Effect of Plastic Mulching on Soil Carbon and Nitrogen Cycling-Related Bacterial Community Structure and Function in a Dryland Spring Maize Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design and Sampling
2.3. Soil Properties Analysis
2.4. DNA Extraction and Sequencing
2.5. Data Analysis
3. Results and Discussion
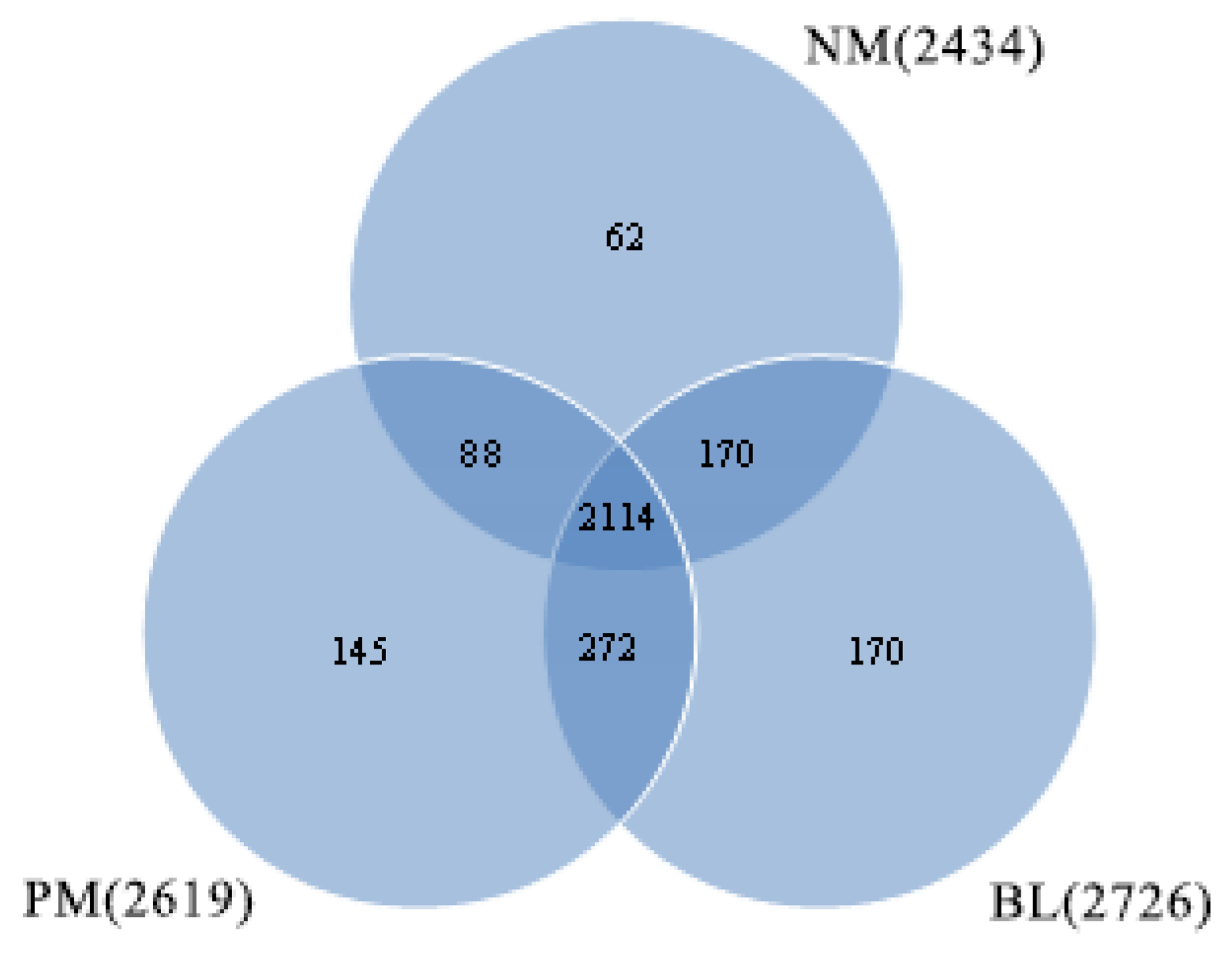
3.1. Soil Bacterial Community
3.2. Diversity of Soil Bacteria
3.3. Functional Group Responses
3.3.1. Soil Carbon Cycling-Related Microbes
3.3.2. Soil Nitrogen Cycling-Related Microbes
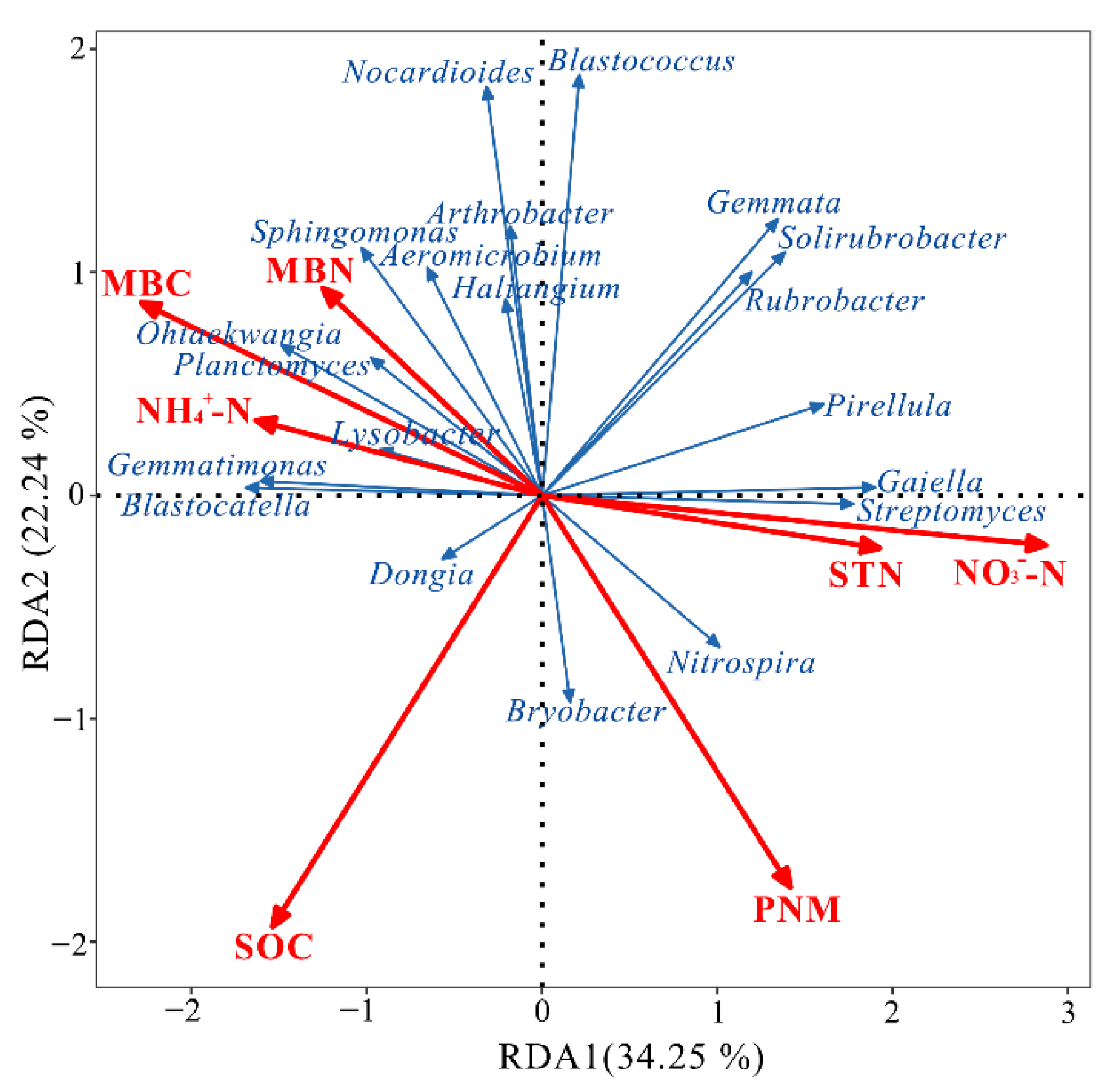
3.4. Relationships between Soil Properties and Bacterial Community Composition
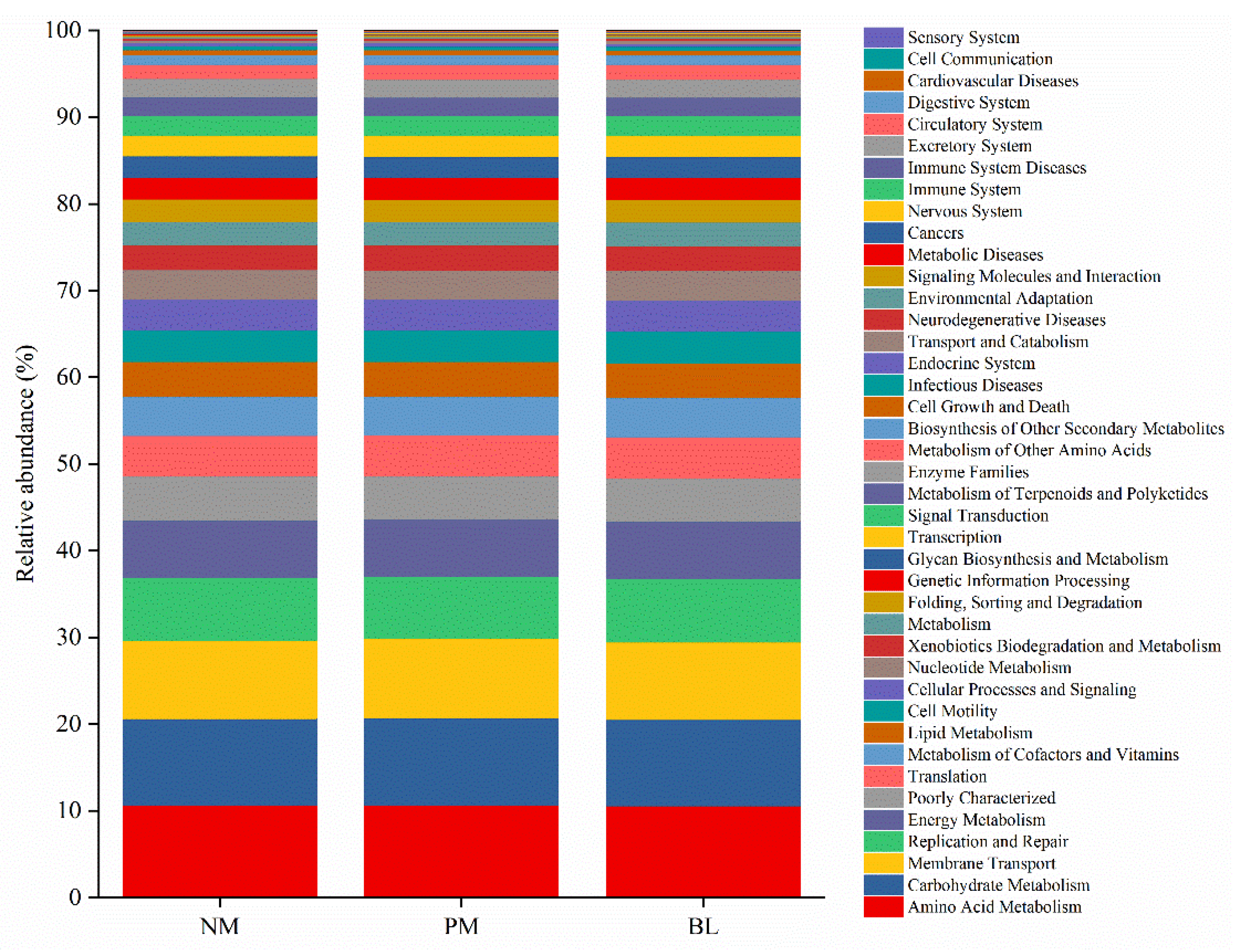
3.5. Prediction of Functional Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Dai, K.; Zhang, D.; Zhang, X.; Wang, Y.; Zhao, Q.; Cai, D.; Hoogmoed, W.; Oenema, O. Dryland maize yields and water use efficiency in response to tillage/crop stubble and nutrient management practices in China. Field Crops Res. 2011, 120, 47–57. [Google Scholar] [CrossRef]
- Zhou, L.; Li, F.; Jin, S.; Song, Y. How two ridges and the furrow mulched with plastic film affect soil water, soil temperature and yield of maize on the semiarid Loess Plateau of China. Field Crop Res. 2009, 113, 41–47. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Zhang, A.; Chen, J.; Cheng, G.; Sun, B.; Pi, X.; Dyck, M.; Si, B.; Zhao, Y. Effects of straw and plastic film mulching on greenhouse gas emissions in Loess Plateau, China: A field study of 2 consecutive wheat-maize rotation cycles. Sci. Total Environ. 2017, 579, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, G.; Guo, T.; Xing, Y.; Mo, F.; Wang, H.; Fan, J.; Zhang, F. Effects of plastic mulch and nitrogen fertilizer on the soil microbial community, enzymatic activity and yield performance in a dryland maize cropping system. Eur. J. Soil Sci. 2021, 72, 400–412. [Google Scholar] [CrossRef]
- Saglam, M.; Sintim, H.Y.; Bary, A.I.; Miles, C.A.; Ghimire, S.; Inglis, D.A.; Flury, M. Modeling the effect of biodegradable paper and plastic mulch on soil moisture dynamics. Agric. Water Manag. 2017, 193, 240–250. [Google Scholar] [CrossRef]
- Li, Z.; Tian, C.; Zhang, R.; Mohamed, I.; Liu, Y.; Zhang, G.; Pan, J.; Chen, F. Plastic mulching with drip irrigation increases soil carbon stocks of natrargid soils in arid areas of northwestern China. Catena 2015, 133, 179–185. [Google Scholar] [CrossRef]
- Ma, D.; Chen, L.; Qu, H.; Wang, Y.; Misselbrook, T.; Jiang, R. Impacts of plastic film mulching on crop yields, soil water, nitrate, and organic carbon in Northwestern China: A meta-analysis. Agric. Water Manag. 2018, 202, 166–173. [Google Scholar] [CrossRef]
- Liu, L.; Hu, C.; Yang, P.; Ju, Z.; Olesen, J.E.; Tang, J. Effects of experimental warming and nitrogen addition on soil respiration and CH4 fluxes from crop rotations of winter wheat–soybean/fallow. Agric. For. Meteorol. 2015, 207, 38–47. [Google Scholar] [CrossRef]
- Nan, W.; Yue, S.; Hang, H.; Li, S.; Shen, Y. Effects of plastic film mulching on soil greenhouse gases (CO2, CH4 and N2O) concentration within soil profiles in maize fields on the Loess Plateau, China. J. Integr. Agric. 2016, 15, 451–464. [Google Scholar] [CrossRef]
- He, J.; Shen, J.; Zhang, L.; Zhu, Y.; Zheng, Y.; Xu, M.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef]
- Gardner, J.G.; Crouch, L.; Labourel, A.; Forsberg, Z.; Bukhman, Y.V.; Vaaje-Kolstad, G.; Gilbert, H.J.; Keating, D.H. Systems biology defines the biological significance of redox-active proteins during cellulose degradation in an aerobic bacterium. Mol. Microbiol. 2014, 94, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Prosser, J.I. Autotrophic nitrification in bacteria. Adv. Microb. Physiol. 1990, 30, 125–181. [Google Scholar]
- Könneke, M.; Bernhard, A.E.; José, R.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef]
- Wang, W.; Han, L.; Zhang, X.; Wei, K. Plastic film mulching affects N2O emission and ammonia oxidizers in drip irrigated potato soil in northwest China. Sci. Total Environ. 2021, 754, 142113. [Google Scholar] [CrossRef] [PubMed]
- Conrad, R. Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol. Rev. 1996, 60, 609–640. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Abatenh, E.; Gizaw, B.; Tsegaye, Z.; Tefera, G. Microbial function on climate change—A review. Open J. Environ. Biol. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, Y.L.; Gong, X.F.; Peng, Y.N.; Wang, Z.Y.; Ji, B. Plastic film mulching improved rhizosphere microbes and yield of rainfed spring wheat. Agric. For. Meteorol. 2018, 263, 130–136. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, X.G.; Hai, L.; Siddique, K.H.M.; Gan, Y.T.; Li, F.M. Film fully-mulched ridge-furrow cropping affects soil biochemical properties and maize nutrient uptake in a rainfed semi-arid environment. Soil Sci. Plant Nutr. 2014, 60, 486–498. [Google Scholar] [CrossRef]
- Akihiko, M.; Satoshi, I.; Nobuaki, F.; Hiroaki, H. Influence for soil environment by continuing use of biodegradable plastic. J. Polym. Environm. 2011, 19, 622–627. [Google Scholar]
- Shen, Y.; Chen, Y.; Li, S. Microbial functional diversity, biomass and activity as affected by soil surface mulching in a semiarid farmland. Plos One 2016, 11, e159144. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Si, P.; Liu, E.; Yan, C.; Zhang, Z.; Zhang, Y. Influence of film mulching on soil microbial community in a rainfed region of northeastern China. Sci Rep. 2017, 7, 8468. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, Z.; Chen, C. Effect of mulching on labile soil organic matter pools, microbial community functional diversity and nitrogen transformations in two hardwood plantations of subtropical Australia. Appl. Soil Ecol. 2008, 40, 229–239. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Chen, R.; Liu, W.; Sainju, U.M. Soil carbon dioxide emissions in response to precipitation frequency in the Loess Plateau, China. Appl. Soil Ecol. 2015, 96, 288–295. [Google Scholar] [CrossRef]
- Aziz, I.; Mahmood, T.; Islam, K.R. Effect of long term no-till and conventional tillage practices on soil quality. Soil Till. Res. 2013, 131, 28–35. [Google Scholar] [CrossRef]
- Fu, X.; Wang, J.; Zhao, D. Effects of plastic film mulching on soil carbon and nitrogen fractions in a dryland spring maize field on the Loess Plateau. J. Soil Water Conserv. 2017, 31, 239–243. [Google Scholar]
- Haney, R.L.; Franzluebbers, A.J.; Porter, E.B.; Hons, F.M.; Zuberer, D.A. Soil carbon and nitrogen mineralization: Influence of drying temperature. Soil Sci. Soc. Am. J. 2004, 68, 489–492. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Hons, F.M.; Zuberer, D.A. Soil organic carbon, microbial biomass, and mineralizable carbon and nitrogen in sorghum. Soil Sci. Soc. Am. J. 1995, 59, 460–466. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1:1–e1:11. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D.A. Brock Biology of Microorganisms, 14th.; Pearson Education: San Francisco, VA, USA, 2015; p. 633. [Google Scholar]
- Pankratov, T.A.; Kirsanova, L.A.; Kaparullina, E.N.; Kevbrin, V.V.; Dedysh, S.N. Telmatobacter bradus gen. nov., sp. nov., a cellulolytic facultative anaerobe from subdivision 1 of the Acidobacteria, and emended description of Acidobacterium capsulatum Kishimoto et al. 1991. Int. J. Syst. Evol. Microbiol. 2012, 62, 430–437. [Google Scholar] [CrossRef]
- Lu, S.P.; Gischkat, S.; Reiche, M.; Akob, D.M.; Hallberg, K.B.; Küsel, K. Ecophysiology of Fe-cycling bacteria in acidic sediments. Appl. Environ. Microbiol. 2010, 76, 8174–8183. [Google Scholar] [CrossRef] [PubMed]
- Piao, Z.; Yang, L.; Zhao, L.; Yin, S. Actinobacterial community structure in soils receiving long-term organic and inorganic amendments. Appl. Environ. Microbiol. 2008, 74, 526–530. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Omiya, T.; Hata, S.; Izui, K. Molecular characterization of a phosphoenolpyruvate carboxylase from a thermophilic cyanobacterium, Synechococcus vulcanus with unusual allosteric properties. Plant Cell Physiol. 2002, 43, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Owttrim, G.W.; Colman, B. Purification and characterization of phosphoenolpyruvate carboxylase from a cyanobacterium. J. Bacteriol. 1986, 168, 207–212. [Google Scholar] [CrossRef]
- Farmer, J.; Zhang, B.; Jin, X.; Zhang, P.; Wang, J. Long-term effect of plastic film mulching and fertilization on bacterial communities in a brown soil revealed by high through-put sequencing. Arch. Agron. Soil Sci. 2017, 63, 230–241. [Google Scholar] [CrossRef]
- Takaichi, S.; Maoka, T.; Takasaki, K.; Hanada, S. Carotenoids of Gemmatimonas aurantiaca (Gemmatimonadetes): Identification of a novel carotenoid, deoxyoscillol 2-rhamnoside, and proposed biosynthetic pathway of oscillol 2, 2′-dirhamnoside. Microbiology 2010, 156, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Aylward, F.O.; McDonald, B.R.; Adams, S.M.; Valenzuela, A.; Schmidt, R.A.; Goodwin, L.A.; Woyke, T.; Currie, C.R.; Suen, G.; Poulsen, M. Comparison of 26 sphingomonad genomes reveals diverse environmental adaptations and biodegradative capabilities. Appl. Environ. Microbiol. 2013, 79, 3724–3733. [Google Scholar] [CrossRef]
- Zhou, J.; Xue, K.; Xie, J.; Deng, Y.; Wu, L.; Cheng, X.; Fei, S.; Deng, S.; He, Z.; Van Nostrand, J.D. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Chang. 2012, 2, 106–110. [Google Scholar] [CrossRef]
- Singh, J.S.; Gupta, S. Plant decomposition and soil respiration in terrestrial ecosystems. Bot. Rev. 1977, 43, 449–528. [Google Scholar] [CrossRef]
- Jang, H.; Chen, K. Production and characterization of thermostable cellulases from Streptomyces transformant T3-1. World J. Microbiol. Biotechnol. 2003, 19, 263–268. [Google Scholar] [CrossRef]
- Ehrt, S.; Schnappinger, D. Mycobacterium tuberculosis virulence: Lipids inside and out. Nat. Med. 2007, 13, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Dreisbach, J.H.; Spain, J.C. Biodegradation of p-nitrophenol via 1, 2, 4-benzenetriol by an Arthrobacter sp. Appl. Environ. Microbiol. 1994, 60, 3030–3032. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K.; Minami, K. Effect of organic matter application on methane emission from some Japanese paddy fields. Soil Sci. Plant Nutr. 1990, 36, 599–610. [Google Scholar] [CrossRef]
- Liebner, S.; Rublack, K.; Stuehrmann, T.; Wagner, D. Diversity of aerobic methanotrophic bacteria in a permafrost active layer soil of the Lena Delta, Siberia. Microb. Ecol. 2009, 57, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Allan, D.L. Exploring the benefits of organic nutrient sources for crop production and soil quality. HortTechnology 2007, 17, 422–430. [Google Scholar] [CrossRef]
- Lynch, R.C.; Darcy, J.L.; Kane, N.C.; Nemergut, D.R.; Schmidt, S.K. Metagenomic evidence for metabolism of trace atmospheric gases by high-elevation desert Actinobacteria. Front. Microbiol. 2014, 5, 698:1–698:13. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, A. Nitrogen oxides and tropical agriculture. Nature 1998, 392, 866–867. [Google Scholar] [CrossRef]
- Huang, B.; Chen, G. Effects of cultivation on N2O emission and seasonal quantitative variations of related microbes in a temperate grassland soil. J. Environ. Sci. 2001, 13, 376–379. [Google Scholar]
- Cuello, J.P.; Hwang, H.Y.; Gutierrez, J.; Kim, S.Y.; Kim, P.J. Impact of plastic film mulching on increasing greenhouse gas emissions in temperate upland soil during maize cultivation. Appl. Soil Ecol. 2015, 91, 48–57. [Google Scholar] [CrossRef]
- Yu, Y.; Tao, H.; Jia, H.; Zhao, C. Impact of plastic mulching on nitrous oxide emissions in China's arid agricultural region under climate change conditions. Atmos. Environ. 2017, 158, 76–84. [Google Scholar] [CrossRef]
- Wu, Z.; Hao, Z.; Sun, Y.; Guo, L.; Huang, L.; Zeng, Y.; Wang, Y.; Yang, L.; Chen, B. Comparison on the structure and function of the rhizosphere microbial community between healthy and root-rot Panax notoginseng. Appl. Soil Ecol. 2016, 107, 99–107. [Google Scholar] [CrossRef]
- Shrestha, P.M.; Kube, M.; Reinhardt, R.; Liesack, W. Transcriptional activity of paddy soil bacterial communities. Environ. Microbiol. 2009, 11, 960–970. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, Y.; Wang, S.; Yan, X.; Zhu, Y.; Jia, Z. Microbial metabolism in typical flooded paddy soils. Acta microbial. Sin. 2014, 54, 1033–1044. [Google Scholar]


| Sample | NM | PM | BL |
|---|---|---|---|
| Nseqs | 24258 ± 3719 a | 28429 ± 4287 a | 40027 ± 4607 a |
| OTUs | 2434 ± 106 a | 2619 ± 202 a | 2726 ± 185 a |
| Chao | 2835.333 ± 87.345 a | 3016.138 ± 169.537 a | 3192.375 ± 140.838 a |
| ACE | 2840.142 ± 82.245 a | 2956.100 ± 160.015 a | 3088.118 ± 137.283 a |
| Coverage | 0.977 ± 0.005 a | 0.981 ± 0.009 a | 0.987 ± 0.007 a |
| Shannon | 6.818 ± 0.051 a | 6.923 ± 0.159 a | 6.866 ± 0.130 a |
| Simpson | 0.00255 ± 0.000 a | 0.00249 ± 0.000 a | 0.00246 ± 0.000 a |
| Function | Microbe | Relative Abundance (%) | ||
|---|---|---|---|---|
| NM | PM | BL | ||
| Carbon cycling | Decomposition of Organics | |||
| Cellvibrio Nocardia | 0 b 0.012 ± 0.003 a | 0.016 ± 0.008 a 0.012 ± 0.001 a | 0.034 ± 0.026 a 0.021 ± 0.006 a | |
| Streptomyces | 0.112 ± 0.016 a | 0.148 ± 0.019 a | 0.071 ± 0.017 b | |
| Bacillus | 0.024 ± 0.005 b | 0.079 ± 0.030 a | 0.024 ± 0.007 b | |
| Pseudomonas | 0.017 ± 0.005 b | 0.022 ± 0.002 b | 0.108 ± 0.062 a | |
| Mycobacterium | 0.134 ± 0.038 a | 0.180 ± 0.041 a | 0.145 ± 0.013 a | |
| Arthrobacter | 0.332 ± 0.054 a | 0.192 ± 0.058 a | 0.272 ± 0.019 a | |
| CO2 fixation | ||||
| Nostoc | 0 a | 0 a | 0.002 ± 0.001 a | |
| Methanotroph | ||||
| Methylobacterium | 0.002 ± 0.001 b | 0.013 ± 0.000 a | 0.005 ± 0.001 b | |
| Nitrogen cycling | Nitrogen fixation | |||
| Rhizomicrobium | 0.025 ± 0.006 a | 0.028 ± 0.009 a | 0.022 ± 0.005 a | |
| Nitrification | ||||
| Nitrosospira | 0.019 ± 0.004 a | 0.023 ± 0.010 a | 0.032 ± 0.018 a | |
| Nitrospira | 0.235 ± 0.017 b | 0.298 ± 0.002 a | 0.191 ± 0.008 c | |
| Planctomyces | 0.319 ± 0.025 a | 0.293 ± 0.107 a | 0.296 ± 0.023 a | |
| Pirellula | 0.508 ± 0.030 a | 0.551 ± 0.063 a | 0.470 ± 0.053 a | |
| Pseudonocardia | 0.040 ± 0.012 a | 0.059 ± 0.004 a | 0.048 ± 0.004 a | |
| Denitrification | ||||
| Bacillus | 0.024 ± 0.005 b | 0.079 ± 0.030 a | 0.024 ± 0.007 b | |
| Pseudomonas | 0.017 ± 0.005 a | 0.022 ± 0.002 a | 0.021 ± 0.002 a | |
| Flavisolibacter | 0.112 ± 0.012 b | 0.100 ± 0.033 b | 0.246 ± 0.044 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ding, L.; Liu, W.; Wang, J.; Qian, Y. Effect of Plastic Mulching on Soil Carbon and Nitrogen Cycling-Related Bacterial Community Structure and Function in a Dryland Spring Maize Field. Agriculture 2021, 11, 1040. https://doi.org/10.3390/agriculture11111040
Wang S, Ding L, Liu W, Wang J, Qian Y. Effect of Plastic Mulching on Soil Carbon and Nitrogen Cycling-Related Bacterial Community Structure and Function in a Dryland Spring Maize Field. Agriculture. 2021; 11(11):1040. https://doi.org/10.3390/agriculture11111040
Chicago/Turabian StyleWang, Sen, Liuyi Ding, Wanyu Liu, Jun Wang, and Yali Qian. 2021. "Effect of Plastic Mulching on Soil Carbon and Nitrogen Cycling-Related Bacterial Community Structure and Function in a Dryland Spring Maize Field" Agriculture 11, no. 11: 1040. https://doi.org/10.3390/agriculture11111040
APA StyleWang, S., Ding, L., Liu, W., Wang, J., & Qian, Y. (2021). Effect of Plastic Mulching on Soil Carbon and Nitrogen Cycling-Related Bacterial Community Structure and Function in a Dryland Spring Maize Field. Agriculture, 11(11), 1040. https://doi.org/10.3390/agriculture11111040










