Quantitative Evaluation of Macro-Nutrient Uptake by Cassava in a Tropical Savanna Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Details
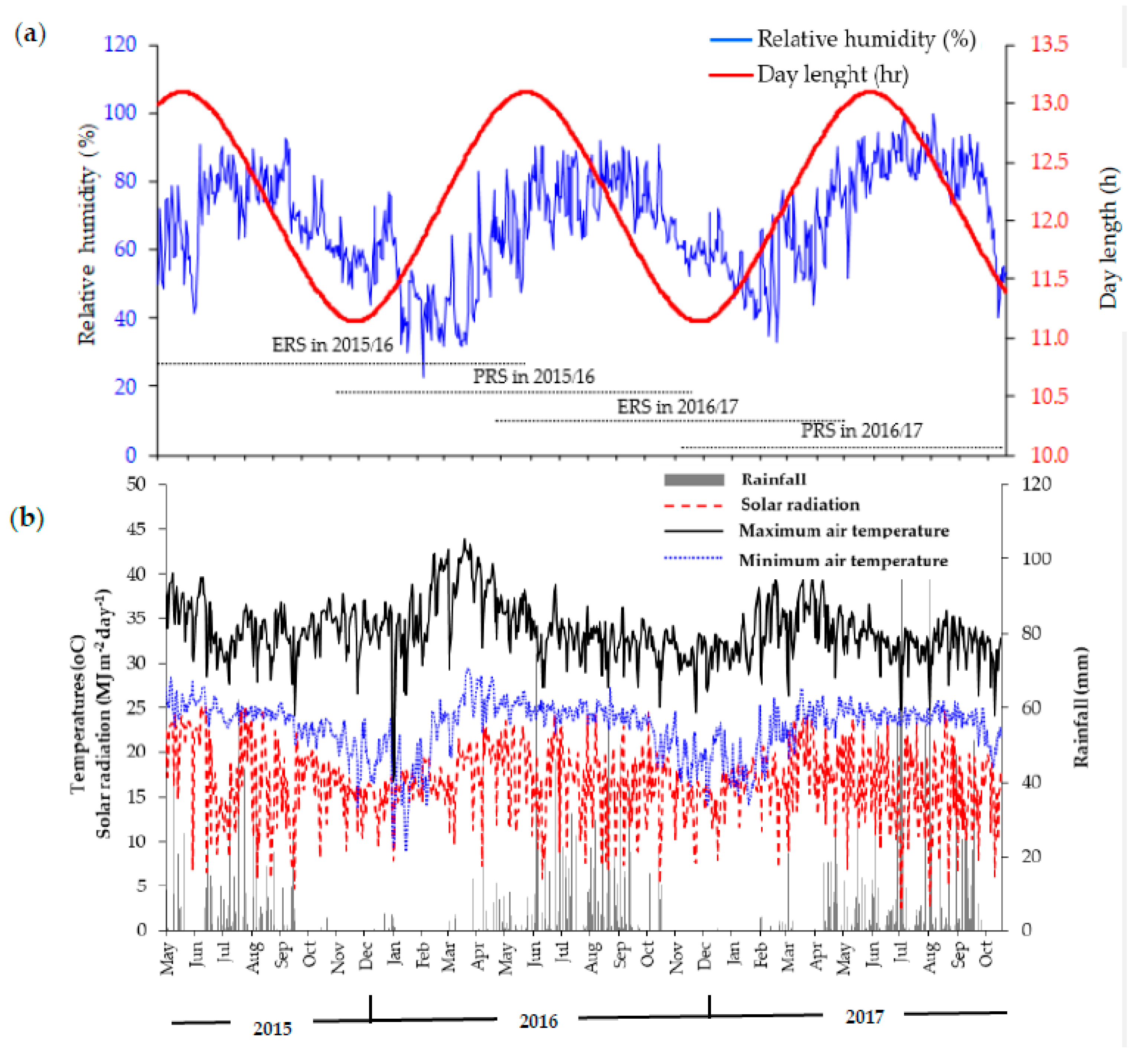
2.2. Description of the Study Site
2.3. Biomass Accumulation and Distribution
2.4. Sample Preparation and Nutrient Analysis
2.5. Statistical Analysis
3. Results
3.1. Combined Analysis of Variance
3.2. Total Nutrient Uptake, Nutrient Uptake by Storage Roots and Their Accumulation Rates
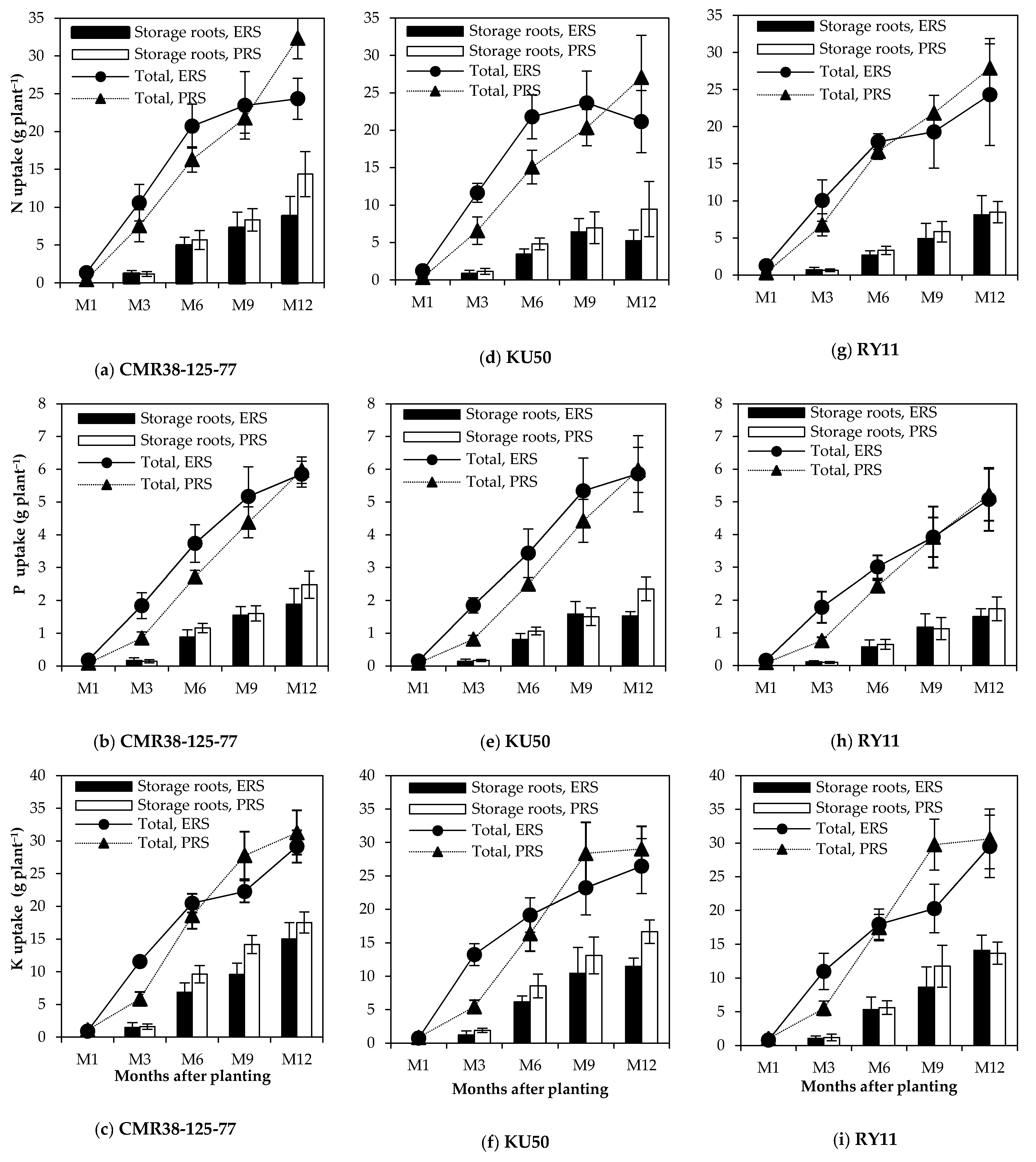
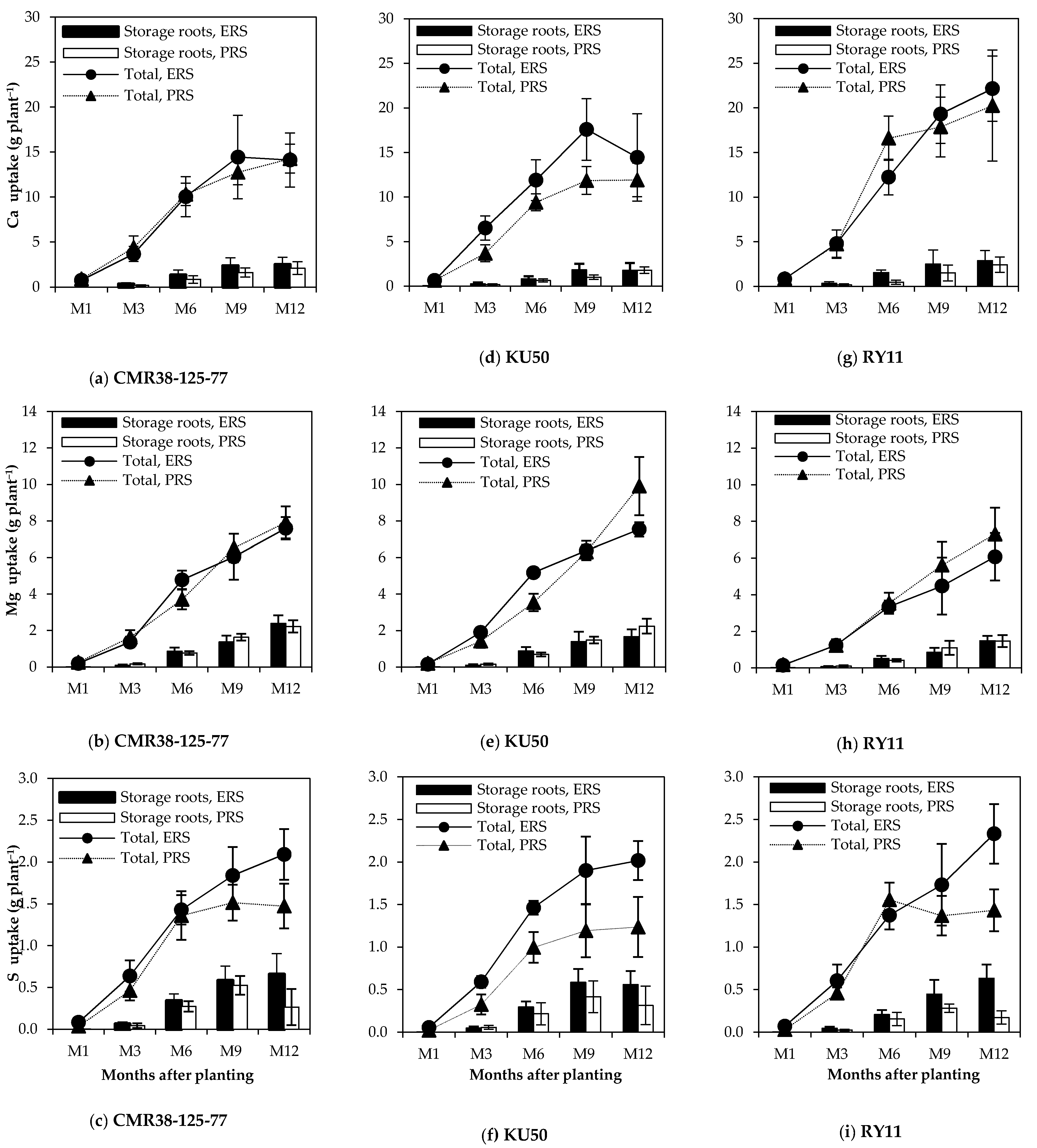
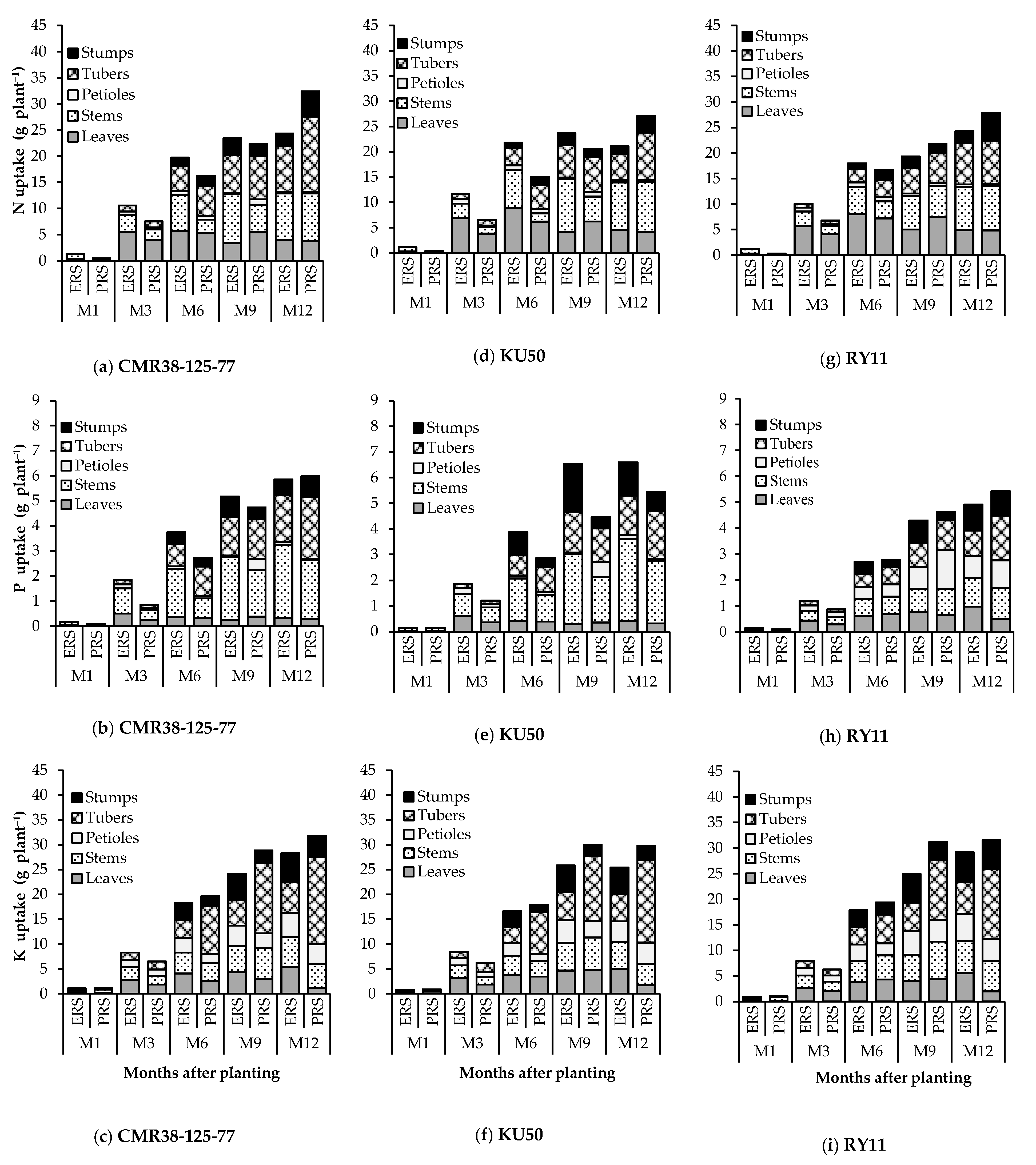
3.3. Nutrient Distribution and Nutrient Removal from the Soil by Storage Roots

3.4. Accumulation of Biomass, Storage Root Dry Weight and NUE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maung Aye, T.; Howeler, R.H. Integrated Crop Management for Cassava Cultivation in Asia. In Achieving Sustainable Cultivation of Cassava Volume 1: Cultivation Techniques; Burleigh Dodds: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Food and Agricultural Organization of the United Nations (FAO). Available online: http://faostat.fao.org (accessed on 4 September 2020).
- Geo-Informatics and Space Technology Development Agency (GISTDA). Available online: https://ecoplant.gistda.or.th/ (accessed on 10 August 2020).
- Akinwumiju, A.S.; Adelodun, A.A.; Orimoogunje, O.I. Agro-climato-Edaphic zonation of Nigeria for a cassava cultivar using GIS-based analysis of data from 1961 to 2017. Sci. Rep. 2020, 10, 1259. [Google Scholar] [CrossRef] [Green Version]
- Janket, A.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Banterng, P.; Kesmala, T.; Theerakulpisut, P.; Jogloy, S. Seasonal variation in starch accumulation and starch granule size in cassava genotypes in a tropical savanna climate. Agronomy 2018, 8, 297. [Google Scholar] [CrossRef] [Green Version]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. Growth rates and yields of cassava at different planting dates in a tropical savanna climate. Sci. Agric. 2019, 76, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. The impact of seasonal environments in a tropical savanna climate on forking, leaf area index, and biomass of cassava genotypes. Agronomy 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Vongcharoen, K.; Santanoo, S.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P. Seasonal variation in photosynthesis performance of cassava at two different growth stages under irrigated and rain-fed conditions in a tropical savanna climate. Photosynthetica 2018, 56, 1398–1413. [Google Scholar] [CrossRef]
- Wongnoi, S.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P. Physiology, growth and yield of different cassava genotypes planted in upland with dry environment during high storage root accumulation stage. Agronomy 2020, 10, 576. [Google Scholar] [CrossRef] [Green Version]
- Pugnaire, F.I.; Morillo, J.A.; Peñuelas, J.; Reich, P.B.; Bardgett, R.D.; Gaxiola, A.; Wardle, D.A.; Van Der Putten, W.H. Climate change effects on plant-soil feedbacks and consequences for biodiversity and functioning of terrestrial ecosystems. Sci. Adv. 2019, 5, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Sharkawy, M.A. International research on cassava photosynthesis, productivity, eco-physiology, and responses to environmental stresses in the tropics. Photosynthetica 2006, 44, 481–512. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015; p. 773. [Google Scholar]
- Fageria, N.K.; Baliger, V.C.; Jones, C.A. Growth and Mineral Nutrition of Field Crops; CRC Press: Boca Raton, FL, USA, 2010; p. 590. [Google Scholar]
- Proebsting, W.M.; Chaplin, M.H. The effect of photoperiod-induced flowering on the nutrient content of pea shoots. J. Plant Nutr. 1981, 4, 419–429. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Lam, S.K.; Zhang, X.; Liu, X.; Pan, G. Changes in nutrient uptake and utilization by rice under simulated climate change conditions: A 2–year experiment in a paddy field. Agric. For. Meteorol. 2018, 250, 202–208. [Google Scholar] [CrossRef]
- Suzuki, M.; Umeda, H.; Matsuo, S.; Kawasaki, Y.; Ahn, D.; Hamamoto, H.; Iwasaki, Y. Effects of relative humidity and nutrient supply on growth and nutrient uptake in greenhouse tomato production. Sci. Hort. 2015, 187, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Howeler, R.H. Cassava mineral nutrition and fertilization. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 115–147. [Google Scholar]
- Howeler, R.H.; Cadavid, L.F. Accumulation and distribution of dry matter and nutrients during a 12-month growth cycle of cassava. Field Crop. Res. 1983, 7, 123–139. [Google Scholar] [CrossRef]
- Byju, G.; Suja, G. Chapter five-mineral nutrition of cassava. Adv. Agron. 1983, 159, 169–235. [Google Scholar] [CrossRef]
- Alves, A.A.C. Cassava botany and physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 66–89. [Google Scholar]
- Janket, A.; Jogloy, S.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Theerakulpisut, P.; Holbrook, C.C.; Kvien, C.K.; Banterng, P. Nutrient uptake and nutrient use efficiency of cassava genotypes with different starch bulking periods as affected by different planting dates. J. Plant Nutr. 2020, 44, 580–599. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons: New York, NY, USA, 1984; pp. 1–657. [Google Scholar]
- Polthanee, A.; Srisutham, M. Supplementary irrigation for cassava planted in the late rainy season of Northeastern Thailand. Asian J. Crop Sci. 2017, 9, 100–108. [Google Scholar] [CrossRef]
- Janket, A.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Theerakulpisut, P.; Holbrook, C.C.; Kvien, C.K.; Jogloy, S.; Banterng, P. Accumulation dynamics of starch and its granule size distribution of cassava genotypes at different growing seasons. Agriculture 2020, 10, 380. [Google Scholar] [CrossRef]
- Shrivastav, P.; Prasad, M.; Singh, T.B.; Yadav, A.; Goyal, D.; Ali, A.; Dantu, P.K. Role of Nutrients in Plant Growth and Development. In Contaminants in Agriculture; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2002; pp. 43–59. [Google Scholar] [CrossRef]
- Rietra, R.P.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef] [Green Version]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2016, 66, 1219–1229. [Google Scholar] [CrossRef]
- Mengel, K.; Kirkby, E.A. Principles of Plant Nutrition; International Potash Institute: Bern, Switzerland, 1987. [Google Scholar]
- Gunes, A.; Inal, A.; Aplaslan, M.; Cakmak, I. Genotypic variation in phosphorus efficiency between wheat cultivars grown under greenhouse and field conditions. Soil Sci. Plant Nutr. 2016, 52, 470–478. [Google Scholar] [CrossRef] [Green Version]
- Putthacharoen, S.; Howeler, R.H.; Jantawat, S.; Vichukit, V. Nutrient uptake and soil erosion losses in cassava and six other crops in a Psamment in eastern Thailand. Field Crop. Res. 1998, 57, 113–126. [Google Scholar] [CrossRef]
- Prajapati, K.; Mod, H.A. The importance of potassium in plant growth—A review. Indian J. Plant Sci. 2012, 1, 177–186. [Google Scholar]
- Bender, R.R.; Haegele, J.W.; Ruffo, M.L.; Below, F.E. Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agron. J. 2013, 105, 161–170. [Google Scholar] [CrossRef] [Green Version]
- Ezui, K.S.; Franke, A.C.; Mando, A.; Ahiabor, B.D.K.; Tetteh, F.M.; Sogbedji, J.; Janssen, B.H.; Giller, K.E. Fertilizer requirements for balanced nutrition of cassava across eight locations in West Africa. Field Crop Res. 2016, 185, 69–78. [Google Scholar] [CrossRef] [Green Version]
- De Tafur, S.M.; El-Sharkawy, M.A.; Calle, F. Photosynthesis and yield performance of cassava in seasonally dry and semiarid environments. Photosynthetica 1997, 33, 249–257. [Google Scholar] [CrossRef]
- Santanoo, S.; Vongcharoen, K.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Roytrakul, S.; Theerakulpisut, P. Seasonal variation in diurnal photosynthesis and chlorophyll fluorescence of four genotypes of cassava (Manihot esculenta Crantz) under irrigation conditions in a tropical savanna climate. Agronomy 2019, 9, 206. [Google Scholar] [CrossRef] [Green Version]
- Cock, J.H.; Porto, M.C.M.; El-Sharkawy, M.A. Water use efficiency of cassava. III. Influence of air humidity and water stress on gas exchange of field grown cassava. Crop Sci. 1985, 25, 265272. [Google Scholar] [CrossRef]
- Keating, B.A.; Evenson, J.P.; Fukai, S. Environment effects on growth and development of cassava (Manihot esculenta Crantz) III. Assimilate distribution and storage organ yield. Field Crops Res. 1982, 5, 293–303. [Google Scholar] [CrossRef]
| Parameters | Digestion Methods | Equipment/Methods |
|---|---|---|
| Soil analysis | ||
| Total nitrogen (%) | Micro-Kjeldahl method (Sulfuric acid; analytical reagent, Merck, Germany) | Auto-Analyzer 3 (Auto Analyzer 3, SEAL Analytical, Norderstedt, Germany) |
| Available phosphorus (mg kg−1) | Bray II (Ammonium fluoride and hydrochloric acid; analytical reagent, Sigma-Aldrich, Darmstadt, Germany) | Spectrophotometer (U-2900UV/VIS, Hitachi, Tokyo, Japan) |
| Exchangeable potassium (mg kg−1) | 1N ammonium acetate (Analytical reagent, Sigma-Aldrich, Darmstadt, Germany) | Flame photometer (M410 flame photometer, Sherwood, Cambridge, UK) |
| Exchangeable calcium (mg kg−1) | 1N ammonium acetate (Analytical reagent, Sigma-Aldrich, Darmstadt, Germany) | Flame photometer (M410 flame photometer, Sherwood, Cambridge, UK) |
| Exchangeable magnesium (mg kg−1) | 1N Ammonium acetate (Analytical reagent, Sigma-Aldrich, Darmstadt, Germany) | Atomic Absorption Spectrophotometer (novAA 350, Analytik Jena, Münster, Germany) |
| Available sulfur (mg kg−1) | Calcium dihydrogen phosphate (Analytical reagent, Sigma-Aldrich, Darmstadt, Germany) | Spectrophotometer (U-2900UV/VIS, Hitachi, Tokyo, Japan) |
| pH | 1:1 H2O | pH meter (F20 Fiveeasy, Mettler-Toledo International Inc., Columbus, OH, USA) |
| Electrical conductivity (dS m−1) | 1:5 H2O | Conductivity meter (Seven Compact S230, Mettler-Toledo International Inc., Columbus, OH, USA) |
| Organic matter (%) | Walkley and Black | |
| Cation exchange capacity (cmol kg−1) | 1N Ammonium acetate/distillation (Analytical reagent, Sigma-Aldrich, Darmstadt, Germany) | |
| Soil texture | Hydrometer | |
| Plant nutrient analysis | ||
| Total nitrogen (%) | Micro-Kjeldahl method (Sulfuric acid; analytical reagent, Merck, Germany) | Auto-Analyzer 3 (Auto Analyzer 3, SEAL Analytical, Norderstedt, Germany) |
| Total phosphorus (%) | Wet oxidation Nitric acid and perchloric acid (analytical reagent, Qrec, New Zealand) | Spectrophotometer (U-2900UV/VIS, Hitachi, Tokyo, Japan) |
| Total potassium (%) | Wet oxidation Nitric acid and perchloric acid (analytical reagent, Qrec, New Zealand) | Flame photometer (M410 flame photometer, Sherwood, Cambridge, UK) |
| Total calcium (%) | Wet oxidation Nitric acid and perchloric acid (analytical reagent, Qrec, New Zealand) | Atomic Absorption Spectrophotometer (novAA 350, Analytik Jena, Münster, Germany) |
| Total magnesium (%) | Wet oxidation Nitric acid and perchloric acid (analytical reagent, Qrec, New Zealand) | Atomic Absorption Spectrophotometer (novAA 350, Analytik Jena, Münster, Germany) |
| Total sulfur (%) | Wet oxidation Nitric acid and perchloric acid (analytical reagent, Qrec, New Zealand) | Spectrophotometer (U-2900UV/VIS, Hitachi, Tokyo, Japan) |
| Soil Physicochemical Properties | ERS in 2015/16 | PRS in 2015/16 | ERS in 2016/17 | PRS in 2016/17 | ||||
|---|---|---|---|---|---|---|---|---|
| 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | 0–30 cm | 30–60 cm | |
| Physical Properties | ||||||||
| Sand (%) | 84.9 | 83.9 | 87.4 | 82.4 | 92.9 | 93.8 | 85 | 76 |
| Silt (%) | 6.8 | 7.9 | 9.9 | 9.0 | 5.9 | 5.1 | 8.9 | 9.9 |
| Clay (%) | 8.3 | 8.2 | 2.7 | 8.6 | 1.2 | 1.1 | 6.1 | 14.1 |
| USDA classification | Loamy sand | Loamy sand | Sand | Loamy sand | ||||
| Chemical Properties at Pre-planting | ||||||||
| Total N (%) | 0.02 | 0.02 | 0.02 | 0.01 | 0.03 | 0.02 | 0.03 | 0.03 |
| Available P (mg kg−1) | 85.6 | 83.0 | 56.0 | 40.7 | 82.7 | 76.8 | 85.1 | 42.1 |
| Exchangeable K (mg kg−1) | 42.7 | 44.9 | 34.7 | 34.5 | 33.5 | 33.9 | 33.5 | 30.7 |
| Exchangeable Ca (mg kg−1) | 415 | 450 | 255 | 270 | 367 | 356 | 380 | 444 |
| Exchangeable Mg (mg kg−1) | 30.0 | 31.4 | 34.2 | 33.5 | 52.3 | 43.6 | 43.1 | 39.2 |
| Available S (mg kg−1) | 54.8 | 45.2 | 43.2 | 49.6 | 41.2 | 41.4 | 39.5 | 43.9 |
| pH (1:1 H2O) | 7.4 | 7.5 | 6.3 | 5.9 | 7.3 | 7.4 | 6.9 | 7.4 |
| EC (dS m−1) | 0.05 | 0.07 | 0.08 | 0.02 | 0.06 | 0.05 | 0.05 | 0.03 |
| OM (%) | 0.42 | 0.44 | 0.45 | 0.31 | 0.51 | 0.44 | 0.51 | 0.32 |
| CEC (cmolc kg−1) | 3.9 | 4.1 | 5.0 | 6.1 | 2.9 | 3.9 | 2.8 | 5.6 |
| Chemical Properties at Post-planting | ||||||||
| Total N (%) | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.03 | 0.03 |
| Available P (mg kg−1) | 85.9 | 81.3 | 44.5 | 37.6 | 62.3 | 52.4 | 81.2 | 35.1 |
| Exchangeable K (mg kg−1) | 34.4 | 28.9 | 37.3 | 30.3 | 28.7 | 25.0 | 28.1 | 28.9 |
| Exchangeable Ca (mg kg−1) | 434 | 429 | 241 | 312 | 406 | 410 | 355 | 400 |
| Exchangeable Mg (mg kg−1) | 40.5 | 41.1 | 39.5 | 38.7 | 39.7 | 39.1 | 47.2 | 47.1 |
| Available S (mg kg−1) | 44.6 | 33.4 | 47.9 | 47.4 | 43.9 | 41.9 | 39.1 | 42.7 |
| pH (1:1 H2O) | 7.1 | 7.0 | 5.8 | 6.2 | 6.9 | 6.8 | 7.1 | 7.2 |
| EC (dS m−1) | 0.05 | 0.05 | 0.03 | 0.03 | 0.04 | 0.03 | 0.03 | 0.02 |
| OM (%) | 0.50 | 0.47 | 0.39 | 0.31 | 0.34 | 0.26 | 0.49 | 0.27 |
| CEC (cmolc kg−1) | 3.3 | 3.7 | 3.5 | 5.6 | 4.4 | 5.1 | 3.3 | 5.3 |
| MAP | N | F-Test | P | F-Test | K | F-Test | Ca | F-Test | Mg | F-Test | S | F-Test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERS | PRS | ERS | PRS | ERS | PRS | ERS | PRS | ERS | PRS | ERS | PRS | |||||||
| g accumulation m−2 day−1 | ||||||||||||||||||
| CMR38-125-77 | ||||||||||||||||||
| 1–3 | 0.15 a | 0.12 a | * | 0.03 a | 0.01 | * | 0.18 a | 0.08 b | ** | 0.05 b | 0.06 a | ns | 0.02 b | 0.02 b | ns | 0.01 | 0.01 | ns |
| 3–6 | 0.11 a | 0.10 a | ns | 0.02 a | 0.02 | ns | 0.10 b | 0.14 a | * | 0.07 a | 0.07 a | ns | 0.04 a | 0.02 b | * | 0.01 | 0.01 | ns |
| 6–9 | 0.03 b | 0.06 b | * | 0.02 b | 0.02 | ns | 0.02 c | 0.10 a | ** | 0.05 b | 0.03 b | * | 0.01 b | 0.03 a | ** | 0.01 | 0.00 | ns |
| 9–12 | 0.01 b | 0.12 a | ** | 0.01 c | 0.02 | ** | 0.08 b | 0.04 c | ** | −0.00 c | 0.02 b | * | 0.02 b | 0.02 b | ns | 0.00 | −0.00 | ns |
| KU50 | ||||||||||||||||||
| 1–3 | 0.17 a | 0.10 a | * | 0.03 a | 0.01 b | ** | 0.21 a | 0.08 b | ** | 0.10 a | 0.05 a | ns | 0.03 a | 0.02 b | ns | 0.01 | 0.01 | ns |
| 3–6 | 0.11 b | 0.09 a | ns | 0.02 b | 0.02 a | ns | 0.07 b | 0.12 a | ** | 0.06 b | 0.06 a | ns | 0.04 a | 0.02 b | * | 0.01 | 0.01 | ns |
| 6–9 | 0.02 c | 0.06 b | * | 0.02 a | 0.02 a | ns | 0.05 b | 0.13 a | ** | 0.06 b | 0.03 b | * | 0.01 b | 0.03 a | ** | 0.01 | 0.00 | ns |
| 9–12 | −0.03 c | 0.08 b | ** | 0.01 c | 0.02 a | * | 0.04 b | 0.01 c | * | −0.04 c | 0.00 c | ns | 0.01 b | 0.04 a | * | 0.00 | 0.00 | ns |
| RY11 | ||||||||||||||||||
| 1–3 | 0.15 a | 0.11 a | * | 0.03 a | 0.01 c | * | 0.17 a | 0.08 b | * | 0.07 a | 0.06 b | ns | 0.02 | 0.02 | ns | 0.01 | 0.01 | ns |
| 3–6 | 0.09 b | 0.10 a | ns | 0.01 b | 0.02 a | ns | 0.08 b | 0.13 a | * | 0.08 a | 0.13 a | * | 0.02 | 0.03 | ns | 0.01 | 0.01 | ns |
| 6–9 | 0.02 c | 0.03 b | * | 0.01 b | 0.02 ab | * | 0.03 b | 0.14 a | ** | 0.08 a | 0.01 c | ** | 0.01 | 0.02 | * | 0.00 | −0.00 | ns |
| 9–12 | 0.06 c | 0.13 a | ** | 0.01 b | 0.01 b | ns | 0.10 b | 0.01 c | * | 0.03 b | 0.03 c | ns | 0.02 | 0.02 | ns | 0.01 | 0.00 | ns |
| MAP | N | F-Test | P | F-Test | K | F-Test | Ca | F-Test | Mg | F-Test | S | F-Test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERS | PRS | ERS | PRS | ERS | PRS | ERS | PRS | ERS | PRS | ERS | PRS | |||||||
| CMR38-125-77 | ||||||||||||||||||
| 3 | 10.9 c | 15.3 c | * | 9.4 c | 17.1 b | ** | 12.7 d | 27.5 b | ** | 8.8 c | 4.1 b | * | 6.7 c | 10.4 b | ns | 9.9 c | 9.4 c | ns |
| 6 | 23.7 b | 34.8 b | ** | 23.7 b | 42.7 a | ** | 33.5 c | 51.8 a | ** | 13.3 b | 8.2 b | * | 18.1 b | 20.7 ab | ns | 23.9 b | 20.1 b | ns |
| 9 | 30.8 a | 38.3 a | * | 30.0 a | 36.6 a | ** | 43.2 b | 51.0 a | * | 16.3 a | 12.7 a | * | 22.8 b | 25.0 a | ns | 31.7 a | 34.7 a | ns |
| 12 | 36.1 a | 44.4 a | * | 32.3 a | 41.6 a | ** | 51.5 a | 56.0 a | ns | 17.6 a | 14.8 a | * | 31.5 a | 28.1 a | ns | 31.5 a | 17.9 b | ** |
| KU50 | ||||||||||||||||||
| 3 | 7.7 c | 17.4 b | ** | 7.8 c | 20.6 c | ** | 9.2 c | 34.6 b | ** | 4.0 b | 5.0 b | ns | 5.6 b | 11.2 b | * | 8.3 c | 17.4 c | ** |
| 6 | 15.8 b | 32.0 a | ** | 23.7 b | 42.6 a | ** | 32.2 b | 52.2 a | ** | 7.0 b | 6.9 b | ns | 17.0 ab | 19.7 ab | ns | 20.2 b | 21.7 b | ns |
| 9 | 27.2 a | 34.3 a | * | 29.6 a | 34.0 b | * | 45.0 a | 46.2 a | * | 10.5 a | 8.5 ab | ns | 22.0 a | 23.5 a | ns | 30.9 a | 34.9 a | ns |
| 12 | 24.7 a | 34.9 a | * | 26.0 a | 39.4 a | * | 43.4 a | 57.3 a | ** | 12.4 a | 15.1 a | * | 22.2 a | 22.6 a | ns | 27.7 a | 25.4 b | ns |
| RY11 | ||||||||||||||||||
| 3 | 7.27 d | 9.5 d | ns | 6.3 d | 12.5 b | * | 9.7 c | 21.6 c | ** | 7.0 b | 3.6 b | * | 5.8 c | 8.1 c | ns | 7.6 c | 5.4 c | ns |
| 6 | 14.93 c | 19.9 c | * | 19.0 c | 26.6 a | * | 29.6 b | 32.1 b | ns | 12.7 a | 2.8 b | ** | 15.3 b | 11.9 b | ns | 15.1 b | 9.9 b | ** |
| 9 | 25.49 b | 26.8 b | ns | 30.0 a | 28.9 a | ns | 42.7 a | 39.5 a | ns | 13.0 a | 8.4 ab | * | 19.0 ab | 19.6 a | ns | 25.8 a | 20.5 a | ns |
| 12 | 33.36 a | 30.4 a | ns | 29.6 a | 33.3 a | * | 47.8 a | 44.7 a | ns | 13.1 a | 12.0 a | * | 24.5 a | 20.2 a | ns | 27.2 a | 12.0 b | ** |
| Genotypes | Storage Root Dry Weight (g m−2) | Biomass (g m−2) | ||||
|---|---|---|---|---|---|---|
| ERS | PRS | F-Test | ERS | PRS | F-Test | |
| CMR38-125-77 | 1810 a | 2253 a | ** | 3280 a | 3824 a | ** |
| KU50 | 1244 b | 2113 a | ** | 2831 b | 3807 a | ** |
| RY11 | 1597 ab | 1604 b | ns | 3046 ab | 3353 b | * |
| Mean | 1550 | 1990 | ** | 3052 | 3661 | ** |
| Element | Genotypes | NUE for Storage Roots | NUE for Biomass | ||||
|---|---|---|---|---|---|---|---|
| ERS | PRS | F-Test | ERS | PRS | F-Test | ||
| N | CMR38-125-77 | 74 a | 70 b | ns | 135 a | 120 | ns |
| KU50 | 59 b | 78 b | * | 113 a | 141 | * | |
| RY11 | 66 ab | 125 a | ** | 58 b | 120 | * | |
| P | CMR38-125-77 | 309 a | 337 a | * | 561 a | 641 | ** |
| KU50 | 212 b | 353 a | ** | 406 b | 637 | ** | |
| RY11 | 315 a | 307 b | ns | 600 a | 642 | * | |
| K | CMR38-125-77 | 62 a | 72 a | * | 112 a | 122 | * |
| KU50 | 47 b | 73 a | ** | 90 b | 131 | * | |
| RY11 | 54 b | 52 b | ns | 103 b | 110 | ns | |
| Ca | CMR38-125-77 | 128 a | 158 a | * | 232 a | 268 a | * |
| KU50 | 86 b | 177 a | ** | 165 b | 319 a | ** | |
| RY11 | 72 b | 79 b | ns | 138 b | 166 b | * | |
| Mg | CMR38-125-77 | 238 a | 285 a | * | 432 a | 483 a | * |
| KU50 | 165 b | 213 b | ** | 316 b | 384 b | * | |
| RY11 | 253 a | 220 b | ns | 499 a | 469 a | ns | |
| S | CMR38-125-77 | 865 a | 1529 | ** | 1568 a | 2595 b | ** |
| KU50 | 617 b | 1708 | ** | 1181 b | 3078 a | ** | |
| RY11 | 685 b | 1120 | ** | 1306 b | 2340 b | ** | |
| Nutrient | Early Rainy Season | Post Rainy Season | ||||
|---|---|---|---|---|---|---|
| CMR38-125-77 | KU50 | RY11 | CMR38-125-77 | KU50 | RY11 | |
| N/K | 0.83 | 0.80 | 0.82 | 1.03 | 0.90 | 0.91 |
| P/K | 0.20 | 0.22 | 0.17 | 0.19 | 0.21 | 0.17 |
| S/P | 0.36 | 0.34 | 0.46 | 0.25 | 0.21 | 0.27 |
| Mg/K | 0.26 | 0.28 | 0.21 | 0.25 | 0.34 | 0.24 |
| Ca/Mg | 1.86 | 1.92 | 3.65 | 1.80 | 1.20 | 2.77 |
| K/Ca | 2.07 | 1.83 | 1.33 | 2.19 | 2.44 | 1.51 |
| P/Ca | 0.41 | 0.41 | 0.23 | 0.42 | 0.50 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janket, A.; Vorasoot, N.; Toomsan, B.; Kaewpradit, W.; Theerakulpisut, P.; Holbrook, C.C.; Kvien, C.K.; Jogloy, S.; Banterng, P. Quantitative Evaluation of Macro-Nutrient Uptake by Cassava in a Tropical Savanna Climate. Agriculture 2021, 11, 1199. https://doi.org/10.3390/agriculture11121199
Janket A, Vorasoot N, Toomsan B, Kaewpradit W, Theerakulpisut P, Holbrook CC, Kvien CK, Jogloy S, Banterng P. Quantitative Evaluation of Macro-Nutrient Uptake by Cassava in a Tropical Savanna Climate. Agriculture. 2021; 11(12):1199. https://doi.org/10.3390/agriculture11121199
Chicago/Turabian StyleJanket, Anon, Nimitr Vorasoot, Banyong Toomsan, Wanwipa Kaewpradit, Piyada Theerakulpisut, Carl Corley Holbrook, Craig K. Kvien, Sanun Jogloy, and Poramate Banterng. 2021. "Quantitative Evaluation of Macro-Nutrient Uptake by Cassava in a Tropical Savanna Climate" Agriculture 11, no. 12: 1199. https://doi.org/10.3390/agriculture11121199
APA StyleJanket, A., Vorasoot, N., Toomsan, B., Kaewpradit, W., Theerakulpisut, P., Holbrook, C. C., Kvien, C. K., Jogloy, S., & Banterng, P. (2021). Quantitative Evaluation of Macro-Nutrient Uptake by Cassava in a Tropical Savanna Climate. Agriculture, 11(12), 1199. https://doi.org/10.3390/agriculture11121199






