Comparison of Protectivity and Safety of Two Vaccines against Actinobacillus pleuropneumoniae in a Field Study
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Production Data
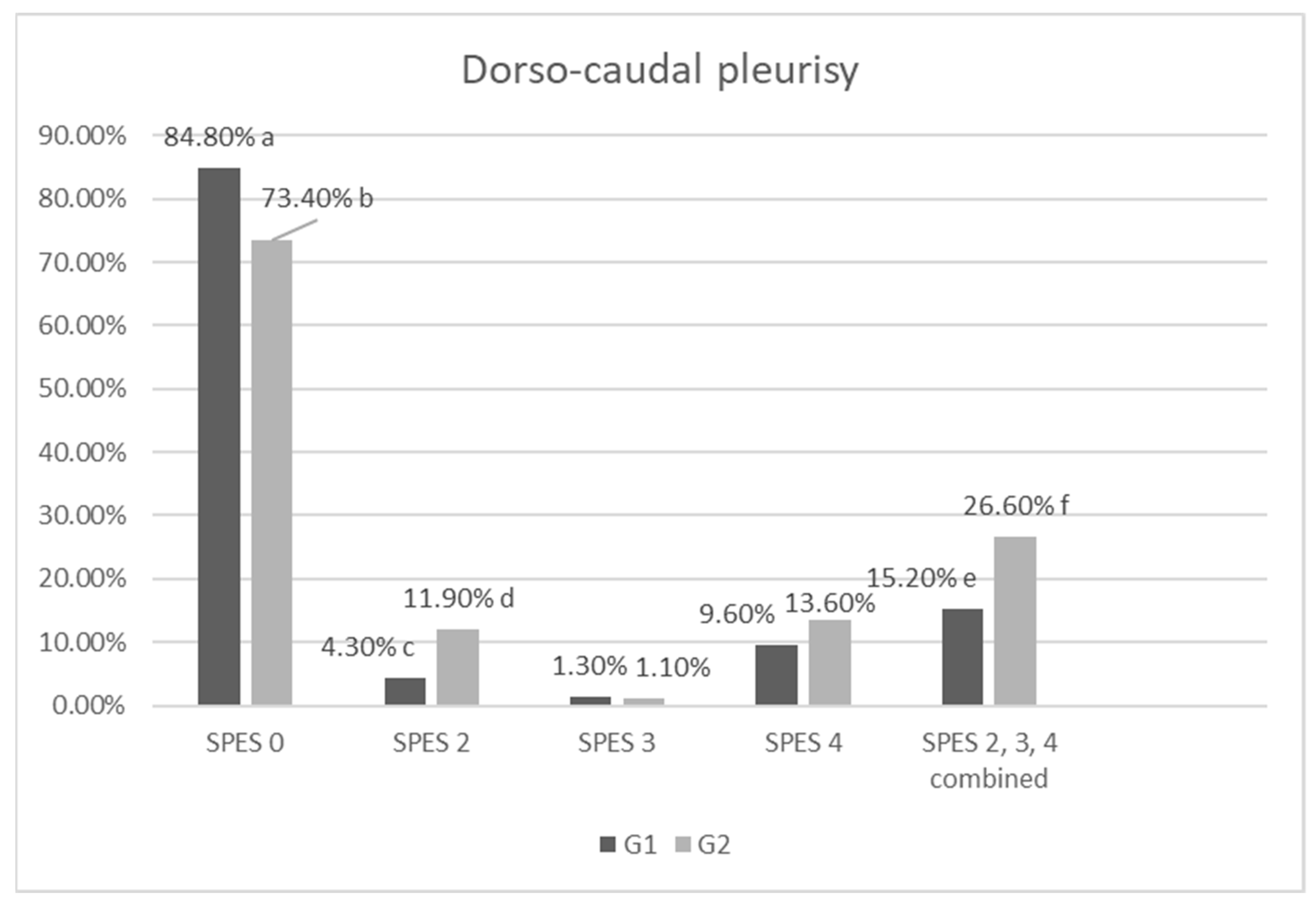
4.2. Lung Lesion Scoring
4.3. Mortality
4.4. Antimicrobial Treatments
4.5. Injection Site and Systemic Adverse Reactions
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Chiers, K.; Donne, E.; Van Overbeke, I.; Ducatelle, R.; Haesebrouck, F. Actinobacillus pleuropneumoniae infections in closed swine herds: Infection patterns and serological profiles. Vet. Immunol. Immunopathol. 2002, 85, 343–352. [Google Scholar] [CrossRef]
- Klinkenberg, D.; Tobias, T.J.; Bouma, A.; van Leengoed, L.A.M.G.; Stegeman, J.A. Simulation study of the mechanisms underlying outbreaks of clinical disease caused by glycoprotein analysis of porcine bronchoalveolar lavage fluid reveals potential biomarkers corresponding to resistance to Actinobacillus pleuropneumoniae infection in finishing pigs. Vet. J. 2014, 202, 99–105. [Google Scholar]
- Gottschalk, M.; Zimmerman, J.J.; Karriker, L.A.; Ramirez, A. Actinobacillosis. In Diseases of Swine, 11th ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 669–749. [Google Scholar]
- Holmgren, N.; Lundeheim, N.; Wallgren, P. Infections with Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae in fattening pigs. Influence of piglet production systems and influence on production parameters. J. Vet. Med. B Infect. Dis. Vet. Public Health 1999, 46, 535–544. [Google Scholar] [CrossRef]
- Bossé, J.T.; Li, Y.; Sàrközi, R.; Fodor, L.; Lacouture, S.; Gottschalk, M.; Amoribieta, M.C.; Angen, O.; Nedbalcova, K.; Holden, M.T.G.; et al. Proposal of serovars 17 and 18 of Actinobacillus pleuropneumoniae based on serological and genotypic analysis. Vet. Microbiol. 2018, 217, 1–6. [Google Scholar] [CrossRef]
- Gottschalk, M. The challenge of detecting herds sub-clinically infected with Actinobacillus pleuropneumoniae. Vet. J. 2015, 206, 30–38. [Google Scholar] [CrossRef]
- Gottschalk, M. Actinobacillus pleuropneumoniae heterogeneity of pathogenicity. In Proceedings of the ESPHM Keynote Lectures, Nantes, France, 22–24 April 2015; pp. 58–60. [Google Scholar]
- Gottschalk, M.; Lacouture, S. Distribution of Streptococcus suis (from 2012 to 2014) and Actinobacillus pleuropneumoniae (from 2011 to 2014) serotypes isolated from diseased pigs. Can. Vet. J. 2015, 56, 1093–1094. [Google Scholar] [PubMed]
- Jacques, M.; Labrie, J.; St Michael, F.; Cox, A.D.; Paradis, M.-A.; Dick, C.P.; Klopfenstein, C.; Broes, A.; Fittipaldi, N.; Gottschalk, M. Isolation of an Atypical Strain of Actinobacillus pleuropneumoniae Serotype 1 with a Truncated Lipopolysaccharide Outer Core and No O-Antigen. J. Clin. Microbiol. 2015, 43, 3522–3525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, M.B.; Angen, Ø.; MacLean, L.L.; Lacouture, S.; Kokotovic, B.; Gottschalk, M. An atypical biotype I Actinobacillus pleuropneumoniae serotype 13 is present in North America. Vet. Microbiol. 2012, 156, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renken, C. Seroprävalenz von Actinobacillus pleuropneumoniae Sowie Zugehöriger Serotypen und Vorkommen von Pleuritiden bei Mastschweinen aus Beständen mit Klinischen Anzeichen Einer Atemwegserkrankung. Inaugural Dissertation, Tierärztliche Fakultät LMU, Münich, Germany, 2017. [Google Scholar]
- Li, L.; Xu, Z.; Zhou, Y.; Sun, L.; Liu, Z.; Chen, H.; Zhou, R. Global Effects of Catecholamines on Actinobacillus pleuropneumoniae Gene Expression. PLoS ONE 2012, 7, e31121. [Google Scholar] [CrossRef]
- Maes, D.; Chiers, K.; Haesebrouck, F.; Laevens, H.; Verdonck, M.; de Kruif, A. Herd factors associated with the seroprevalences of Actinobacillus pleuropneumoniae serovars 2, 3 and 9 in slaughter pigs from farrow-to-finish pig herds. Vet. Res. 2001, 32, 409–419. [Google Scholar] [CrossRef] [Green Version]
- Sassu, E.L.; Bossé, J.T.; Tobias, T.J.; Gottschalk, M.; Langford, P.R.; Hennig-Pauka, I. Update on Actinobacillus pleuropneumoniae—knowledge, gaps and challenges. Transbound. Emerg. Dis. 2018, 65, 72–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiers, K.; De Waele, T.; Pasmans, F.; Ducatelle, R.; Haesebrouck, F. Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet. Res. 2010, 41, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Beck, M.; van den Bosch, J.F.; Jongenelen, I.M.; Loeffen, P.L.; Nielsen, R.; Nicolet, J.; Frey, J. RTX toxin genotypes and phenotypes in Actinobacillus pleuropneumoniae field strains. J. Clin. Microbiol. 1994, 32, 2749–2754. [Google Scholar] [CrossRef] [Green Version]
- Dom, P.; Haesebrouck, F. Comparative virulence of NAD-dependent and NAD-independent Actinobacillus pleuropneumoniae strains. Zent. Vet. B. 1992, 9, 303–306. [Google Scholar] [CrossRef]
- Frey, J. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 1995, 3, 257–261. [Google Scholar] [CrossRef]
- Schaller, A.; Kuhnert, P.; de la Puente-Redondo, V.A.; Nicolet, J.; Frey, J. Apx toxins in Pasteurellaceae species from animals. Vet. Microbiol. 1995, 74, 365–376. [Google Scholar] [CrossRef]
- Sárközi, R.; Makrai, L.; Fodor, L. Identification of a proposed new serovar of Actinobacillus pleuropneumoniae: Serovar 16. Acta Vet. Hung. 2015, 63, 444–450. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Pin, C.; Haibing, G.; Yang, C.; Hui, L.; Qigai, H. Loop mediated isothermal amplification targeting the apxIVA gene for detection of Actinobacillus pleuropneumoniae. FEMS Microbiol. Lett. 2009, 300, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Dreyfus, A.; Schaller, A.; Nivollet, S.; Segers, R.P.; Kobisch, M.; Mieli, L.; Soerensen, V.; Hüssy, D.; Miserez, R.; Zimmermann, W.; et al. Use of recombinant ApxIV in serodiagnosis of Actinobacillus pleuropneumoniae infections, development and prevalidation of the ApxIV ELISA. Vet. Microbiol. 2004, 99, 227–238. [Google Scholar] [CrossRef]
- Fenwick, B.; Osburn, B. Immune responses to the lipopolysaccharides and capsular polysaccharides of Haemophilus pleuropneumoniae in convalescent and immunized pigs. Infect. Immun. 1986, 54, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Ramjeet, M.; Deslandes, V.; St Michael, F.; Cox, A.; Kobisch, M.N.; Gottschalk, M.; Jacques, M. Truncation of the lipopolysaccharide outer core affects susceptibility to antimicrobial peptides and virulence of Actinobacillus pleuropneumoniae serotype 1. J. Biol. Chem. 2005, 280, 39104–39114. [Google Scholar] [CrossRef] [Green Version]
- Auger, E.; Deslandes, V.; Ramjeet, M.; Contreras, I.; Nash, J.H.E.; Harel, J.; Gottschalk, M.; Olivier, M.; Jacques, M. Host-pathogen interactions of Actinobacillus pleuropneumoniae with porcine lung and tracheal epithelial cells. Infect. Immun. 2009, 77, 1426–1441. [Google Scholar] [CrossRef] [Green Version]
- Fraile, L.; Alegre, A.; López-Jiménez, R.; Nofrarías, M.; Segalés, J. Risk factors associated with pleuritis and cranio-ventral pulmonary consolidation in slaughter-aged pigs. Vet. J. 2010, 184, 326–333. [Google Scholar] [CrossRef]
- Brewster, V.R.; Maiti, H.C.; Tucker, A.W.; Nevel, A. Associations between EP-like lesions and pleuritis and post trimming carcass weights of finishing pigs in England. Livest. Sci. 2017, 201, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Straw, B.E.; Shin, S.J.; Yeager, A.E. Effect of pneumonia on growth rate and feed efficiency of minimal disease pigs exposed to Actinobacillus pleuropneumoniae and Mycoplasma hyopneumoniae. Prev. Vet. Med. 1990, 9, 287–294. [Google Scholar] [CrossRef]
- Bossé, J.T.; Li, Y.; Rogers, J.; Fernandez Crespo, R.; Li, Y.; Chaudhuri, R.R.; Langford, P.R. Whole genome sequencing for surveillance of antimicrobial resistance in Actinobacillus pleuropneumoniae. Front. Microbiol. 2017, 8, 311. [Google Scholar] [CrossRef]
- Vanni, M.M.; Merenda, G.; Barigazzi, C.; Garbarino, A.; Luppi, R.; Tognetti, L.; Intorre, L. Antimicrobial resistance of Actinobacillus pleuropneumoniae isolated from swine. Vet. Microbiol. 2012, 23, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Bossé, J.T.; Li, Y.; Atherton, T.G.; Walker, S.; Williamson, S.M.; Rogers, J. Characterisation of a mobilizable plasmid conferring florfenicol and chloramphenicol resistance in Actinobacillus pleuropneumoniae. Vet. Microbiol. 2015, 178, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha da Silva, G.; Rossi, C.C.; Santana, M.F.; Langford, P.R.; Bosse, J.T.; Bazzolli, D.M. p518, a small floR plasmid from a South American isolate of Actinobacillus pleuropneumoniae. Vet. Microbiol. 2017, 204, 129–132. [Google Scholar] [CrossRef]
- Van Overbeke, I.; Chiers, K.; Ducatelle, R.; Haesebrouck, F. Effect of endobronchial challenge with Actinobacillus pleuropneumoniae serotype 9 of pigs vaccinated with a vaccine containing Apx toxins and transferrin-binding proteins. J. Vet. Medicine. B Infect. Dis. Vet. Public Health 2001, 48, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ramjeet, M.; Deslandes, V.; Goure, J.; Jacques, M. Actinobacillus pleuropneumoniae vaccines: From bacterins to new insights into vaccination strategies. Anim. Health Res. Rev. 2008, 9, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Wang, Y.; Wang, C.; Guo, Y.; Peng, Y.; Liu, J.; Liu, S. Evaluation of multicomponent recombinant vaccines against Actinobacillus pleuropneumoniae in mice. Acta Vet. Scand. 2010, 52, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevenon, J.; Ivoc, M.; Rozsnyay, Z.; Alapi, I.; Imre, A.; Tenk, M. Coglapix, an Actinobacillus pleuropneumoniae inactivated vaccine induce high levels of anti-Apx and anti-capsular antibodies. In Proceedings of the European Symposium of Porcine Health Management, Sorrento, Italy, 7–9 May 2014; Volume 6, p. 245. [Google Scholar]
- Tumamao, J.Q.; Bowles, R.E.; Bosch, H.; Klaasen, H.; Fenwick, B.W.; Storie, G.J.; Blackall, P.J. Comparison of the efficacy of a subunit and a live streptomycin-dependent porcine pleuropneumonia vaccine. Aust. Vet. J. 2004, 82, 370–374. [Google Scholar] [CrossRef] [Green Version]
- van den Bosch, H.; Frey, J. Interference of outer membrane protein PalA with protective immunity against Actinobacillus pleuropneumoniae infections in vaccinated pigs. Vaccine 2003, 2, 3601–3607. [Google Scholar] [CrossRef]
- Brackmann, J.; Beckmann, K.; Baier, S. Zur Verbreitung und Diagnostik von Actinobacillus pleuropneumoniae. Prakt. Tierarzt 2015, 96, 372–381. [Google Scholar]
- Dottori, M.; Nigrelli, A.D.; Bonilauri, P.; Merialdi, G.; Gozio, S.; Cominotti, F. Proposta di un nuovo sistema di punteggiatura delle pleuriti suine in sede di macellazione. La griglia s.p.e.s. (slaughterhouse pleuritis evaluation system). Large Anim. Rev. 2007, 13, 161–165. [Google Scholar]
- Christensen, G.; Sørensen, V.; Mousing, J. Diseases of the respiratory system. In Diseases of Swine 8th; Wiley-Blackwell: Hoboken, NJ, USA, 1999; pp. 927–928. [Google Scholar]
- Madec, F.; Kobisch, M. Bilan lésionnel des poumons de porcs charcutiers à l‘abattoir. Journ. Rech Porc. En Fr. 1982, 14, 405–412. [Google Scholar]
- Tucker, A.W.; McKinley, T.J.; Jaeger, H.J. Pleurisy in Pigs: Associated Risk Factors and Impact on Health, Welfare and Performance; Bpex: Warwickshire, UK, 2009; pp. 1–94. [Google Scholar]
- Cleveland Nielsen, A.; Nielsen, E.; Ersbøll, A. Chronic pleuritis in Danish slaughter pig herds. Prev. Vet. Medicine 2002, 55, 121–135. [Google Scholar] [CrossRef]
- Cruijsen, T.; van Leengoed, L.A.; Kamp, E.M.; Hunneman, W.A.; Riepema, K.; Bartelse, A.; Verheijden, J.H. Prevalence and development of antibodies neutralizing the haemolysin and cytotoxin of Actinobacillus pleuropneumoniae in three infected pig herds. Vet. Q. 1995, 17, 96–100. [Google Scholar] [CrossRef]
- Sjölund, M.; Fossum, C.; Martin de la Fuente, A.J.; Alava, M.; Juul-Madsen, H.R.; Lampreave, F.; Wallgren, P. Effects of different antimicrobial treatments on serum acute phase responses and leucocyte counts in pigs after a primary and a secondary challenge infection with Actinobacillus pleuropneumoniae. Vet. Rec. 2011, 169, 70. [Google Scholar] [CrossRef]
- Mbow, M.L.; De Gregorio, E.; Ulmer, J.B. Alum’s adjuvant action: Grease is the word. Nat. Med. 2011, 17, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Cárceles, S.; Cuestas, F.; Celma, S.; Oliver-Ferrando, S.; Del Carmen, P.; Carmona, M.; Lasierra, M.; Espigares, D. In Proceedings of the Porcine Pleuropneumonia Control with Coglapix® Vaccination under Field Conditions, Utrecht, The Netherlands, 22–24 May 2019.
| Groups | N | Average ± Standard Deviation (kg) | p-Value | |
|---|---|---|---|---|
| Weight at weaning | Group 1 | 300 | 6.00 ± 1.16 | 0.899 |
| Group 2 | 300 | 6.00 ± 1.16 | ||
| Weight at nursery at second vaccination (kg) | Group 1 | 253 | 25.80 ± 4.73 | 0.755 |
| Group 2 | 261 | 25.70 ± 5.05 | ||
| Weight at end of finishing (kg) | Group 1 | 253 | 100.53 ± 9.99 | 0.109 |
| Group 2 | 261 | 99.00 ± 11.53 | ||
| ADWG weaning to end of finishing (kg) | Group 1 | 253 | 0.705 ± 0.07 | 0.095 |
| Group 2 | 261 | 0.693 ± 0.08 | ||
| ADWG weaning to second vaccination (kg) | Group 1 | 253 | 0.411 ± 0.09 | 0.801 |
| Group 2 | 261 | 0.410 ± 0.10 | ||
| ADWG from second vaccination to end of finishing (kg) | Group 1 | 253 | 0.869 ± 0.11 | 0.124 |
| Group 2 | 261 | 0.852 ± 0.13 |
| Groups | First Slaughter Group | Second Batch | Third Batch | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APP Index | Number of Evaluated Pigs | p-Value | Index | Number of Evaluated Pigs | p-Value | Index | Number of Evaluated Pigs | p-Value | Index | n | p-Value | |
| Group 1 | 0.26 | 47 | 0.038 | 0.08 | 99 | 0.003 | 1.15 | 84 | 0.368 | 0.51 | 230 | 0.024 |
| Group 2 | 0.78 | 18 | 0.37 | 91 | 1.41 | 68 | 0.81 | 177 | ||||
| First Vaccination | Second Vaccination | ||||||
|---|---|---|---|---|---|---|---|
| Mean Value (°C) | Standard Deviation | p-Value | Mean Value (°C) | Standard Deviation | p-Value | ||
| Temperature before vaccination | Group 1 | 39.45 | 0.5 | 0.316 | 39.49 | 0.6 | 0.329 |
| Group 2 | 39.34 | 0.4 | 39.64 | 0.6 | |||
| Temperature 6 h after vaccination | Group 1 | 40.08 | 0.5 | 0.977 | 40.31 | 1.0 | 0.928 |
| Group 2 | 40.08 | 0.5 | 40.34 | 0.8 | |||
| Temperature 24 h after vaccination | Group 1 | 39.14 | 0.6 | 0.954 | 39.04 | 0.6 | 0.714 |
| Group 2 | 39.14 | 0.5 | 38.99 | 0.5 | |||
| First Vaccination | Second Vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | p-Value | Score 0 | Score 1 | Score 2 | p-Value | ||
| ISR before vaccination | Group 1 | 99% | 1% | 0 | 0.997 | 99.3% | 0.7% | 0 | 0.261 |
| Group 2 | 99% | 1% | 0 | 98.3% | 1.7% | 0 | |||
| ISR 6 h after vaccination | Group 1 | 97.7% | 2% | 0 | 0.578 | 96% | 4% | 0 | 0.131 |
| Group 2 | 98.3% | 1.7% | 0 | 95% | 3.7% | 1.3% | |||
| ISR 24 h after vaccination | Group 1 | 98% | 2% | 0 | 0.761 | 99% | 1% | 0 | 0.606 |
| Group 2 | 98.3% | 1.7% | 0 | 98.8% | 1% | 0.1% | |||
| SAR before vaccination | Group 1 | 99% | 1% | 0 | 0.477 | 99.7% | 0.3% | 0 | 0.317 |
| Group 2 | 98.3% | 1.7% | 0 | 100% | 0 | 0 | |||
| SAR 6 h after vaccination | Group 1 | 98.3% | 1.7% | 0 | 0.423 | 96% | 3.7% | 0.3% | 0.590 |
| Group 2 | 97% | 2.7% | 0.3% | 96.7% | 3.3% | 0 | |||
| SAR 24 h after vaccination | Group 1 | 98.3% | 1.7% | 0 | 0.560 | 99% | 1% | 0 | 0.325 |
| Group 2 | 97.7% | 2.3% | 0 | 99.7% | 0.3% | 0 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hölzen, P.; Warnck, T.; Hoy, S.; Schlegel, K.; Hennig-Pauka, I.; Gaumann, H. Comparison of Protectivity and Safety of Two Vaccines against Actinobacillus pleuropneumoniae in a Field Study. Agriculture 2021, 11, 1143. https://doi.org/10.3390/agriculture11111143
Hölzen P, Warnck T, Hoy S, Schlegel K, Hennig-Pauka I, Gaumann H. Comparison of Protectivity and Safety of Two Vaccines against Actinobacillus pleuropneumoniae in a Field Study. Agriculture. 2021; 11(11):1143. https://doi.org/10.3390/agriculture11111143
Chicago/Turabian StyleHölzen, Peter, Tobias Warnck, Steffen Hoy, Kathleen Schlegel, Isabel Hennig-Pauka, and Horst Gaumann. 2021. "Comparison of Protectivity and Safety of Two Vaccines against Actinobacillus pleuropneumoniae in a Field Study" Agriculture 11, no. 11: 1143. https://doi.org/10.3390/agriculture11111143
APA StyleHölzen, P., Warnck, T., Hoy, S., Schlegel, K., Hennig-Pauka, I., & Gaumann, H. (2021). Comparison of Protectivity and Safety of Two Vaccines against Actinobacillus pleuropneumoniae in a Field Study. Agriculture, 11(11), 1143. https://doi.org/10.3390/agriculture11111143






