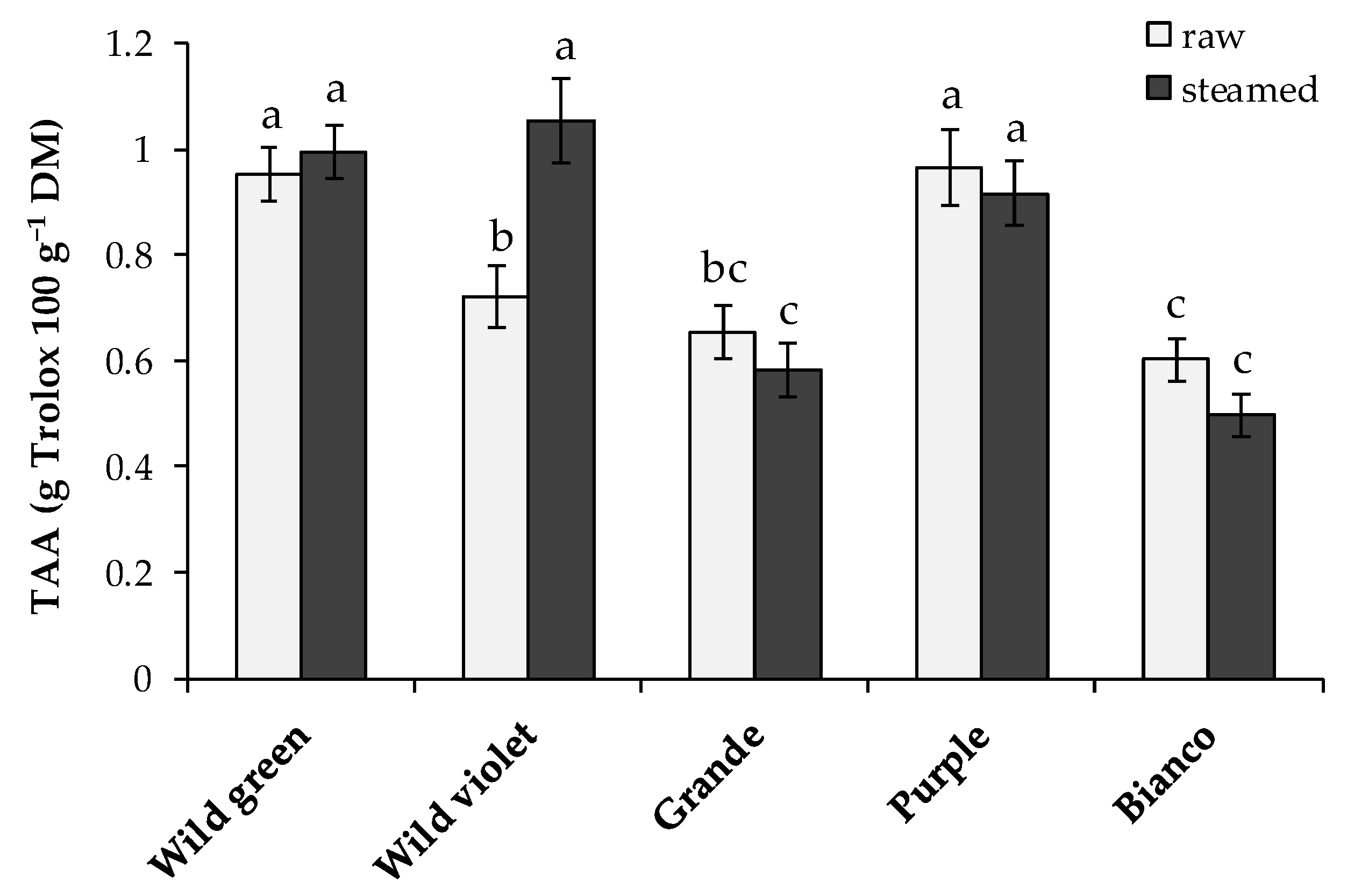
3.2. Antioxidant Activity and Phenol Content
3.3. Chlorophyll and Carotenoid Content
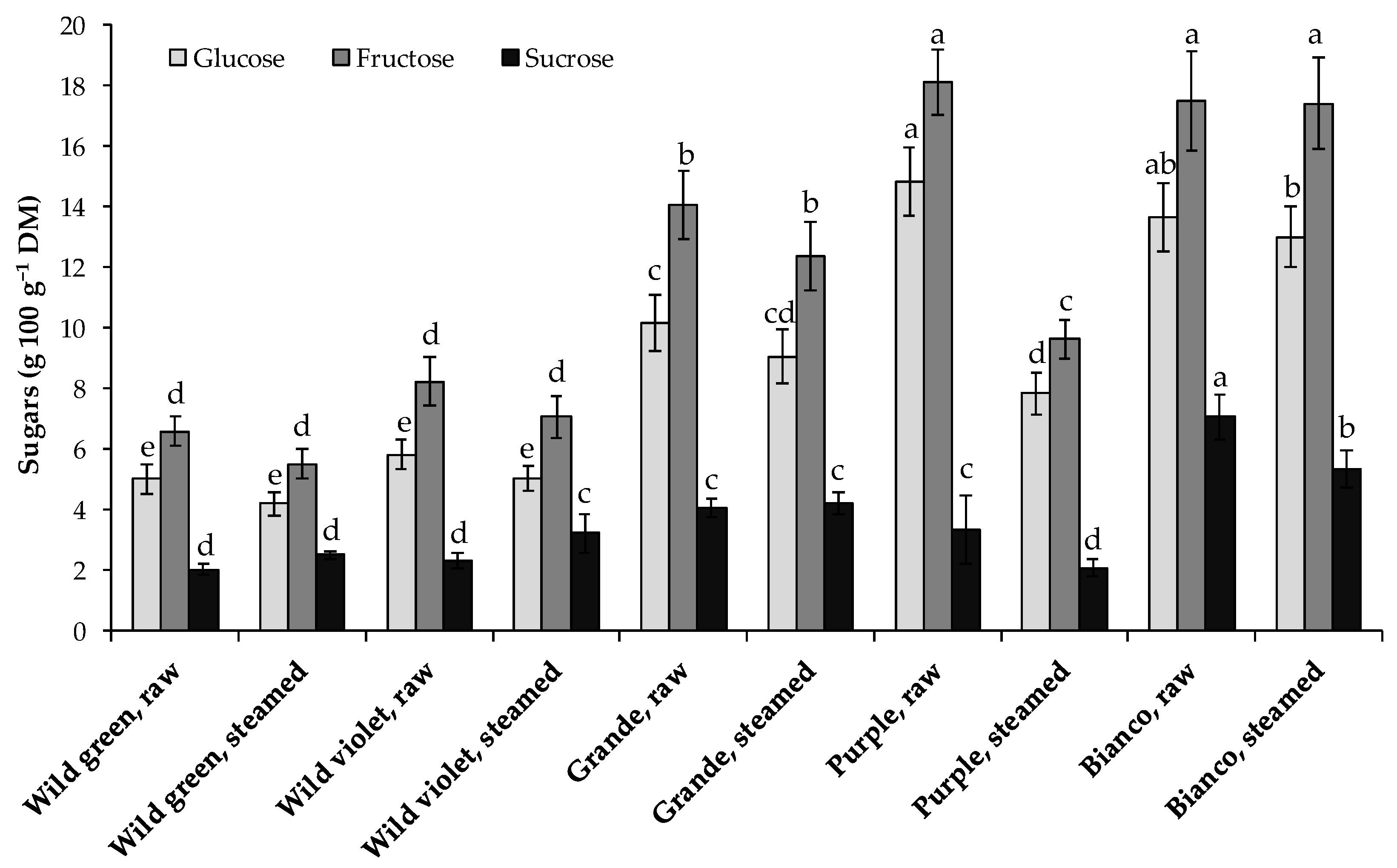
3.4. Sugar Content
3.5. Color Traits
3.6. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Herrera, P.; Morales, P.; Cámara, M.; Fernández-Ruiz, V.; Tardío, J.; Sánchez-Mata, M.C. Nutritional and phytochemical composition of mediterranean wild vegetables after culinary treatment. Foods 2020, 9, 1761. [Google Scholar] [CrossRef]
- Solana, M.; Boschiero, I.; Dall’Acqua, S.; Bertucco, A. A comparison between supercritical fluid and pressurized liquid extraction methods for obtaining phenolic compounds from Asparagus officinalis L. J. Supercrit. Fluids 2015, 100, 201–208. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.H.; Yu, I.H.; Gorinstein, S.; Bae, J.H.; Ku, Y.G. Bioactive compounds, antioxidant and binding activities and spear yield of Asparagus officinalis L. Plant Foods Hum. Nutr. 2014, 69, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L.; Dosi, R.; Di Maro, A.; Guida, V.; Cefarelli, G.; Pacifico, S.; Mastellone, C.; Fiorentino, A.; Rosati, A.; Parente, A. Nutritional values, metabolic profile and radical scavenging capacities of wild asparagus (A. acutifolius L.). J. Food Compos. Anal. 2011, 24, 326–333. [Google Scholar] [CrossRef]
- Słupski, J.; Korus, A.; Lisiewska, Z.; Kmiecik, W. Content of amino acids and the quality of protein in as-eaten green asparagus (Asparagus officinalis L.) products. Int. J. Food Sci. Technol. 2010, 45, 733–739. [Google Scholar] [CrossRef]
- Fuentes-Alventosa, J.M.; Rodríguez-Gutiérrez, G.; Jaramillo-Carmona, S.; Espejo-Calvo, J.A.; Rodríguez-Arcos, R.; Fernández-Bolaños, J.; Guillén-Bejarano, R.; Jiménez-Araujo, A. Effect of extraction method on chemical composition and functional characteristics of high dietary fibre powders obtained from asparagus by-products. Food Chem. 2009, 113, 665–671. [Google Scholar] [CrossRef]
- Sergio, L.; Cantore, V.; Spremulli, L.; Pinto, L.; Baruzzi, F.; Di Venere, D.; Boari, F. Effect of cooking and packaging conditions on quality of semi-dried green asparagus during cold storage. LWT-Food Sci. Technol. 2018, 89, 712–718. [Google Scholar] [CrossRef]
- Mazzeo, T.; Paciulli, M.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Ganino, T.; Pellegrini, N. Impact of the industrial freezing process on selected vegetables -Part II. Colour and bioactive compounds. Food Res. Int. 2015, 75, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Horvat, J.; Jakse, M.; Sircelj, H. Game of tones: Sugars, organic acids, and phenolics in green and purple asparagus (Asparagus officinalis L.) cultivars. Turk. J. Agric. For. 2018, 42, 55–66. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Waring, R.H. Asparagusic acid. Phytochemistry 2014, 97, 5–10. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhao, J.; Zhang, W.; Pang, X. Saponins extracted from by-product of Asparagus officinalis L. suppress tumour cell migration and invasion through targeting Rho GTPase signalling pathway. J. Sci. Food Agric. 2013, 93, 1492–1498. [Google Scholar] [CrossRef]
- Jaramillo, S.; Muriana, F.J.G.; Guillen, R.; Jimenez-Araujo, A.; Rodriguez-Arcos, R.; Lopez, S. Saponins from edible spears of wild asparagus inhibit AKT, p70S6K, and ERK signalling, and induce apoptosis through G0/G1 cell cycle arrest in human colon cancer HCT-116 cells. J. Funct. Foods 2016, 26, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Palfi, M.; Tomic-obrdalj, H.; Horvat, D. Healthy vegetables for healthy heart: Asparagus. Cardiol. Croat. 2014, 9, 142–149. [Google Scholar]
- Hafizur, R.M.; Kabir, N.; Chishti, S. Asparagus officinalis extract controls blood glucose by improving insulin secretion and β-cell function in streptozotocin-induced type 2 diabetic rats. Br. J. Nutr. 2012, 108, 1586–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarrera, P.M.; Savo, V. Perceived health properties of wild and cultivated food plants in local and popular traditions of Italy: A review. J. Ethnopharmacol. 2013, 146, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Ghirardini, M.P.; Carli, M.; del Vecchio, N.; Rovati, A.; Cova, O.; Valigi, F.; Agnetti, G.; Macconi, M.; Adamo, D.; Traina, M.; et al. The importance of a taste. A comparative study on wild food plant consumption in twenty-one local communities in Italy. J. Ethnobiol. Ethnomed. 2007, 3, 22. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Naranjo, R.; Guzmán-Troncoso, A.J.; Gómez-Melara, J. The persistence of wild edible plants in agroforestry systems: The case of wild asparagus in Southern Extremadura (Spain). Agrofor. Syst. 2020, 94, 2391–2400. [Google Scholar] [CrossRef]
- Maeda, T.; Honda, K.; Sonoda, T.; Motoki, S.; Inoue, K.; Suzuki, T.; Oosawa, K.; Suzuki, M. Light condition influences rutin and polyphenol contents in asparagus spears in the mother-fern culture system during the summer-autumn harvest. J. Jpn. Soc. Hortic. Sci. 2010, 79, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Lo Porto, C.; Sergio, L.; Boari, F.; Logrieco, A.F.; Cantore, V. Cold plasma pretreatment improves the germination of wild asparagus (Asparagus acutifolius L.) seeds. Sci. Hortic. 2019, 256, 108554. [Google Scholar] [CrossRef]
- Benincasa, P.; Tei, F.; Rosati, A. Plant density and genotype effects on wild asparagus (Asparagus acutifolius L.) spear yield and quality. HortScience 2007, 42, 1163–1166. [Google Scholar] [CrossRef] [Green Version]
- Conversa, G.; Elia, A. Effect of seed age, stratification, and soaking on germination of wild asparagus (Asparagus acutifolius L.). Sci. Hortic. 2009, 119, 241–245. [Google Scholar] [CrossRef]
- Conversa, G.; Lazzizera, C.; Elia, A. Effects of after-ripening, stratification and GA3 on dormancy release and on germination of wild asparagus (Asparagus acutifolius L.) seeds. Sci. Hortic. 2010, 125, 196–202. [Google Scholar] [CrossRef]
- Fanasca, S.; Rouphael, Y.; Venneria, E.; Azzini, E.; Durazzo, A.; Maiani, G. Antioxidant properties of raw and cooked spears of green asparagus cultivars. Int. J. Food Sci. Technol. 2009, 44, 1017–1023. [Google Scholar] [CrossRef]
- Sergio, L.; Gonnella, M.; Renna, M.; Linsalata, V.; Gatto, M.A.; Boari, F.; Di Venere, D. Biochemical traits of asparagus cultivars and quality changes in two differently coloured genotypes during cold storage. LWT 2019, 101, 427–434. [Google Scholar] [CrossRef]
- Boari, F.; Cefola, M.; Di Gioia, F.; Pace, B.; Serio, F.; Cantore, V. Effect of cooking methods on antioxidant activity and nitrate content of selected wild Mediterranean plants. Int. J. Food Sci. Nutr. 2013, 64, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Sergio, L.; Boari, F.; Pieralice, M.; Linsalata, V.; Cantore, V.; Di Venere, D. Bioactive phenolics and antioxidant capacity of some wild edible greens as affected by different cooking treatments. Foods 2020, 9, 1320. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Gatto, M.A.; Sergio, L.; Ippolito, A.; Di Venere, D. Phenolic extracts from wild edible plants to control postharvest diseases of sweet cherry fruit. Postharvest Biol. Technol. 2016, 120, 180–187. [Google Scholar] [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azinobis(3-ethylenebenzothiazoline-6- sulfonic acid radical cation decolorization assay. Methods Enzymol. 1998, 299, 379–389. [Google Scholar] [CrossRef]
- Gonnella, M.; Durante, M.; Caretto, S.; D’Imperio, M.; Renna, M. Quality assessment of ready-to-eat asparagus spears as affected by conventional and sous-vide cooking methods. LWT-Food Sci. Technol. 2018, 92, 161–168. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Di Maro, A.; Pacifico, S.; Fiorentino, A.; Galasso, S.; Gallicchio, M.; Guida, V.; Severino, V.; Monaco, P.; Parente, A. Raviscanina wild asparagus (Asparagus acutifolius L.): A nutritionally valuable crop with antioxidant and antiproliferative properties. Food Res. Int. 2013, 53, 180–188. [Google Scholar] [CrossRef]
- Papoulias, E.; Siomos, A.S.; Koukounaras, A.; Gerasopoulos, D.; Kazakis, E. Effects of genetic, pre- and post-harvest factors on phenolic content and antioxidant capacity of White Asparagus spears. Int. J. Mol. Sci. 2009, 10, 5370–5380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danowska-Oziewicz, M.; Narwojsz, A.; Draszanowska, A.; Marat, N. The effects of cooking method on selected quality traits of broccoli and green asparagus. Int. J. Food Sci. Technol. 2020, 55, 127–135. [Google Scholar] [CrossRef]
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Velioglu, Y.S. Effects of cooking methods on chlorophylls, pheophytins and colour of selected green vegetables. Int. J. Food Sci. Technol. 2006, 41, 281–288. [Google Scholar] [CrossRef]
- Tenorio, M.; Villanueva, M.; Sagardoy, M. Changes in carotenoids and chlorophylls in fresh green asparagus (Asparagus officinalis L.) stored under modified atmosphere packaging. J. Sci. Food Agric. 2004, 84, 357–365. [Google Scholar] [CrossRef]
- Pénicaud, C.; Achir, N.; Dhuique-Mayer, C.; Dornier, M.; Bohuon, P. Degradation of β-carotene during fruit and vegetable processing or storage: Reaction mechanisms and kinetic aspects: A review. Fruits 2011, 66, 417–440. [Google Scholar] [CrossRef] [Green Version]
- Granato, D.; Masson, M.L. Instrumental color and sensory acceptance of soy-based emulsions: A response surface approach. Ciênc. Tecnol. Aliment. 2010, 30, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Renna, M.; Pace, B.; Cefola, M.; Santamaria, P.; Serio, F.; Gonnella, M. Comparison of two jam making methods to preserve the quality of colored carrots. LWT-Food Sci. Technol. 2013, 53, 547–554. [Google Scholar] [CrossRef]
- Shin, S.; Bhowmik, S.R. Thermal kinetics of color changes in pea puree. J. Food Eng. 1995, 24, 77–86. [Google Scholar] [CrossRef]
- Lozano, J.E.; Ibarz, A. Colour changes in concentrated fruit pulp during heating at high temperatures. J. Food Eng. 1997, 31, 365–373. [Google Scholar] [CrossRef]
- Barrett, D.M.; Beaulieu, J.C.; Shewfelt, R. Color, flavor, texture, and nutritional quality of fresh-cut fruits and vegetables: Desirable levels, instrumental and sensory measurement, and the effects of processing. Crit. Rev. Food Sci. Nutr. 2010, 50, 369–389. [Google Scholar] [CrossRef] [PubMed]




| Genotype | Type | Value | Chlorogenic Acid | Chicoric Acid | Rutin | I-3-Rutinoside |
|---|---|---|---|---|---|---|
| (mg 100 g−1 DM) | ||||||
| Wild green | Raw | Mean | 66.76 b | 82.08 a | 80.41 e | 95.69 d |
| SD | 6.01 | 7.88 | 7.94 | 7.02 | ||
| Steamed | Mean | 77.11 b | 79.20 a | 116.32 d | 137.15 c | |
| SD | 6.95 | 6.9 | 9.88 | 10.03 | ||
| Wild violet | Raw | Mean | 70.16 b | 30.94 b | 203.03 c | 154.16 b |
| SD | 7.04 | 3.25 | 20.11 | 11.38 | ||
| Steamed | Mean | 84.63 a | 78.46 a | 220.19 bc | 206.42 a | |
| SD | 8.04 | 7.01 | 20.8 | 15.01 | ||
| ‘Grande’ | Raw | Mean | n.d. | n.d. | 241.17 b | tr. |
| SD | - | - | 22 | - | ||
| Steamed | Mean | n.d. | n.d. | 205.21 c | tr. | |
| SD | - | - | 15.15 | - | ||
| ‘Purple Passion’ | Raw | Mean | n.d. | n.d. | 310.40 a | tr. |
| SD | - | - | 24.2 | - | ||
| Steamed | Mean | n.d. | n.d. | 318.04 a | tr. | |
| SD | - | - | 24.75 | - | ||
| ’Bianco di Bassano del Grappa’ | Raw | Mean | n.d. | n.d. | n.d. | n.d. |
| SD | - | - | - | - | ||
| Steamed | Mean | n.d. | n.d. | n.d. | n.d. | |
| SD | - | - | - | - | ||
| Significance | * | *** | * | *** | ||
| Genotype | Type | Value | L | a* | b* | C | h° |
|---|---|---|---|---|---|---|---|
| Wild green | Raw | Mean | 46.94 d | −16.47 f | 32.42 a | 36.37 a | 116.92 b |
| SD | 0.94 | 0.76 | 0.13 | 0.46 | 0.98 | ||
| Steamed | Mean | 40.15 e | −14.03 e | 28.82 b | 32.06 b | 115.93 b | |
| SD | 1.43 | 1.41 | 1.53 | 1.96 | 1.23 | ||
| Wild violet | Raw | Mean | 26.77 g | 8.17 a | 9.16 f | 12.28 d | 48.27 e |
| SD | 1.57 | 0.11 | 0.34 | 0.31 | 0.83 | ||
| Steamed | Mean | 32.05 f | −10.80 d | 19.11 d | 21.95 c | 119.47 b | |
| SD | 0.92 | 0.12 | 0.34 | 0.33 | 0.37 | ||
| ‘Grande’ | Raw | Mean | 52.15 c | −16.12 f | 32.97 a | 36.69 a | 116.05 b |
| SD | 1.14 | 0.41 | 0.2 | 0.36 | 0.44 | ||
| Steamed | Mean | 41.71 e | −13.81 e | 27.34 c | 30.63 b | 116.78 b | |
| SD | 0.55 | 1.03 | 1.06 | 1.41 | 0.83 | ||
| ‘Purple Passion’ | Raw | Mean | 25.86g | 9.02 a | 1.81 h | 9.21 e | 11.46 f |
| SD | 0.43 | 0.86 | 0.34 | 1.38 | 2.71 | ||
| Steamed | Mean | 31.22 f | −3.43 c | 11.98 e | 12.47 d | 105.79 c | |
| SD | 1.25 | 1.04 | 1.16 | 1.38 | 3.29 | ||
| ‘Bianco di Bassano del Grappa’ | Raw | Mean | 78.85 a | −1.22 b | 11.23 e | 11.30 d | 96.21 d |
| SD | 1.06 | 0.13 | 0.23 | 0.22 | 0.79 | ||
| Steamed | Mean | 63.43 b | −4.34 c | 6.00 g | 7.40 f | 125.86 a | |
| SD | 1.1 | 0.19 | 0.15 | 0.23 | 0.53 | ||
| Significance | *** | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergio, L.; Boari, F.; Di Venere, D.; Gonnella, M.; Cantore, V.; Renna, M. Quality Evaluation of Wild and Cultivated Asparagus: A Comparison between Raw and Steamed Spears. Agriculture 2021, 11, 1213. https://doi.org/10.3390/agriculture11121213
Sergio L, Boari F, Di Venere D, Gonnella M, Cantore V, Renna M. Quality Evaluation of Wild and Cultivated Asparagus: A Comparison between Raw and Steamed Spears. Agriculture. 2021; 11(12):1213. https://doi.org/10.3390/agriculture11121213
Chicago/Turabian StyleSergio, Lucrezia, Francesca Boari, Donato Di Venere, Maria Gonnella, Vito Cantore, and Massimiliano Renna. 2021. "Quality Evaluation of Wild and Cultivated Asparagus: A Comparison between Raw and Steamed Spears" Agriculture 11, no. 12: 1213. https://doi.org/10.3390/agriculture11121213
APA StyleSergio, L., Boari, F., Di Venere, D., Gonnella, M., Cantore, V., & Renna, M. (2021). Quality Evaluation of Wild and Cultivated Asparagus: A Comparison between Raw and Steamed Spears. Agriculture, 11(12), 1213. https://doi.org/10.3390/agriculture11121213









