Growth and Yield Responses of Pot-Grown Long Bean and Luffa to Nitrogen Rates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivation
2.2. Leaf Sampling and SPAD
2.3. Aboveground Plant Dry Biomass
2.4. Root and Nodule Sampling for Long Bean
2.5. Yield and NUE
2.6. Statistical Analysis
3. Results
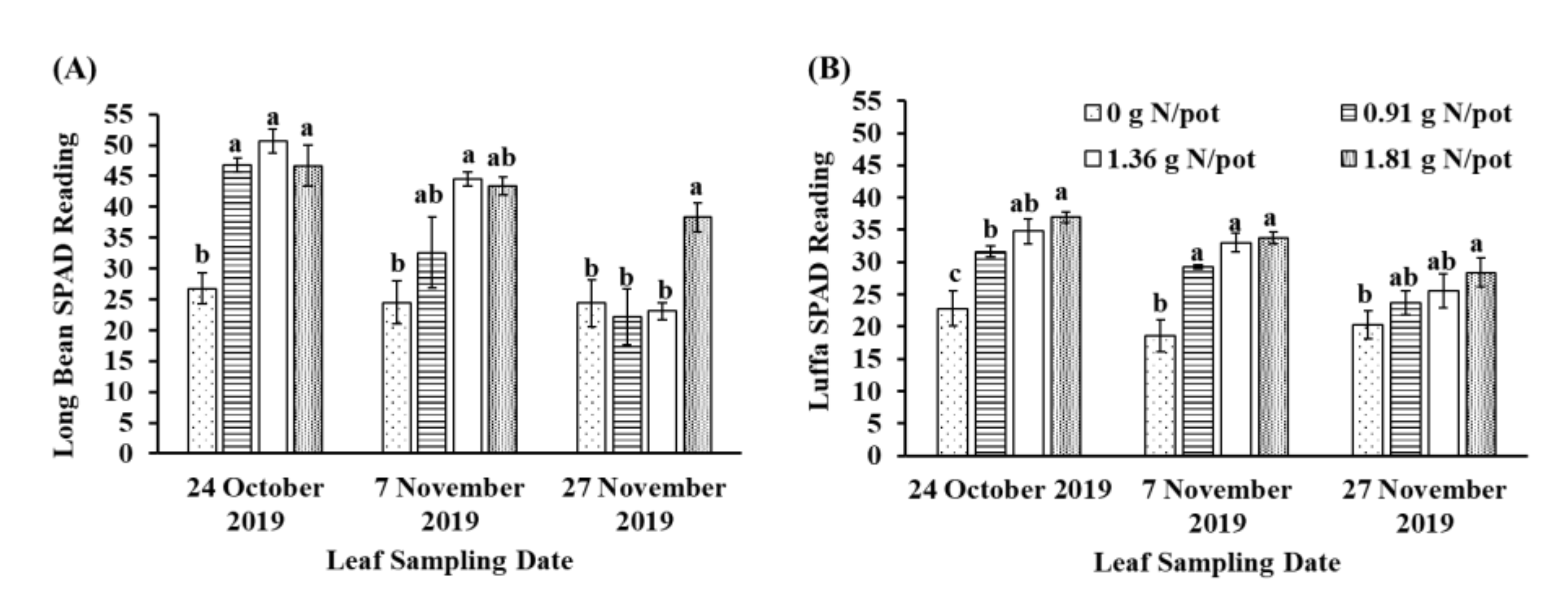
3.1. Leaf SPAD Readings
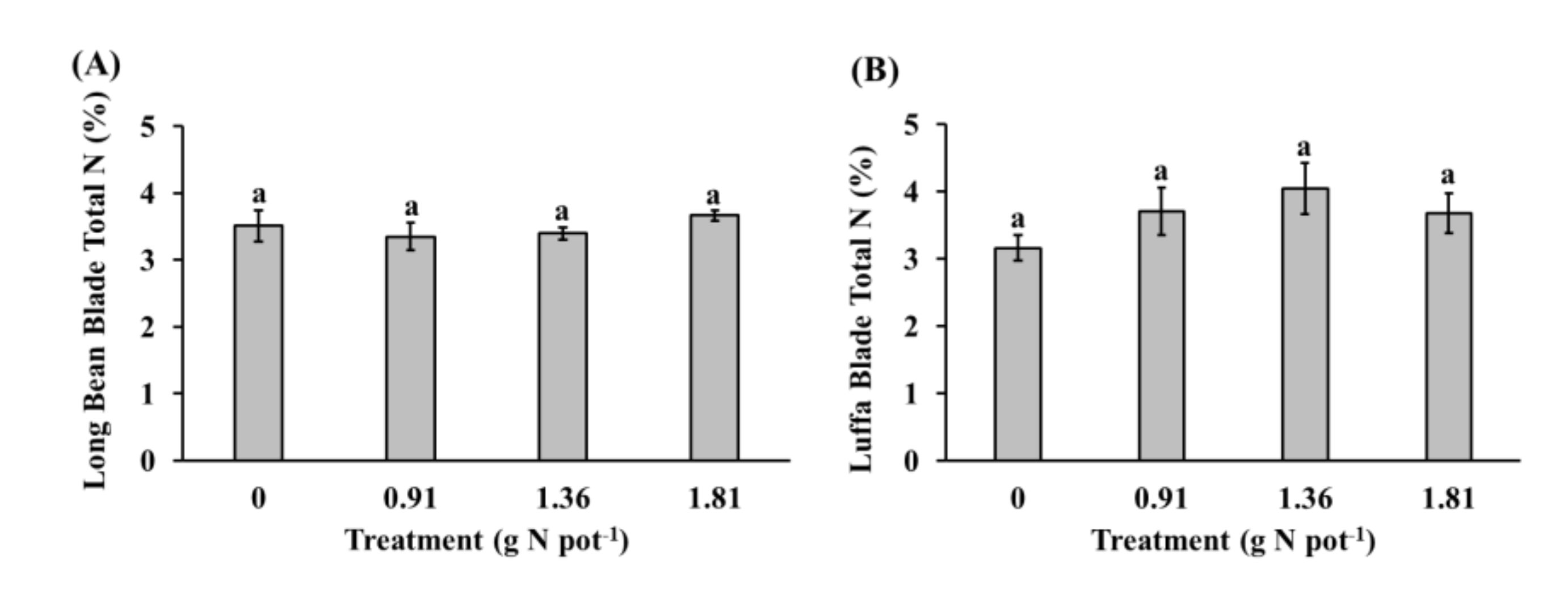
3.2. Aboveground Plant Dry Biomass and Total Nitrogen Content
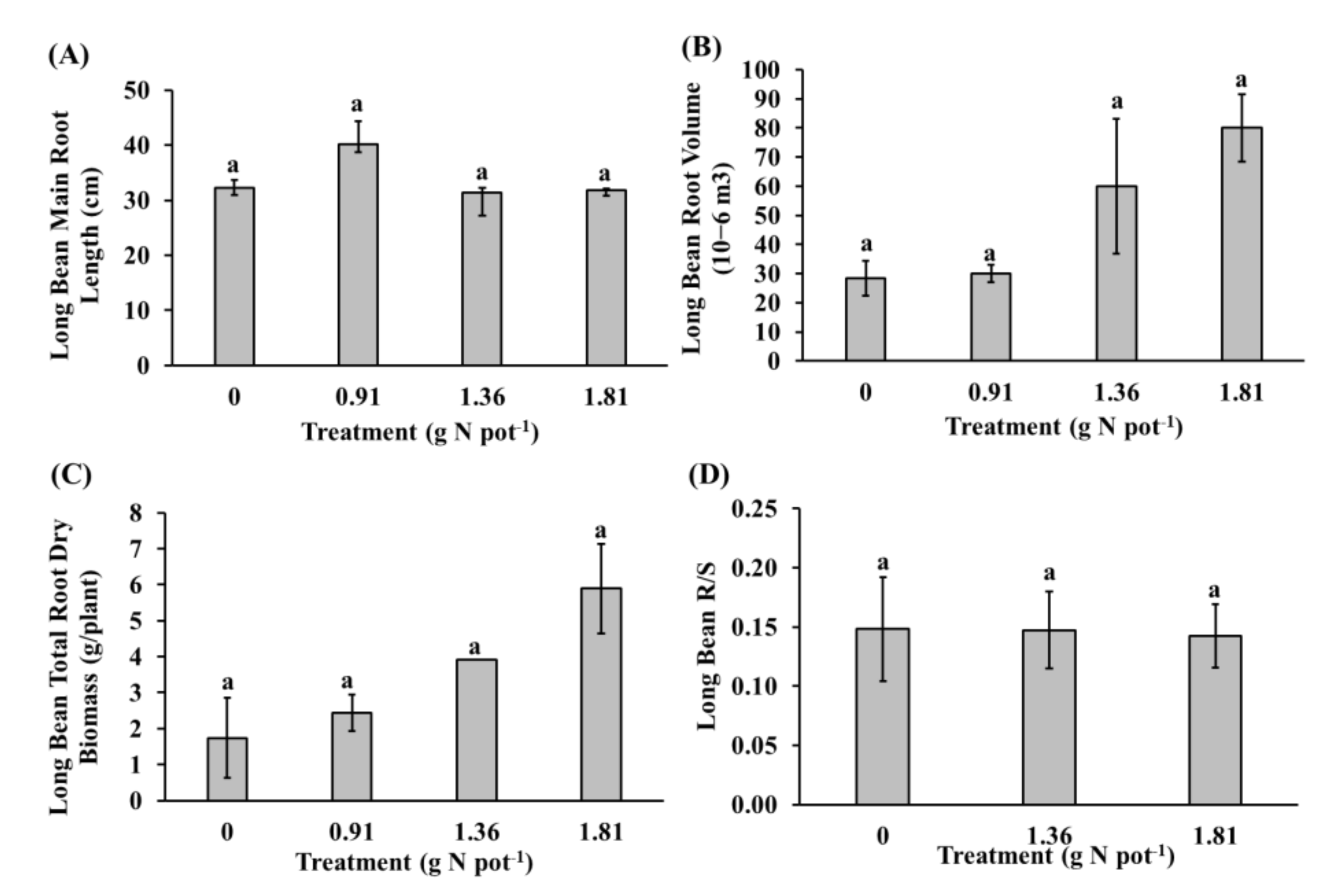
3.3. Roots and Nodules of Long Bean
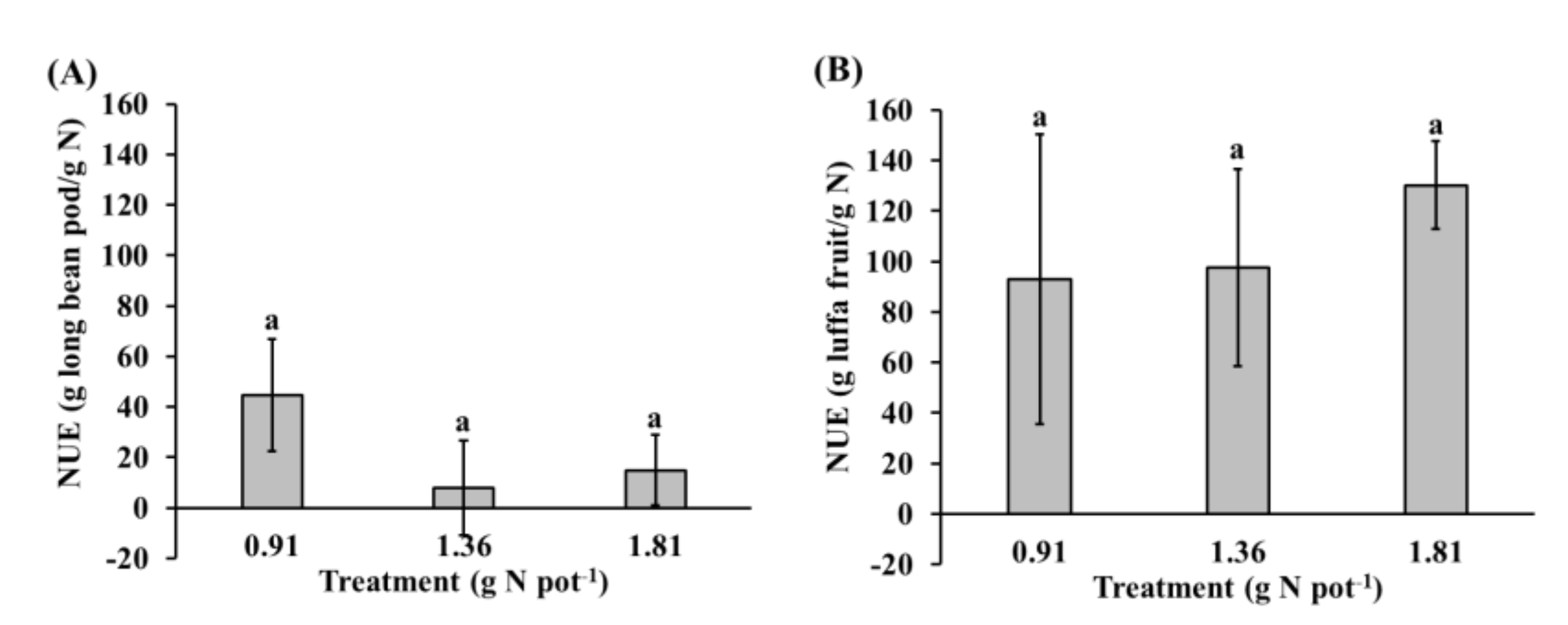
3.4. Nitrogen Use Efficienct (NUE)
4. Discussion
4.1. Nitrogen and Carbon Partitioning and Plant Growth
4.2. Effects of N Inputs on Long Bean Nodules
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, M.M.; Wang, Y.; Liu, G.D. Asian Vegetables Rapidly Emerging in Florida. VSCNews: Cegetable and Specialty Crop News. 2020. Available online: https://vscnews.com/asian-vegetable-crop-interest-increasing-in-florida/ (accessed on 2 November 2021).
- Barik, N.; Phookan, D.B.; Kumar, V.; Millik, T.T.; Nath, D.J. Organic cultivation of ridge gourd (Luffa acutangula Roxb.). Curr. J. Appl. 2018, 26, 1–6. [Google Scholar] [CrossRef]
- Ju, X.T.; Liu, X.J.; Zhang, F.S. Accumulation and movement of NO3−-N in soil profile in winter wheat-summer maize rotation system. Acta Pedol. Sin. 2003, 40, 538–546. [Google Scholar]
- Balasubramanian, V.; Alves, B.; Aulakh, M.; Bekunda, M.; Cai, Z.; Drinkwater, L.; Oenema, O. Crop, enviromental, and management factors affecting nitrogen use efficiency. In Agriculture and the Nitrogen Cycle; Mosier, A.R., Syers, J.K., Freney, J., Eds.; SCOPE: Washington, DC, USA, 2004; Volume 65, pp. 19–33. [Google Scholar]
- Ano, A.O.; Ubochi, C.I. Nutrient composition of climbing and prostrate vegetable cowpea accessions. Afr. J. Biotechnol. 2008, 7, 3795–3798. [Google Scholar]
- Wang, W.; Xue, Z.; Zhu, F.; Chen, X.; Yao, Y.; Hong, C. Study of characteristics of accumulation and distribution of nutritional elements’ uptake of asparagus bean. J. Soil Water Conserv. 2013, 27, 158–171. [Google Scholar]
- Martin, F.W.; Ruberté, R.M. Techniques and plants for the tropical subsistence farm. Agric. Res. 1980, 8, 22. [Google Scholar]
- Bland, R.G.; Knausenberger, W.I. Predators and parasites of insect pests on cantaloupe and asparagus bean. Agric. Econ. 1984. [Google Scholar] [CrossRef]
- Sinsiri, W.; Homchan, J. Effect of rhizobial management upon rhizobial population, nodulation and growth of yard long beans (Vigna sesquipedalis L.): A new approach to maximize benefits from Rhizobium. Pak. J. Biol. Sci. 2002, 5, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Gajete, T.D. Nodulation, Nitrogen Fixation and Yield of Five Varieties of Pole Sitao (Vigna Unguiculata L. Walp Subsp. Sesquipedalis) Inoculated with Different Strains of Rhizobia; University of the Philippines at Los Baños: Los Baños, Philippines, 1984. [Google Scholar]
- Parsons, R.; Stanforth, A.; Raven, J.A.; Sprent, J.I. Nodule growth and activity may be regulated by a feedback mechanism involving phloem nitrogen. Plant Cell Environ. 1993, 16, 125–136. [Google Scholar] [CrossRef]
- Heiser, C.B. The Gourd Book; University of Oklahoma Press: Norman, OK, USA, 2016; pp. 1–266. [Google Scholar]
- Ravella, R.; Reddy, M.R.; Taylor, K.O.; Miller, M. Evaluation of sustainable production practices for Asian vegetables (luffa and bitter gourd) and their mineral nutrient analysis in a piedmont soil of North Carolina. J. Exp. Agric. Int. 2015, 475–481. [Google Scholar] [CrossRef]
- Joshi, B.K.; KC, H.B.; Tiwari, R.K.; Ghale, M.; Sthapit, B.R. Descriptors for Sponge Gourd (Luffa cylindrica (L.) Roem.). NARC, LIBIRD and IPGRI. 2014. Available online: https://idl-bnc-idrc.dspacedirect.org/bitstream/handle/10625/31459/122785.pdf?sequence=1 (accessed on 13 November 2021).
- Pimple, B.P.; Kadam, P.V.; Patil, M.J. Antidiabetic and antihyperlipidemic activity of Luffa acutangula fruit extracts in streptozotocin induced NIDDM rats. Asian J. Pharm. Clin. Res. 2011, 4, 156–163. [Google Scholar]
- Okusanya, O.T. The effects of light and temperature on germination and growth of Luffa aegyptiaca. Physiol. Plant. 1978, 44, 429–433. [Google Scholar] [CrossRef]
- Hilli, J.S.; Vyakarnahal, B.S.; Biradar, D.P.; Hunje, R. Influence of method of trailing and fertilizer levels on seed yield of ridgegourd (Luffa acutangula L. Roxb). Karnataka J. Agric. Sci. 2010, 22, 47–52. [Google Scholar]
- Okusanya, O.T.; Ola-Adams, B.A.; Bamidele, J.F. Variations in size, leaf morphology, and fruit characters among 25 populations of Luffa aegyptiaca. Can. J. Bot. 1981, 59, 2618–2627. [Google Scholar] [CrossRef]
- Okusanya, O.T.; Lakanmi, O.O. The growth of Luffa aegyptiaca in response to various nitrogen sources and concentrations. Can. J. Bot. 1985, 63, 2283–2287. [Google Scholar] [CrossRef]
- Soil Survey Staff. Web Soil Survey; National Soil Survey Center: Lincoln, NE, USA, 2017. Available online: http://websoilsurvey.nrcs.usda.gov/ (accessed on 2 November 2021).
- Islam, M.A.; Boyce, A.N.; Rahman, M.M.; Azirun, M.S.; Ashraf, M.A. Effects of organic fertilizers on the growth and yield of bush bean, winged bean and yard long bean. Braz. Arch. Biol. Technol. 2016, 59, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Okusanya, O.T. Experimental studies on some observed variations in Luffa aegyptiaca. Can. J. Bot. 1983, 61, 202–210. [Google Scholar] [CrossRef]
- Okusanya, O.T. The mineral nutrition of Luffa aegyptiaca. Can. J. Bot. 1983, 61, 2124–2132. [Google Scholar] [CrossRef]
- Swain, D.K.; Sandip, S.J. Development of SPAD values of medium-and long-duration rice variety for site-specific nitrogen management. J. Agron. 2010, 9, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Derivation of sufficiency values of a chlorophyll meter to estimate cucumber nitrogen status and yield. Comput. Electron. Agric. 2017, 141, 54–64. [Google Scholar] [CrossRef]
- Howe, J. Effect of Soil Moisture Manipulation and Nitrogen Application on Leaf Gas Exchange, Fruit Composition, and Carbohydrate Storage of Pinot Noir and Chardonnay Grapevines in the Willamette Valley. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2017. Available online: https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/rn301427s (accessed on 3 November 2021).
- Abdallah, M.; Dubousset, L.; Meuriot, F.; Etienne, P.; Avice, J.C.; Ourry, A. Effect of mineral sulphur availability on nitrogen and sulphur uptake and remobilization during the vegetative growth of Brassica napus L. J. Exp. Bot. 2010, 61, 2635–2646. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Zhai, B.; Zhu, H. Relationship between leaf SPAD values and the nitrate content and nitrate reductase activity in cucumber at different nitrogen rates. J. Northwest A F Univ. Nat. Sci. Ed. 2009, 37, 189–198. [Google Scholar]
- Wallace, S.U.; Blanchet, R.; Bouniols, A.; Gelfi, N. Influence of nitrogen fertilization on morphological development of indeterminate and determinate soybeans. J. Plant Nutr. 1990, 13, 1523–1537. [Google Scholar] [CrossRef]
- Egli, D.B.; Guffy, R.D.; Leggett, J.E. Partitioning of assimilate between vegetative and reproductive growth in soybean 1. Agron. J. 1985, 77, 917–922. [Google Scholar] [CrossRef]
- Ryle, G.J.A.; Arnott, R.A.; Powell, C.E. Distribution of dry weight between root and shoot in white clover dependent on N 2 fixation or utilizing abundant nitrate nitrogen. Plant Soil 1981, 60, 29–39. [Google Scholar] [CrossRef]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ciampitti, I.A.; Salvagiotti, F. New insights into soybean biological nitrogen fixation. J. Agron. 2015, 110, 1185–1196. [Google Scholar] [CrossRef] [Green Version]
- Hungria, M.; Franchini, J.C.; Campo, R.J.; Crispino, C.C.; Moraes, J.Z.; Sibaldelli, R.N.; Mendes, I.C.; Arihara, J. Nitrogen nutrition of soybean in Brazil: Contributions of biological N2 fixation and N fertilizer to grain yield. Can. J. Plant Sci. 2006, 86, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.R.; Desai, U.T.; Pawar, B.G.; Patil, B.T. Effects of NPK doses on growth and yield of bottle gourd cv. Samrat. J. Maharashtra Agric. Univ. 1996, 21, 65–66. [Google Scholar]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Trinchant, J.C.; Rigaud, J. Nitrite inhibition of nitrogenase from soybean bacteroids. Arch. Microbiol. 1980, 124, 49–54. [Google Scholar] [CrossRef]
- Trinchant, J.C.; Rigaud, J. Nitrogen fixation in French-beans in the presence of nitrate: Effect on bacteroid respiration and comparison with nitrite. J. Plant Physiol. 1984, 116, 209–217. [Google Scholar] [CrossRef]
- Davidson, I.A.; Robson, M.J. Effect of contrasting patterns of nitrate application on the nitrate uptake, N2-fixation, nodulation, and growth of white clover. Ann. Bot. 1986, 57, 331–338. [Google Scholar] [CrossRef]
- Stevenson, F.J. Organic forms of soil nitrogen. In Nitrogen in Agricultural Soils; American Society of Agronomy: New York, NY, USA, 1982; Volume 22, pp. 67–122. [Google Scholar]
- Mahon, J.D.; Child, J.J. Growth response of inoculated peas (Pisum sativum) to combined nitrogen. Can. J. Bot. 1979, 57, 1687–1693. [Google Scholar] [CrossRef]
- Pankhurst, C.E. Effect of plant nutrient supply on nodule effectiveness and Rhizobium strain competition for nodulation of Lotus pedunculatus. Plant Soil 1981, 60, 325–339. [Google Scholar] [CrossRef]
- Harper, J.E. Soil and symbiotic nitrogen requirements for optimum soybean production 1. Crop Sci. 1974, 14, 255–260. [Google Scholar] [CrossRef]
- Hill-Cottingham, D.G.; Lloyd-Jones, C.P. The influence of nitrate supply on nitrogen fixation during growth of the field bean Vicia faba in sand. Physiol. Plant. 1980, 48, 116–120. [Google Scholar] [CrossRef]
- Rabie, R.K.; Kumazawa, K. Effect of nitrate application and shade treatment on the nitrogen fixation and yield of soybean plant. Soil Sci. Plant Nutr. 1979, 25, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Dazzo, F.B.; Brill, W.J. Regulation by fixed nitrogen of host-symbiont recognition in the Rhizobium-clover symbiosis. Plant Physiol. 1978, 62, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Truchet, G.L.; Dazzo, F.B. Morphogenesis of lucerne root nodules incited by Rhizobium meliloti in the presence of combined nitrogen. Planta 1982, 154, 352–360. [Google Scholar] [CrossRef] [PubMed]




| Crop | Biomass | Blade Nitrogen | Yield | NUE | Number of Fruits | SPAD Reading | Root | Root/Shoot | Nodule | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (A) | Date (B) | A × B | Biomass | Length | Volume | Number | Biomass | |||||||
| Long Bean | 0.008 | 0.275 | 0.626 | 0.241 | N/A | 0.413 | <0.001 | <0.001 | 0.065 | 0.338 | 0.089 | 0.883 | 0.117 | 0.229 |
| Luffa | 0.312 | 0.347 | 0.007 | 0.830 | 0.079 | <0.001 | <0.001 | 0.281 | N/A | N/A | N/A | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Maltais-Landry, G.; Rathinasabapathi, B.; Sargent, S.A.; Liu, G. Growth and Yield Responses of Pot-Grown Long Bean and Luffa to Nitrogen Rates. Agriculture 2021, 11, 1145. https://doi.org/10.3390/agriculture11111145
Wang Y, Maltais-Landry G, Rathinasabapathi B, Sargent SA, Liu G. Growth and Yield Responses of Pot-Grown Long Bean and Luffa to Nitrogen Rates. Agriculture. 2021; 11(11):1145. https://doi.org/10.3390/agriculture11111145
Chicago/Turabian StyleWang, Yanlin, Gabriel Maltais-Landry, Bala Rathinasabapathi, Steven A. Sargent, and Guodong Liu. 2021. "Growth and Yield Responses of Pot-Grown Long Bean and Luffa to Nitrogen Rates" Agriculture 11, no. 11: 1145. https://doi.org/10.3390/agriculture11111145
APA StyleWang, Y., Maltais-Landry, G., Rathinasabapathi, B., Sargent, S. A., & Liu, G. (2021). Growth and Yield Responses of Pot-Grown Long Bean and Luffa to Nitrogen Rates. Agriculture, 11(11), 1145. https://doi.org/10.3390/agriculture11111145






