Arbuscular Mycorrhizal Communities in the Roots of Sago Palm in Mineral and Shallow Peat Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Soil Physicochemical Properties
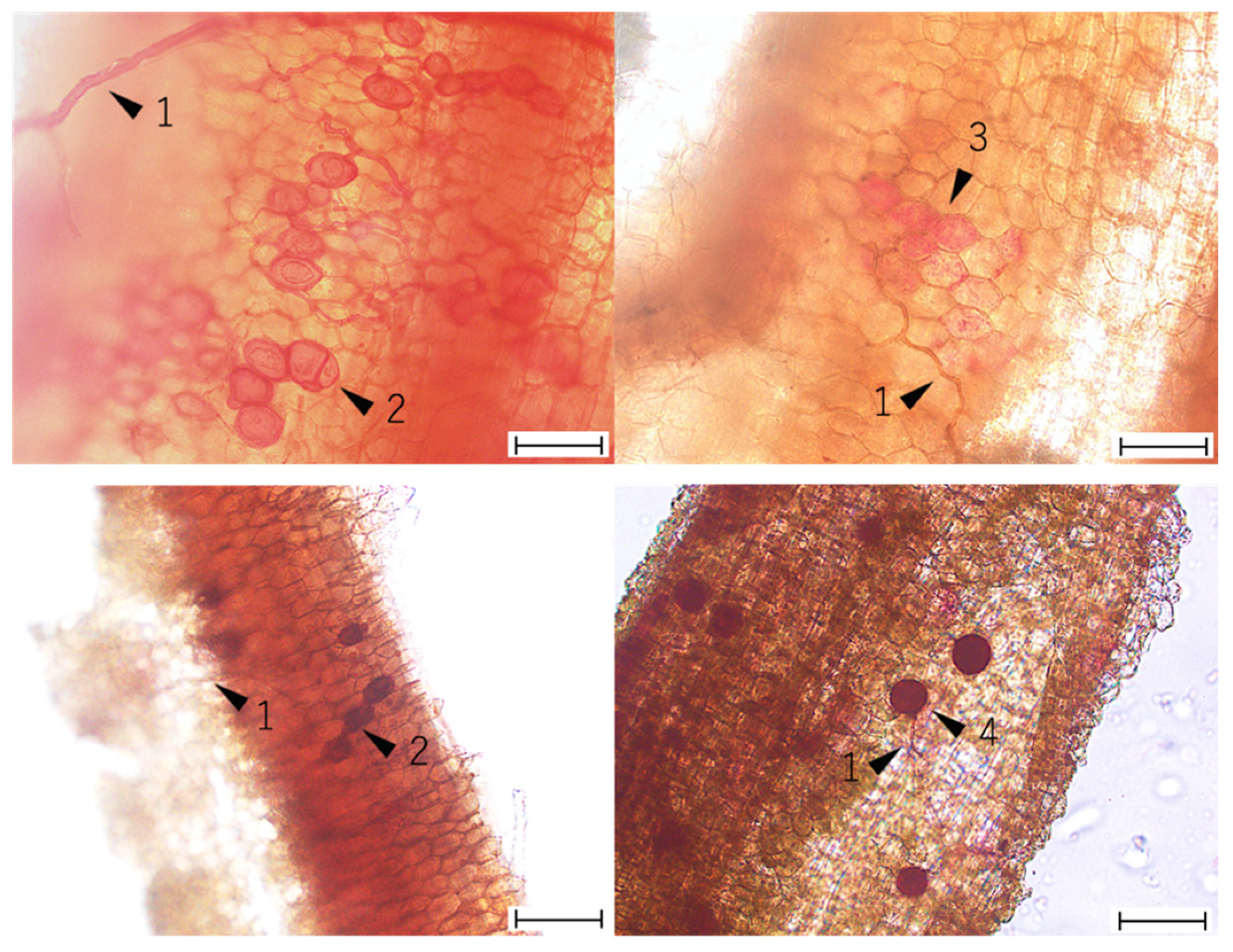
2.3. Visual Assessment of AMF Colonization
2.4. DNA Extraction and Sequencing
2.5. Bioinformatic Processing
3. Results and Discussion
3.1. Soil Physicochemical Properties
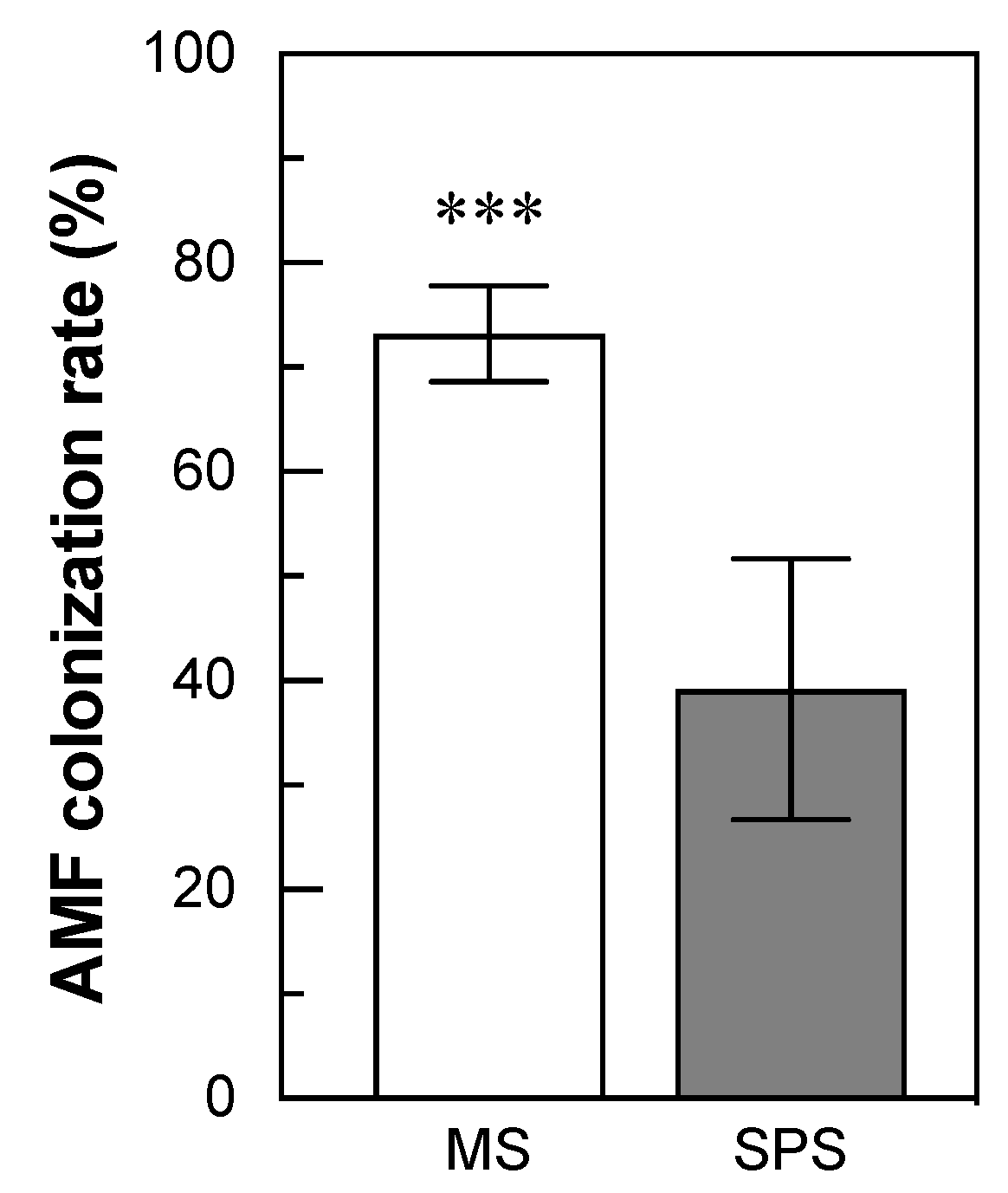
3.2. The Abundance of AMF Colonization
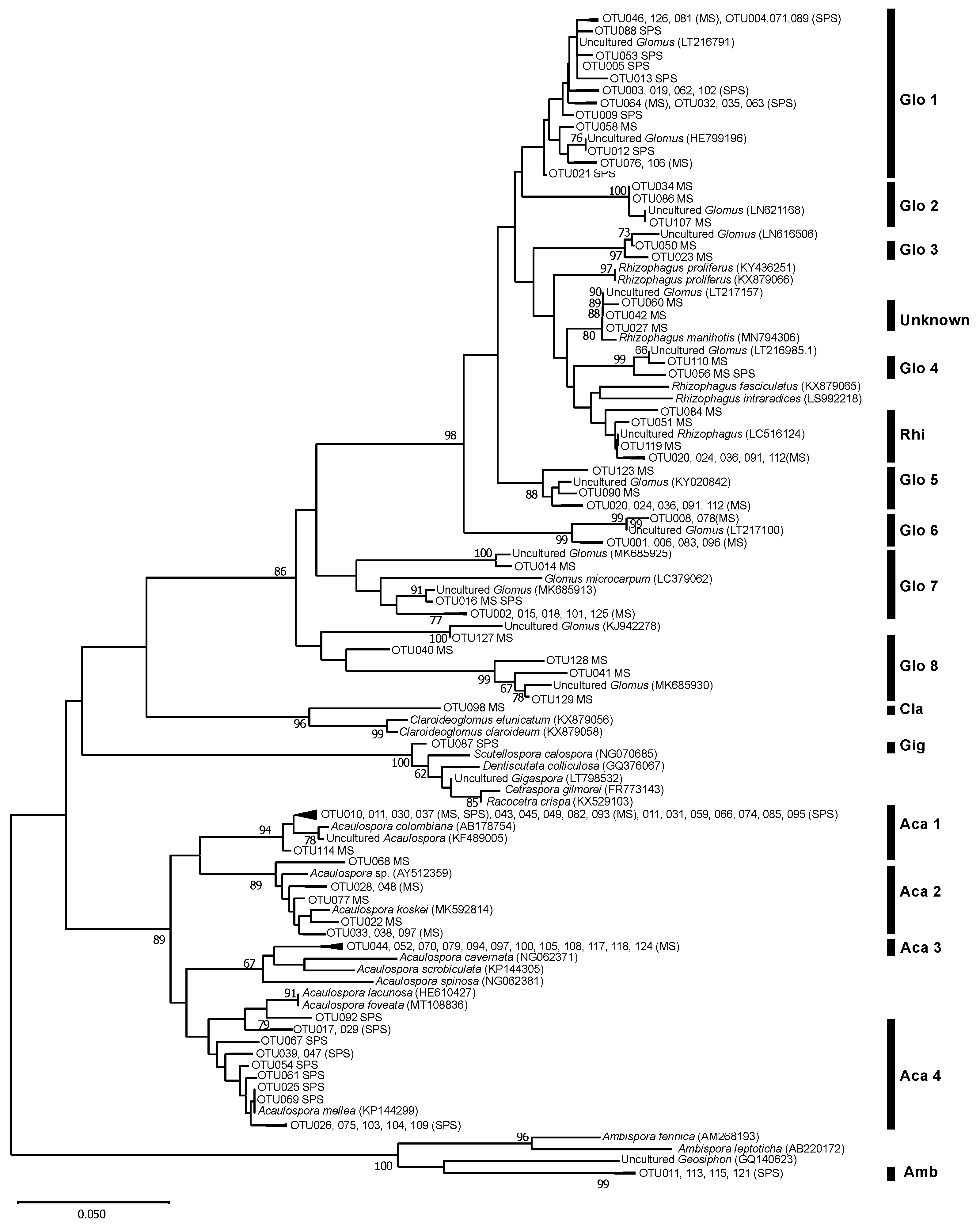
3.3. AMF Community Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehara, H. Genetic variation and agronomic features of Metroxylon palms in Asia and Pacific. In Sago Palm: Multiple Contributions to Food Security and Sustainable Livelihoods; Ehara, H., Toyoda, Y., Johnson, D.V., Eds.; Springer: Singapore, 2018; pp. 1–330. [Google Scholar] [CrossRef] [Green Version]
- Ming, R.Y.C.; Sobeng, Y.; Zaini, F.; Busri, N. Suitability of peat swamp areas for commercial production of sago palms: The Sarawak experience. In Sago Palm: Multiple Contributions to Food Security and Sustainable Livelihoods; Ehara, H., Toyoda, Y., Johnson, D.V., Eds.; Springer: Singapore, 2018; pp. 91–108. [Google Scholar] [CrossRef] [Green Version]
- Ehara, H. Geographical distribution and specification of Metroxylon palms. Jpn. J. Trop. Agric. 2006, 50, 229–233. [Google Scholar] [CrossRef]
- Kueh, H.; Tie, Y.; Robert, E.; Ung, C.; Osman, H. The feasibility of plantation production of sago (Metroxylon sagu) on an organic soil in Sarawak. In Proceedings of the Towards Greater Advancement of the Sago Industry in the 90’s: Proceedings of the Fourth International Sago Symposium, Kuching, Sarawak, Malaysia, 6–9 August 1990; Lee Ming Press: Kuching, Malaysia, 1991; pp. 127–136. [Google Scholar]
- Kakuda, K.; Ando, H.; Yoshida, T.; Yamamoto, Y.; Nitta, Y.; Ehara, H.; Goto, Y.; Purwanto, B.H. Soil characteristics in sago palm grown area: Factors associated with fate of inorganic nitrogen in soil. Sago Palm 2000, 8, 9–16. [Google Scholar]
- Gianinazzi, S.; Gollotte, A.; Binet, M.N.; van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazszoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Maldonado-Mendoza, I.; Lopez-Meyer, M.; Cheung, F.; Town, C.D.; Harrison, M.J. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J. 2007, 50, 529–544. [Google Scholar] [CrossRef]
- Oehl, F.; Alves a Silva, G.; Goto, B.T.; Costa Maia, L.; Sieverding, E. Glomeromycota: Two new classes and a new order. Mycotaxon 2011, 116, 365–379. [Google Scholar] [CrossRef]
- Schüβler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Redecker, D.; Schüssler, A.; Stockinger, H.; Stürmer, S.L.; Morton, J.B.; Walker, C. An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 2013, 23, 515–531. [Google Scholar] [CrossRef]
- Morton, J.B.; Msiska, Z. Phylogenies from genetic and morphological characters do not support a revision of Gigasporaceae (Glomeromycota) into four families and five genera. Mycorrhiza 2010, 20, 483–496. [Google Scholar] [CrossRef]
- Bills, R.J.; Morton, J.B. A combination of morphology and 28S rRNA gene sequences provide grouping and ranking criteria to merge eight into three Ambispora species (Ambisporaceae, Glomeromycota). Mycorrhiza 2015, 25, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, E.; van der Heijden, M.G.A.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. N. Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? N. Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Crossay, T.; Majorel, C.; Redecker, D.; Gensous, S.; Medevielle, V.; Durrieu, G.; Cavaloc, Y.; Amir, H. Is a mixture of arbuscular mycorrhizal fungi better for plant growth than single-species inoculants? Mycorrhiza 2019, 29, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G. Application of mycorrhizae in sustainable date palm cultivation. Emir. J. Food Agric. 2013, 25, 854. [Google Scholar] [CrossRef] [Green Version]
- Phosri, C.; Rodriguez, A.; Sanders, I.R.; Jeffries, P. The role of mycorrhizas in more sustainable oil palm cultivation. Agric. Ecosyst. Environ. 2010, 135, 187–193. [Google Scholar] [CrossRef]
- Chan, M.K.Y.; Liew, G.M.; Zaliha, C.; Halim, A. Detection of vesicular-arbuscular mycorrhiza of sago palm (Metroxylon sagu Rottboll). In Proceedings of the Development of our Natural Resources for Economic and Environmental Properties, Conference Chemical Congress, Kuching, Malaysia, 14 December 2002; MCC: Kuala Lumpur, Malaysia, 2002. [Google Scholar]
- Asano, K.; Isoi, T.; Murano, H.; Azhar, A.; Pasolon, Y.B.; Ehara, H. Colonization of roots in sago palm seedlings associated with commercial mycorrhizal inocula. Sago Palm 2019, 27, 9–14. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. N. Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Porebski, S.L.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Sato, K.; Suyama, Y.; Saito, M.; Sugawara, K. A new primer for discrimination of arbuscular mycorrhizal fungi with polymerase chain reaction-denature gradient gel electrophoresis. Grassl. Sci. 2005, 51, 179–181. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tommerup, I.C. Spore dormancy in vesicular-arbuscular mycorrhizal fungi. Trans. Br. Mycol. Soc. 1983, 81, 37–45. [Google Scholar] [CrossRef]
- Daniels, B.A.; Trappe, J.M. Factors affecting spore germination of the vesicular-arbuscular mycorrhizal fungus, Glomus epigaeus. Mycologia 1980, 72, 457–471. [Google Scholar] [CrossRef]
- Siqueira, J.O.; Hubbell, D.H.; Schenck, N.C. Spore germination and germ tube growth of a vesicular-arbuscular mycorrhizal fungus in vitro. Mycologia 1982, 74, 952. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Miller, S.P. Arbuscular mycorrhizal colonization of semi-aquatic grasses along a wide hydrologic gradient. N. Phytol. 2000, 145, 145–155. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Q.; Yang, Z.; Hu, Z.; Tam, N.F.-Y.; Xin, G. Arbuscular mycorrhizal fungi in two mangroves in South China. Plant Soil 2010, 331, 181–191. [Google Scholar] [CrossRef]
- Parvin, S.; van Geel, M.; Yeasmin, T.; Lievens, B.; Honnay, O. Variation in arbuscular mycorrhizal fungal communities associated with lowland rice (Oryza sativa) along a gradient of soil salinity and arsenic contamination in Bangladesh. Sci. Total Environ. 2019, 686, 546–554. [Google Scholar] [CrossRef]
- Xiao, D.; Tan, Y.; Liu, X.; Yang, R.; Zhang, W.; He, X.; Wang, K. Effects of different legume species and densities on arbuscular mycorrhizal fungal communities in a karst grassland ecosystem. Sci. Total Environ. 2019, 678, 551–558. [Google Scholar] [CrossRef]
- Higo, M.; Tatewaki, Y.; Iida, K.; Yokota, K.; Isobe, K. Amplicon sequencing analysis of arbuscular mycorrhizal fungal communities colonizing maize roots in different cover cropping and tillage systems. Sci. Rep. 2020, 10, 6039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chubo, J.K.; Huat, O.K.; Jais, H.M.; Mardatin, N.F.; Majid, N.M.N.A. Genera of arbuscular mycorrhiza occurring within the rhizospheres of Octomeles sumatrana and Anthocephalus chinensis in Niah, Sarawak, Malaysia. Sci. Asia 2009, 35, 340. [Google Scholar] [CrossRef]
- Auliana; Kaonongbua, W. Preliminary study on biodiversity of arbuscular mycorrhizal fungi (AMF) in oil palm (Elaeis guineensis Jacq.) plantations in Thailand. IOP Conf. Ser. Earth Environ. Sci. 2018, 144, 012010. [Google Scholar] [CrossRef]
- Al-Yahya’ei, M.N.; Oehl, F.; Vallino, M.; Lumini, E.; Redecker, D.; Wiemken, A.; Bonfante, P. Unique arbuscular mycorrhizal fungal communities uncovered in date palm plantations and surrounding desert habitats of Southern Arabia. Mycorrhiza 2011, 21, 195–209. [Google Scholar] [CrossRef] [Green Version]
- Hazard, C.; Gosling, P.; van der Gast, C.J.; Mitchell, D.T.; Doohan, F.M.; Bending, G.D. The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J. 2013, 7, 498–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deepika, S.; Kothamasi, D. Soil moisture—A regulator of arbuscular mycorrhizal fungal community assembly and symbiotic phosphorus uptake. Mycorrhiza 2015, 25, 67–75. [Google Scholar] [CrossRef]
- Oehl, F.; Jansa, J.; Ineichen, K.; Mäeder, P.; van der Heijden, M. Arbuscular mycorrhizal fungi as bioindicators in Swiss agricultural soils. Agrar. Schweiz 2011, 18, 304–311. [Google Scholar]
- Oehl, F.; Schneider, D.; Sieverding, E.; Burga, C.A. Succession of arbuscular mycorrhizal communities in the foreland of the retreating Morteratsch glacier in the Central Alps. Pedobiologia 2011, 54, 321–331. [Google Scholar] [CrossRef]
- Hepper, C.M. Regulation of spore germination of the vesicular-arbuscular mycorrhizal fungus Acaulospora laevis by soil pH. Trans. Br. Mycol. Soc. 1984, 83, 154–156. [Google Scholar] [CrossRef]
- Clark, R.B. Arbuscular mycorrhizal adaptation, spore germination, root colonization, and host plant growth and mineral acquisition at low pH. Plant Soil 1997, 192, 15–22. [Google Scholar] [CrossRef]
- Sahmat, S.S.; Chan, M.K.Y. Spore production of indigenous mycorrhiza of sago palm (Metroxylon sagu Rottboll) in culture media. In Proceedings of the 2011 UiTM Sarawak Conference, Samarahan, Malaysia, 18 October 2011; Universiti Teknologi MARA: Kota Samarahan, Malaysia, 2011. [Google Scholar]
- Ait-El-Mokhtar, M.; Laouane, R.B.; Anli, M.; Boutasknit, A.; Wahbi, S.; Meddich, A. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hortic. 2019, 253, 429–438. [Google Scholar] [CrossRef]
- Benhiba, L.; Fouad, M.O.; Essahibi, A.; Ghoulam, C.; Qaddoury, A. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees 2015, 29, 1725–1733. [Google Scholar] [CrossRef]
| Soil Type | Depth | pH (H2O) | Moisture Content | Bulk Density | C Content | N Content | P2O5 |
|---|---|---|---|---|---|---|---|
| (Bray II) | |||||||
| (cm) | (%) | (g cm−3) | (kg m−3) | (g m−3) | |||
| MS | 0–15 | 4.6 | 38.1 | 1.03 | 66.2 | 16.9 | 1.6 |
| SPS | 0–15 | 4.1 | 79.8 | 0.20 | 69.7 | 2.7 | 1.9 |
| 15–30 | 4.0 | 77.7 | 0.23 | 64.9 | 1.4 | 3.2 | |
| 30–50 | 4.3 | 50.6 | 0.96 | 52.7 | 18.7 | 4.3 | |
| MS | SPS | |||
|---|---|---|---|---|
| Clades | No. of OTUs | RA (%) | No. of OTUs | RA (%) |
| Aca 1 | 10 | 3.7 | 11 | 25.3 |
| Aca 2 | 7 | 7.1 | 0 | 0.0 |
| Aca 3 | 11 | 2.0 | 0 | 0.0 |
| Aca 4 | 0 | 0.0 | 15 | 11.8 |
| Amb | 2 | 0.2 | 3 | 0.1 |
| Cla | 1 | 0.1 | 0 | 0.0 |
| Gig | 0 | 0.0 | 1 | 0.2 |
| Glo 1 | 7 | 1.7 | 16 | 53.4 |
| Glo 2 | 3 | 1.4 | 0 | 0.0 |
| Glo 3 | 2 | 2.8 | 0 | 0.0 |
| Glo 4 | 5 | 1.2 | 2 | 9.2 |
| Glo 5 | 7 | 6.3 | 0 | 0.0 |
| Glo 6 | 6 | 36.5 | 0 | 0.0 |
| Glo 7 | 6 | 32.0 | 2 | 0.0 |
| Glo 8 | 5 | 1.3 | 0 | 0.0 |
| Rhi | 3 | 0.7 | 0 | 0.0 |
| Unknown | 3 | 2.9 | 0 | 0.0 |
| Total | 78 | 100 | 50 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asano, K.; Kagong, W.V.A.; Mohammad, S.M.B.; Sakazaki, K.; Talip, M.S.A.; Sahmat, S.S.; Chan, M.K.Y.; Isoi, T.; Kano-Nakata, M.; Ehara, H. Arbuscular Mycorrhizal Communities in the Roots of Sago Palm in Mineral and Shallow Peat Soils. Agriculture 2021, 11, 1161. https://doi.org/10.3390/agriculture11111161
Asano K, Kagong WVA, Mohammad SMB, Sakazaki K, Talip MSA, Sahmat SS, Chan MKY, Isoi T, Kano-Nakata M, Ehara H. Arbuscular Mycorrhizal Communities in the Roots of Sago Palm in Mineral and Shallow Peat Soils. Agriculture. 2021; 11(11):1161. https://doi.org/10.3390/agriculture11111161
Chicago/Turabian StyleAsano, Koki, Willy Vincent Anak Kagong, Siraj Munir Bin Mohammad, Kurumi Sakazaki, Muhamad Syukrie Abu Talip, Siti Sahmsiah Sahmat, Margaret Kit Yok Chan, Toshiyuki Isoi, Mana Kano-Nakata, and Hiroshi Ehara. 2021. "Arbuscular Mycorrhizal Communities in the Roots of Sago Palm in Mineral and Shallow Peat Soils" Agriculture 11, no. 11: 1161. https://doi.org/10.3390/agriculture11111161
APA StyleAsano, K., Kagong, W. V. A., Mohammad, S. M. B., Sakazaki, K., Talip, M. S. A., Sahmat, S. S., Chan, M. K. Y., Isoi, T., Kano-Nakata, M., & Ehara, H. (2021). Arbuscular Mycorrhizal Communities in the Roots of Sago Palm in Mineral and Shallow Peat Soils. Agriculture, 11(11), 1161. https://doi.org/10.3390/agriculture11111161







