Combined Therapy of Probiotic Supplementation and Rehydration Improves Blood Dehydration Parameters and Decreases Mortality of Neonatal Piglets Naturally Infected with Porcine Epidemic Diarrhea Virus: A Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Farm
2.2. Rehydration Solutions and Probiotics
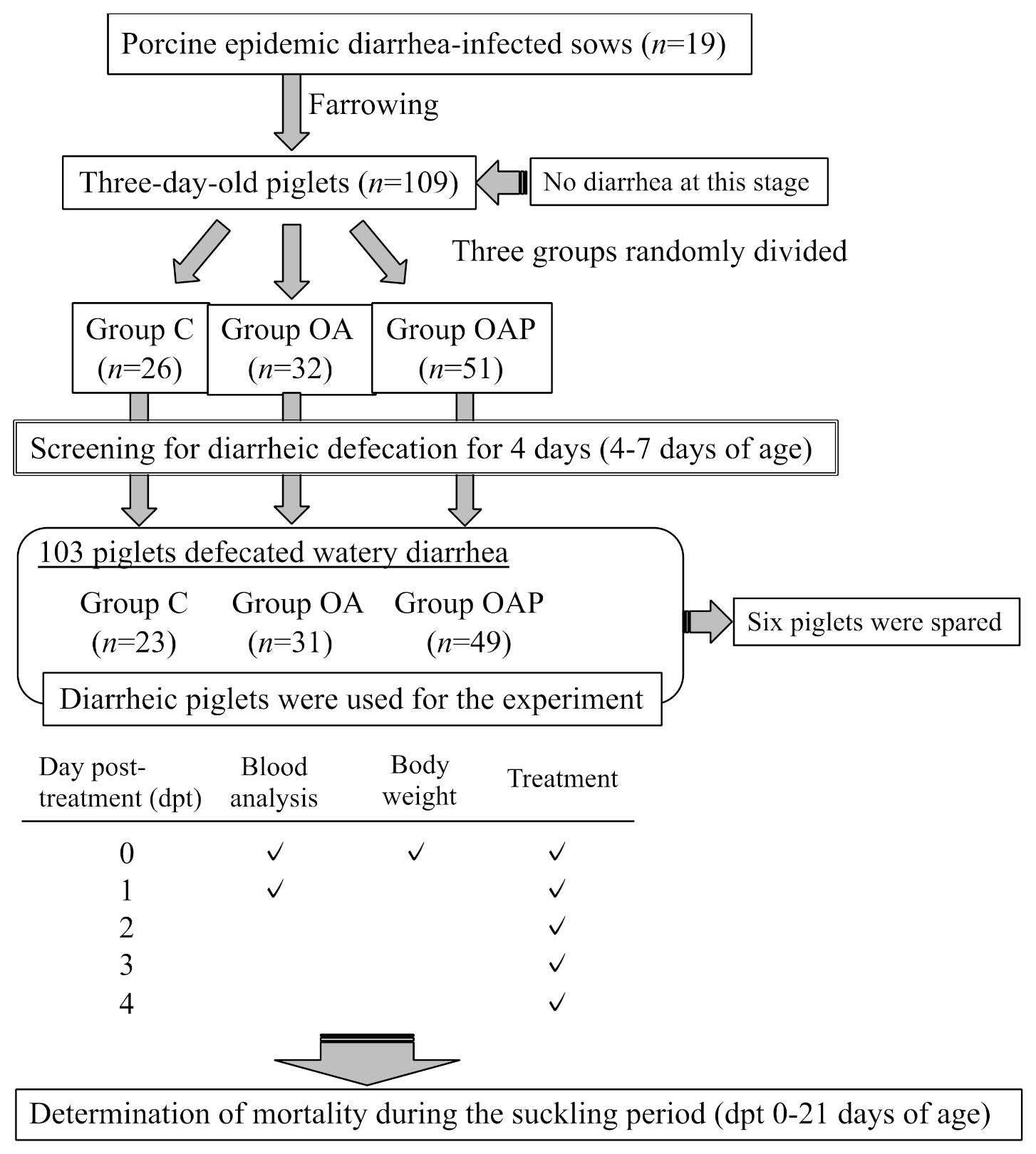
2.3. Animals and Experimental Design
2.4. Blood Collection and Analysis, and Monitoring of Mortality
2.5. Statistical Analysis
3. Results
3.1. Blood Dehydration Parameters
3.2. Mortality of PED-Infected Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sasaki, Y.; Alvarez, J.; Sekiguchi, S.; Sueyoshi, M.; Otake, S.; Perez, A. Epidemiological factors associated to spread of porcine epidemic diarrhea in Japan. Prev. Vet. Med. 2016, 123, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Murakami, S.; Takahashi, O.; Kodera, A.; Masuda, T.; Itoh, S.; Miyazaki, A.; Ohashi, S.; Tsutsui, T. Molecular characterization of pig epidemic diarrhoea viruses isolated in Japan from 2013 to 2014. Infect. Genet. Evol. 2015, 36, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Murphy, F.; Gibbs, E.; Horzinek, M.; Studdert, M. Coronaviridae. In Veterinary Virology, 3rd ed.; Murphy, F., Gibbs, E., Horzinek, M., Studdert, M., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 495–508. [Google Scholar]
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef]
- Sueyoshi, M.; Tsuda, T.; Yamazaki, K.; Yoshida, K.; Nakazawa, M.; Sato, K.; Minami, T.; Iwashita, K.; Watanabe, M.; Suzuki, Y.; et al. An immunohistochemical investigation of porcine epidemic diarrhoea. J. Comp. Pathol. 1995, 113, 59–67. [Google Scholar] [CrossRef]
- Shibata, I.; Tsuda, T.; Mori, M.; Ono, M.; Sueyoshi, M.; Uruno, K. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet. Microbiol. 2000, 72, 173–182. [Google Scholar] [CrossRef]
- Khin Maung, U.; Greenough, W.B., 3rd. Cereal-based oral rehydration therapy. I. Clinical studies. J. Pediatrics 1991, 118, S72–S79. [Google Scholar] [CrossRef]
- Lexmond, W.S.; Rufo, P.A.; Fiebiger, E.; Lencer, W.I. Electrophysiological studies into the safety of the anti-diarrheal drug clotrimazole during oral rehydration therapy. PLoS Negl. Trop. Dis. 2015, 9, e0004098. [Google Scholar] [CrossRef] [Green Version]
- Drolet, R.; Morin, M.; Fontaine, M. Fluid therapy trials in neonatal piglets infected with transmissible gastroenteritis virus. Can. J. Comp. Med. 1985, 49, 357–360. [Google Scholar]
- Silanikove, N. Effects of oral, intraperitoneal and intrajugular rehydrations on water retention, rumen volume, kidney function and thirst satiation in goats. Comp. Biochem. Physiol. A Comp. Physiol. 1991, 98, 253–258. [Google Scholar] [CrossRef]
- Silanikove, N. Comparison among the effects of oral, intraperitoneal and intravenous fluid-loading on kidney function and drinking in goats. Comp. Biochem. Physiol. Part A Physiol. 1994, 109, 749–754. [Google Scholar] [CrossRef]
- Basu, S.; Paul, D.K.; Ganguly, S.; Chatterjee, M.; Chandra, P.K. Efficacy of high-dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: A randomized controlled trial. J. Clin. Gastroenterol. 2009, 43, 208–213. [Google Scholar] [CrossRef]
- Depoorter, L.; Vandenplas, Y. Probiotics in Pediatrics. A Review and Practical Guide. Nutrients 2021, 13, 2176. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Szajewska, H.; Mrukowicz, J.Z. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: A systematic review of published randomized, double-blind, placebo-controlled trials. J. Pediatr. Gastroenterol. Nutr. 2001, 33 (Suppl. 2), S17–S25. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, S.; Chattha, K.S.; Vlasova, A.N.; Rajashekara, G.; Saif, L.J. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes 2014, 5, 639–651. [Google Scholar] [CrossRef]
- Tsukahara, T.; Nakanishi, N.; Matsubara, N.; Itoh, M.; Ushida, K. The effect of Enterococcus faecalis cell preparation (EC-12) against the diarrhea in the nursing and weaning piglets under the clinical condition. Proc. Jpn. Pig Vet. Soc. 2006, 48, 19–23. [Google Scholar]
- Tsuruta, T.; Inoue, R.; Tsukahara, T.; Matsubara, N.; Hamasaki, M.; Ushida, K. A cell preparation of Enterococcus faecalis strain EC-12 stimulates the luminal immunoglobulin A secretion in juvenile calves. Anim. Sci. J. 2009, 80, 206–211. [Google Scholar] [CrossRef]
- Sakai, Y.; Tsukahara, T.; Bukawa, W.; Matsubara, N.; Ushida, K. Cell preparation of Enterococcus faecalis strain EC-12 prevents vancomycin-resistant enterococci colonization in the cecum of newly hatched chicks. Poult. Sci. 2006, 85, 273–277. [Google Scholar] [CrossRef]
- Inatomi, T.; Amatatsu, M.; Romero-Pérez, G.A.; Inoue, R.; Tsukahara, T. Dietary probiotic compound improves reproductive performance of porcine epidemic diarrhea virus-infected sows reared in a Japanese commercial swine farm under vaccine control condition. Front. Immunol. 2017, 8, 1877. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, T.; Inatomi, T.; Otomaru, K.; Amatatsu, M.; Romero-Pérez, G.A.; Inoue, R. Probiotic supplementation improves reproductive performance of unvaccinated farmed sows infected with porcine epidemic diarrhea virus. Anim. Sci. J. 2018, 89, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Kong, M.S.; Lai, M.W.; Chao, H.C.; Chang, K.W.; Chen, S.Y.; Huang, Y.C.; Chiu, C.H.; Li, W.C.; Lin, P.Y.; et al. Probiotics have clinical, microbiologic, and immunologic efficacy in acute infectious diarrhea. Pediatr. Infect. Dis. J. 2010, 29, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Liu, P.Y.; Chen, Y.Y.; Nong, B.R.; Huang, I.F.; Hsieh, K.S.; Chen, K.T. Three-combination probiotics therapy in children with salmonella and rotavirus gastroenteritis. J. Clin. Gastroenterol. 2014, 48, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Otsuka, M.; Nishi, K.; Nishi, Y.; Tsukano, K.; Noda, J.; Higuchi, H.; Suzuki, K. Evaluation of probiotic therapy for calf diarrhea with serum diamine oxidase activity as an indicator. Jpn. J. Vet. Res. 2019, 67, 305–311. [Google Scholar] [CrossRef]
- Hayakawa, T.; Masuda, T.; Kurosawa, D.; Tsukahara, T. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim. Sci. J. 2016, 87, 1501–1510. [Google Scholar] [CrossRef]
- Kweon, C.H.; Kwon, B.J.; Woo, S.R.; Kim, J.M.; Woo, G.H.; Son, D.H.; Hur, W.; Lee, Y.S. Immunoprophylactic effect of chicken egg yolk immunoglobulin (Ig Y) against porcine epidemic diarrhea virus (PEDV) in piglets. J. Vet. Med. Sci. 2000, 62, 961–964. [Google Scholar] [CrossRef] [Green Version]
- Shibata, I.; Ono, M.; Mori, M. Passive protection against porcine epidemic diarrhea (PED) virus in piglets by colostrum from immunized cows. J. Vet. Med. Sci. 2001, 63, 655–658. [Google Scholar] [CrossRef] [Green Version]
- Schwedhelm, L.; Kirchner, D.; Klaus, B.; Bachmann, L. Experimentally induced hyperchloremic and DL-lactic acidosis in calves: An attempt to study the effects of oral rehydration on acid-base status. J. Dairy Sci. 2013, 96, 2464–2475. [Google Scholar] [CrossRef] [Green Version]
- Shaoul, R.; Okev, N.; Tamir, A.; Lanir, A.; Jaffe, M. Value of laboratory studies in assessment of dehydration in children. Ann. Clin. Biochem. 2004, 41, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Martini, W.Z.; Pusateri, A.E.; Uscilowicz, J.M.; Delgado, A.V.; Holcomb, J.B. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J. Trauma Acute Care Surg. 2005, 58, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Cieza, J.A.; Hinostroza, J.; Huapaya, J.A.; León, C.P. Sodium chloride 0.9% versus Lactated Ringer in the management of severely dehydrated patients with choleriform diarrhoea. J. Infect. Dev. Ctries. 2013, 7, 528–532. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, B.; Duncker, S.; Barth, S.; Bauerfeind, R.; Gruber, A.D.; Deppenmeier, S.; Breves, G. Preventive effects of the probiotic Escherichia coli strain Nissle 1917 on acute secretory diarrhea in a pig model of intestinal infection. Dig. Dis. Sci. 2006, 51, 724–731. [Google Scholar] [CrossRef]
- Bernardeau, M.; Lehtinen, M.J.; Forssten, S.D.; Nurminen, P. Importance of the gastrointestinal life cycle of Bacillus for probiotic functionality. J. Food Sci. Technol. 2017, 54, 2570–2584. [Google Scholar] [CrossRef]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The use of lactic Acid bacteria as a probiotic in Swine diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Weström, B.R.; Svendsen, J.; Karlsson, B.W. Protease inhibitor levels in porcine mammary secretions. Biol. Neonate 1982, 42, 185–194. [Google Scholar] [CrossRef]
- Ali, R. The Use of Probiotic with ORS and ORS Only in Children with Acute Diarrhea. J. Coll. Physicians Surg. Pak. 2019, 29, 1179–1182. [Google Scholar] [CrossRef]
- Inoue, R.; Tsukahara, T.; Nakatani, M.; Okutani, M.; Nishibayashi, R.; Ogawa, S.; Harayama, T.; Nagino, T.; Hatanaka, H.; Fukuta, K.; et al. Weaning Markedly Affects Transcriptome Profiles and Peyer’s Patch Development in Piglet Ileum. Front. Immunol. 2015, 6, 630. [Google Scholar] [CrossRef] [Green Version]
- Suresh, K.P. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inatomi, T.; Tsukahara, T.; Romero-Pérez, G.A.; Inoue, R. Combined Therapy of Probiotic Supplementation and Rehydration Improves Blood Dehydration Parameters and Decreases Mortality of Neonatal Piglets Naturally Infected with Porcine Epidemic Diarrhea Virus: A Clinical Trial. Agriculture 2021, 11, 1058. https://doi.org/10.3390/agriculture11111058
Inatomi T, Tsukahara T, Romero-Pérez GA, Inoue R. Combined Therapy of Probiotic Supplementation and Rehydration Improves Blood Dehydration Parameters and Decreases Mortality of Neonatal Piglets Naturally Infected with Porcine Epidemic Diarrhea Virus: A Clinical Trial. Agriculture. 2021; 11(11):1058. https://doi.org/10.3390/agriculture11111058
Chicago/Turabian StyleInatomi, Takio, Takamitsu Tsukahara, Gustavo A. Romero-Pérez, and Ryo Inoue. 2021. "Combined Therapy of Probiotic Supplementation and Rehydration Improves Blood Dehydration Parameters and Decreases Mortality of Neonatal Piglets Naturally Infected with Porcine Epidemic Diarrhea Virus: A Clinical Trial" Agriculture 11, no. 11: 1058. https://doi.org/10.3390/agriculture11111058
APA StyleInatomi, T., Tsukahara, T., Romero-Pérez, G. A., & Inoue, R. (2021). Combined Therapy of Probiotic Supplementation and Rehydration Improves Blood Dehydration Parameters and Decreases Mortality of Neonatal Piglets Naturally Infected with Porcine Epidemic Diarrhea Virus: A Clinical Trial. Agriculture, 11(11), 1058. https://doi.org/10.3390/agriculture11111058





