Abstract
The flora of willow (Salix viminalis L.) plantations consists of various plant groups, including plants related to arable land, called segetal plants. Knowledge of this flora is important for maintaining biodiversity in agroecosystems. The aim of the study was to assess the segetal flora of the willow plantations in central Poland, depending on the land use before the establishment of the plantations (arable land or fallow) and the age of the plantations. Moreover, the aim was also to check for the presence of invasive, medicinal, poisonous and melliferous species. The vegetation accompanying willow was identified based on an analysis of 60 phytosociological relevés performed using the Braun-Blanquet method. For each species, the following parameters were determined: the phytosociological class; family; geographical and historical group; apophyte origin; biological stability; life-form; and status as an invasive, medicinal (herbs), poisonous or melliferous species. The results were statistically processed. Segetal species accounted for 38% of the flora accompanying willow. The plantations on former arable land were richer in segetal species than those on fallow. Mostly, short-lived and native species dominated. In line with the age of the plantations, the number of segetal species decreased. The share of apophytes increased, and anthropophytes decreased. Furthermore, many valuable plants were found among the flora accompanying willow.
1. Introduction
Climate change caused by increasing emissions of greenhouse gases into the atmosphere, the depletion of fossil fuel resources and the rising demand for energy [1,2] has led the world to take an interest in renewable energy sources [3,4]. Legal regulations coming into being oblige states to increase the proportion of energy derived from renewable sources [5,6,7,8]. According to Directive 2009/28/EC of the European Parliament and of the Council (of 23 April 2009 on the promotion of the use of energy from renewable sources, repealing Directives 2001/77/EC and 2003/30/EC), by 2020, the share of energy from renewable sources (RES) in Poland should be 15% of total energy consumption. In 2018, this share was 11.3% of gross final energy consumption and was 73% of the expected share of RES [9].
One of the sources of renewable energy is biomass obtained from energy plants such as shrubs and trees that grow quickly after cutting, e.g., poplar (Populus sp.), willow (Salix viminalis L.) and black locust (Robinia pseudoacacia L.); perennial forbs, e.g., Virginia mallow Sida hermaphrodita (L.) Rusby; and perennial grasses, e.g., Miscanthus sp. From these plants, in the northern and central parts of Europe, including Poland, the main energy crop is willow [10]. Poland’s willow plantations were established mainly after 2000, and at present, willow is cultivated in Poland on about 8700 ha [11], including about 230 ha in Łódź Province ([12]; authors’ estimations). Plantations of Salix viminalis L. are established on various soils, especially poor, light (class IV–VI) arable land, including fallow lands, which can be managed in this way [13,14]. These plantations are located in field depressions, among arable land; they are bordered by small rivers, forests and grasslands, thus enriching the agricultural landscape and becoming an important element of the environment for numerous fauna and flora [15,16,17]. Moreover, the cultivation of Salix viminalis L. for energy purposes is one of the activities contributing to stopping climate change (carbon sequestration) [18,19]
The flora of the energy willow plantation includes different groups of plants: meadow, woodland, shrub, ruderal and segetal species, as well as shrubs and tree seedlings. Segetal plants, which are the subject of this work, are plants mainly associated with arable land, especially with cereal crops and root crops. Their occurrence is influenced by a number of factors: climatic, geo-environmental, soil and anthropogenic [20,21,22]. The introduction of new cultivars of crops; the use of seed material well cleared of diaspora weeds; and improvements in tillage technology, herbicides and mineral fertilizers, as well as changes in the structure of sown crops and the simplification of crop rotation, all define the structure of segetal flora [23,24,25,26,27]. Its biodiversity decreases with the intensification of agricultural production and the emergence of monocultural, large-area crops, and as research findings show, many species have already disappeared irretrievably [28]. On Salix viminalis L. plantations, segetal plants have different conditions for their development than in typical fields due to the different technology for cultivating this species. The long period of time (2–3 years) between successive willow harvests often has an effect on the limited light conditions, and also on the thermal and humidity conditions, which prevents the plant community from stabilizing [29]. Moreover, the flora of willow plantations grown for energy purposes depends on factors such as crop age and soil conditions [30,31], as well as the land use before the plantation was established [30,32].
Although Salix sp. has been cultivated in Poland for many years, to date, segetal plants occurring on willow plantations have only been dealt with sporadically. The vegetation accompanying willow was usually assessed in the first years of cultivation and presented as a whole [33,34,35]. The flora of older Salix viminalis L. plantations is particularly poorly researched. Knowledge of segetal plants present on willow plantations is important for maintaining the biodiversity of native flora in agroecosystems [27]. Moreover, they are an important element of agrobiocenoses, as many are valuable medicinal and melliferous species [36,37]. The aims of this study were as follows: (1) to determine and analyse, in a broad sense, the species composition of segetal flora on plantations of Salix viminalis L. in central Poland, depending on the land use before establishing plantations (arable land and fallow land); (2) to assess the formation of segetal flora depending on the age of Salix viminalis L. plantations; (3) to analyse the flora accompanying energy willow crops and the choice of factors that are important for biodiversity plant species: invasive, medicinal, poisonous and melliferous.
2. Materials and Methods
2.1. Study Area and Characteristics of the Plantations
The study area is located in Łódź Province in the central part of Poland. It is, like most of the Łódź region, situated on a plain area. The study was carried out on eight commercial Salix viminalis L. plantations, in five localities: Dmosin (1 plantation: N 51.542775; E 19.451714; altitude: 157 m a.s.l. (above sea level)), Okołowice (1 plantation: N 51.431806; E 19.193768; altitude: 180 m a.s.l.), Piwaki (2 plantations: N 51.124068; E 19.453671; altitude: 224 m a.s.l. and N 51.131197; E 19.461013; altitude: 222 m a.s.l.), Podole (2 plantations: N 51.532193; E 19.30404; altitude: 166 m a.s.l. and N 51.533847; E 19.303854; altitude: 162 m. a.s.l.), Świątniki (2 plantations: N 51.425982; E 19.191872; altitude: 188 m a.s.l. and N 51.424868; E 19.194597; altitude: 183 m a.s.l.) (Figure 1). In total, the study was carried out in approx. 60% of all the localities of Łódź Province, where energy willow plantations, established on former arable (land under temporary crops) and fallow (arable land temporarily, for at least two growing seasons, excluded from agricultural production) lands, were located.
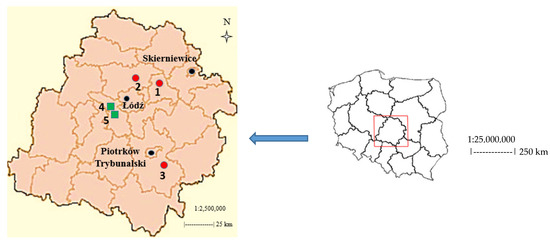
Figure 1.
Location of the study area in Poland and Łódź Province. Explanations:  Plantations established on former arable lands (AR): 1—Dmosin; 2—Podole; 3—Piwaki.
Plantations established on former arable lands (AR): 1—Dmosin; 2—Podole; 3—Piwaki.  Plantations established on former fallow lands (FA): 4—Okołowice; 5—Świątniki.
Plantations established on former fallow lands (FA): 4—Okołowice; 5—Świątniki.
 Plantations established on former arable lands (AR): 1—Dmosin; 2—Podole; 3—Piwaki.
Plantations established on former arable lands (AR): 1—Dmosin; 2—Podole; 3—Piwaki.  Plantations established on former fallow lands (FA): 4—Okołowice; 5—Świątniki.
Plantations established on former fallow lands (FA): 4—Okołowice; 5—Świątniki.
The energy willow plantations that were studied differed in terms of age and the type of land use before their establishment. Five plantations were established on former arable lands (in 2004–2005) and three, on former fallow lands (in 2006–2008) (Figure 1). Plantations established on former arable land are hereinafter abbreviated as AR, and plantations established on former fallow land, as FA. Before the establishment of the Salix viminalis L. plantations (AR and FA), cereals (rye and oats) were mainly cultivated and an extensive farming system was used. One-year sprouts, at a density of 26,000 sprouts per ha, were planted on each plantation. Before planting the sprouts, the lands were ploughed and harrowed. The AR plantations were located among arable land, whereas the FA plantations were located among arable land and mixed forests. In subsequent years, all the plantations were managed in a similar manner. During the study period, they were not fertilized and no pesticides were used. There were also no treatments such as ploughing or mowing. Willow was harvested every 2–3 years in the same years on all the plantations.
The study was carried out between 2011 and 2015 and in 2018 on the same plantations in the subsequent years of research (Table 1). The species composition of the segetal flora depending on the land use before establishing the plantation was analysed on the basis of an assessment of AR plantations in 2011–2012 and 2013–2014 (6–7 years old and 9–10 years old, respectively) and FA plantations in 2014–2015 (6–7 years old) and 2018 (10 years old). The different study periods in the FA plantations (6–7 and 9–10 years old) compared to the AR, which was due to the later year of their establishment, did not affect the results (the number of species or coverage), because the vegetation developed in similar weather conditions (Table 2). Only 2013 was characterized by a higher sum of precipitation, although only single relevés (three) were included from this year.

Table 1.
Number of phytosociological relevés carried out in individual localities of energy willow plantations.

Table 2.
Weather conditions in the growing seasons in the years 2011–2015 and 2018 (Meteorological Station in Bratoszewice).
The dynamics of segetal flora were analysed on FA plantations starting in 2011 (3–4 years old) for 7 consecutive years (2011–2018). The paper presents the results of flora from these plantations when they were 3–4 years old, 5 years old, 6–7 years old and 9–10 years old.
2.2. Soil Conditions
All the Salix viminalis L. plantations were located on soils that, according to Polish Soil Classification 2019 [38] and WRB Classification 2015 [39], were categorised as Cambisols. According to “Particle size distribution and textural classes of soils and mineral material-Classification of Polish Society of Soil Science 2008” [40], these soils were sand (FA) and loamy sand (AR). The soils had a very acid reaction (pH KCl 4.1–4.4), with a medium to high phosphorous content (53.1–81.9 mg·kg−1 soil), but were very poor and poor in their potassium (30.7–63.0 mg·kg−1 soil) and magnesium (11.0–17.0 mg·kg−1 soil) concentrations. The content of organic matter ranged from 1.97 to 2.28%.
The soils on which the AR plantations were located belonged to the soil agricultural complexes 5 and 6, whereas the soils, on which the FA plantations were located were classified into complexes 6 and 7. Soil agriculture complex 5 (soils suitable for rye) includes soils slightly sensitive to droughts. This is underlying loam material that reduces the permeability and retains the water supply in the soil, thus providing favourable conditions for plants. Soil agriculture complex 6 (soils less suitable for rye) includes soils often too dry. On complex 6 soils, plants only provide satisfactory yields in wet years. Soil agriculture complex 7 (soils very bad for rye) includes very dry sandy soils.
The information about the soil type and soil agriculture complex is based on agricultural maps on a scale of 1:5000 obtained from the Voivodeship Geodesy Office in Łódź (Solna St. 14, 91–423 Łódź, Poland), the Łódź Voivodeship Geoportal [41] and Polish Soil Classification 2019 [38]. Soil samples were taken with an Egner–Riehm stick (up to a depth of 20 cm) in accordance with the methods for soil study material. Topsoil analysis was carried out at the Regional Chemistry–Agriculture Station in Łódź and Warsaw. The contents of available phosphorus and available potassium were determined according to the Egner–Riehm (DL) method in calcium lactate extract, and the magnesium content was determined according to the Schatschabel method in calcium chloride extract. In addition, the soil pH in KCl and the content of organic matter according to the Tiurin method were identified. The soil texture according to research procedure (PB 40 ed. 3, 14.02.2011) was also identified.
2.3. Weather Conditions
The weather conditions during the study period were determined based on the dates recorded at the Meteorological Station of the Institute of Soil Science and Plant Cultivation, located in Bratoszewice, near Łódź. The long-term data were recorded for Łódź. The sum of precipitation in the vegetation period in the years 2011, 2012, 2014 and 2018 was similar and was 400.0–421.7 mm (Table 2). However, in 2013, it was about 40% higher than the long-term mean, and in 2015, it was about 24% lower than the long-term mean (Table 1). The greatest sum of precipitation was noted in May and June of that year (2015). The mean air temperatures between April and October in 2011–2015 were similar to each other (14.1–14.6 °C) and about 0.8–1.3 °C higher than the long-term mean. In 2018, the temperature was about 2.9 °C higher than the long-term mean. Detailed data regarding the average monthly air temperatures and the sums of monthly precipitation are shown in Table 2.
2.4. Methods
The vegetation accompanying willow (Salix viminalis L.) energy crops was identified based on an analysis of 60 phytosociological relevés (descriptions of vegetation patches—phytocenoses), i.e., ten relevés carried out in each group of plantations mentioned above. The number of phytosociological relevés carried out on an individual plantation depended on its area and soil diversity; i.e., if the plantation was located on two different soil complexes, separate relevés were taken on each of them. Details of the number of relevés taken on individual plantation is presented in Table 1. Each relevé had an area of 100 m2, was roughly square-shaped, and was made using the Braun-Blanquet [42] method. This method simultaneously captures the number and degree of coverage of a given species in what is called a phytosociological relevé. When producing such a relevé, the first stage involved selecting a uniform, typical patch of vegetation, i.e., one without any unusual places, e.g., hills and hollows. In turn, a list was made of all the species that were present in the area, and the coverage of a given plant species was estimated (plant image on the surface) using the Braun-Blanquet scale (1–5, +, r); the meaning of individual symbols is presented in Table 3 [43,44]. The research was mainly carried out in June and July each year.

Table 3.
Quantitative scale of Braun-Blanquet coverings.
Plant communities were distinguished by characteristic and differential species according to Matuszkiewicz [45], affiliated to phytosociological classes, and analysed in each group where they occurred. Subsequently, the number and proportions of species were determined. The proportion of each plant species was determined based on constancy class (S): V—80–100% of all phytosociological relevés; IV—60–80%; III—40–60%; II—20–40%; I—0.01–20%. The cover coefficient (D) was calculated according to the following formula: the sum of the average percentage of species cover that occurred in all the phytosociological relevés divided by the total number of phytosociological relevés and multiplied by 100 [43].
For each species, the following parameters were determined: family; geographical and historical groups; apophyte (i.e., synanthropic species of local origin) origin; biological stability; life-form; and status as an invasive, medicinal (herb), poisonous or melliferous species. The geographical and historical groups, apophyte origins, biological stability and life-form were mainly identified based on the following sources: Anioł-Kwiatkowska [46], Korniak [47], Mirek et al. [48], Rutkowski [49], Sowa and Warcholińska [50], Szafer et al. [51], Zając and Zając [52,53], and Zając [54]. Invasive species status was determined based on Tokarska-Guzik et al. [55]. Medicinal and poisonous plants were identified based on Rutkowski [49]. Melliferous plants were determined based on Sulborska [56]. Tree seedlings were not classified as melliferous plants. The Latin names of the vascular plants are in accordance with those presented by Mirek et al. [48], and the phytosociological classifications follow Matuszkiewicz [45].
2.5. Statistical Analysis
Canonical correspondence analysis (CCA, constrained ordination) was used to visualize the main differences in species composition occurring in the six groups of plantations (the age of the plantations and the type of land use before establishing the plantations) that were studied [57,58]. The relative species abundance was analysed by this method. The explanatory variable (categorical) was the phytosociological relevé’s affiliation to one of the six groups. The significance of constraints (with plantation types treated as the dummy variables) was tested by means of the permutation test [59].
Cluster analysis based on the mean species coverage was conducted for each of the six plantation groups. The Euclidean measure and Ward method were used. The coverage of each species was averaged for each of the plantation types. This 2-dimensional table (plantation type by species) was analysed by cluster analysis. The similarities between the age influence and previous land use influence on the plant composition are shown in the dendrogram chart.
The species richness was calculated according to the first-order jackknife species richness estimator [60,61]. This statistic introduces a correction for species not recorded in the abundance table during the study.
These statistical analyses were conducted with the use of the R software [62]. The CCA analysis was performed by the “cca” function contained in the R “vegan” package. The cluster analysis was performed by the “dist” and “hclust” functions. The “specpool” function from the “vegan” package was used for the estimated species richness.
3. Results
The flora of all eight Salix viminalis L. plantations that were studied totalled 114 vascular plant species. A large proportion of them were segetal species, i.e., 43 species (38%). The characteristics of this group of plants on 6–7-year-old and on 9–10-year-old energy willow plantations established on former arable (AR) and fallow (FA) lands are presented below. Then, the dynamics of the segetal flora on 3–10-year-old willow plantations established on former fallow lands were analysed in terms of four age groups (3–4, 5, 6–7 and 9–10 years old). In turn, the invasive, medicinal, poisonous and melliferous species accompanying the Salix viminalis L. plantations were discussed.
3.1. Characteristics of the Segetal Flora Depending on the Land Use before Establishing Plantations
The flora of the Salix viminalis L. plantations (6–7-year-old and 9–10-year-old) established on former arable (AR) and fallow land (FA) had a total of 103 vascular plant species. The species richness estimated by the jackknife method for the 6–7-year-old and 9–10-year-old plantations established on the arable land (AR) was 87.1 and 82.1, respectively. That is, the estimated species number included missed species was a little higher for 6–7-year-old plantations. Among the observed species, 61 and 56 (for 6–7-year-old and 9–10-year-old plantations, respectively) segetal species, which occurred mainly in cereal and root crops, accounted for 37% (38 species) (Table 4). On the plantations, the species characteristics for cereal crops such as Anthoxanthum aristatum Boiss., Matricaria maritima subsp. inodora (L.) Dostál, Vicia hirsuta (L.) Gray, Vicia tetrasperma (L.) Schreb. and Vicia villosa Roth. were recorded, while the species characteristics were also recorded for root crops including Chenopodium album L. and Sonchus arvensis L. The segetal species belonged to 15 botanical families. The most numerous family was Asteraceae (eight species), Fabaceae (seven species) and Poaceae (five species) (Table 4).

Table 4.
Constancy classes, botanical families, phytosociological classes, life-forms, and geographical and historical groups of segetal species occurring on 6–7-year-old and 9–10-year-old Salix viminalis L. plantations established on former arable land and fallow.
The study shows that more segetal species were on the AR plantations than on the FA (Table 4). On 6–7-year-old AR plantations, their number was 30% higher than on the FA, but on 9–10-year-old plantations, the difference was greater (41%) (Table 4). In both groups of 6–7-year-old plantations (AR and FA), the same 12 species were recorded, i.e., 32% of the segetal flora, and on older plantations (9–10 years old), eight species (21% of the segetal flora) were the same. These were mostly perennial plants, mainly reproducing vegetatively through rhizomes or stolons, such as Achillea millefolium L. s.str., Artemisia vulgaris L., Cirsium arvense (L.) Scop., Convolvulus arvensis L., Elymus repens (L.), Rumex acetosella L., and two short-lived (1–2 years) species, i.e., Trifolium arvense (L.) and invasive species Conyza canadensis (L.) Cronquist, characterised by high seed production. The remaining species occurred differently on both types of plantations (Table 4).
In line with the age of the plantations, the number of segetal plants decreased in both groups of plantations—on the AR, from 27 to 22, and on the FA, from 19 to 13 species. Only in younger plantations (6–7 years old) were there species such as Anthoxanthum aristatum Boiss., Galeopsis bifida Boenn., Matricaria maritima subsp. inodora (L.) Dostál, Medicago lupulina L., Setaria pumila (Poir.), Vicia tetrasperma (L.) and Vicia villosa Roth. They were mainly short-lived, photophilous species. On plantations established on former arable land, 37% of the species disappeared, while nearly half (47%) disappeared on formerly fallow land. They were replaced with new species (found only on older 9–10-year-old plantations), which constituted 23% of each group of plantations (Table 4). They were also mainly short-lived species reproducing from seeds, e.g., Polygonum hydropiper L., Sonchus asper L. and Veronica arvensis L. on AR and Thlaspi arvense L. and Chenopodium album L. on FA.
In the segetal flora, regardless of the type of land use before establishing the energy willow plantations, species of native origin (apophytes) dominated. With older plantations, the proportion of native species increased and that of anthropophytes decreased; this was more visible on the FA plantations (Figure 2).
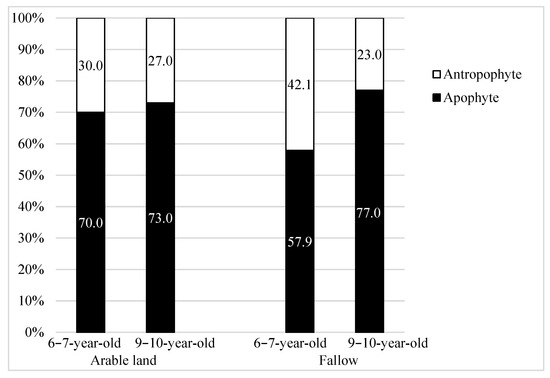
Figure 2.
The origin of segetal species (%) occurring on 6–7-year-old and 9–10-year-old Salix viminalis L. plantations established on former arable land and fallow.
Woody-shrub apophytes (native species of natural forest and shrub community origin, well-established in anthropogenic habitats) dominated in the structure of the agrophytocenoses in both types of plantation. On AR, their share was slightly higher than on FA (Figure 3). Besides woodland-shrub apophytes, meadow apophytes make up a high proportion on AR plantations, and sandyside apophytes (native species of sandy habitat, where soils are light, dry, low in nutrients and often acidic) constitute a high proportion on FA plantations. Among these, the following have the highest coverage: Achillea millefolium L. s.str and Artemisia vulgaris L. (Table 5).
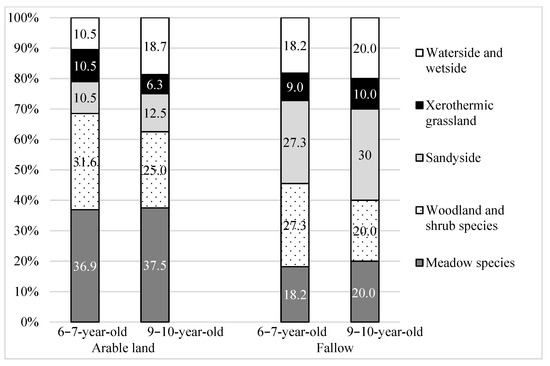
Figure 3.
The origin of segetal apophytes (%) occurring on 6–7-year-old and 9–10-year-old Salix viminalis L. plantations established on former arable land and fallow.

Table 5.
Constancy classes (S) and cover coefficient (D) of segetal species, which occurred with the highest constancy classes in all groups of Salix viminalis L. plantations established on fallow and arable land.
The study shows considerable diversity in the flora in terms of the share of life forms and biological stability. Regardless of the type of former land use, before the establishment of willow plantations, therophytes dominated in the communities (Figure 4). However, on AR, their proportion was higher than on FA, especially on older plantations (9–10 years old) by approx. 35% (i.e., by nine species). Taking into account the biological stability of plants, on AR plantations, over 63% of the species were short-lived species (Figure 5), whereas on the FA ones, the proportion of short-lived species was lower, especially on older plantations. The shading of the soil caused by the willow plants and the lack of arable land use (ploughing) meant that perennial plants dominated in line with the age, and on 9–10-year-old FA, these plants constituted approx. 54% of plants. On AR plantations, the proportion of perennial plants was at a similar level.
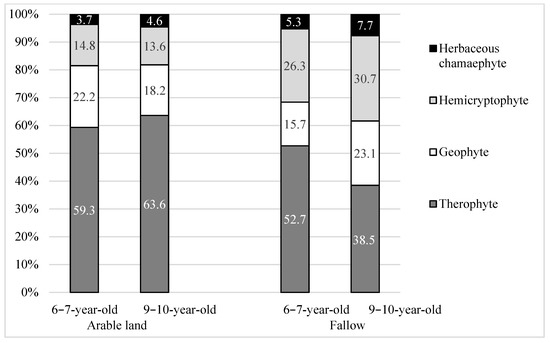
Figure 4.
Raunkier life-forms (%) of segetal species occurring on 6–7-year-old and 9–10-year-old Salix viminalis L. plantations established on former arable land and fallow.
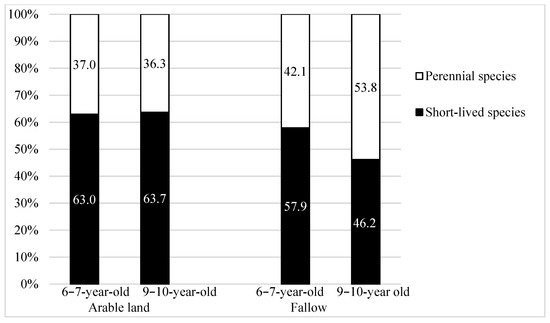
Figure 5.
Biological stability (%) of segetal species occurring on 6–7-year-old and 9–10-year-old Salix viminalis L. plantations established on former arable land and fallow.
The vast majority of the segetal plants on both types of plantations belonged to the Stellarietea mediae class, which is characteristic of arable land (with an average of about 43%) (Table 4). Species from the Artemisietea vulgaris class characteristic of nitrophilous communities, perennials and climbers found in ruderal habitats were noted more often on AR plantations than on FA (Figure 6). The next groups, in order, were species from the Molinio-Arrhenatheretea class, characteristic of semi-natural and anthropogenic meadow communities, and species from the Agropyretea intermedio-repentis class, characteristic of rhizome and stolon plants. Moreover, the willow plantations were accompanied by several species from other classes: Bidentetea tripartiti; Koelerio glauce—Corynephoretea canescentis; and species not classified as being in any of the phytosociological classes but enriching the floristic composition of communities (Figure 6).
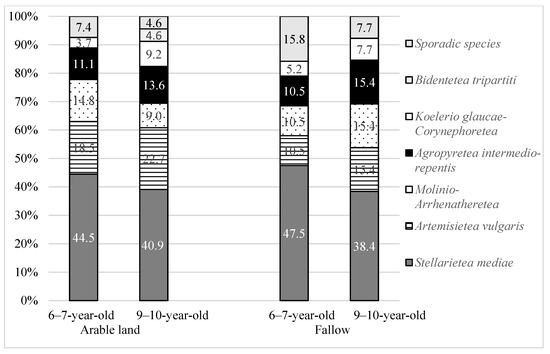
Figure 6.
Phytosociological classes (%) of segetal species occurring on 6–7-year-old and 9–10-year-old Salix viminalis L. plantations established on former arable land and fallow.
The species from the Agropyretea intermedio-repentis class had the highest share of soil coverage on AR plantations, among which Elymus repens (L.) Gould played an important role. On the other hand, on FA, the highest share was of species from the Molinio-Arrhenatheretea and Stellarietea mediae classes, the coverage of which was mainly determined by Achillea millefolium L. s. str. and Rumex acetosella L. (Table 5 and Table 6). Species from the Stellarietea mediae class, despite making up a considerable proportion, were characterised by a low cover coefficient. Among the species from this class, Rumex acetosella L. occurred more frequently and with greater coverage on FA plantations, and Vicia hirsuta (L.) Gray and Conyza canadensis (L.) Cronquist. occurred more frequently on AR plantations (Table 5).

Table 6.
Cover coefficients (D) of phytosociological classes and their shares (%) in phytosociological classes on Salix viminalis L. plantations established on fallow and arable land.
An analysis of the constancy classes and the cover coefficients showed that most of the segetal species in both types of plantations were in the I and II constancy classes. Only 25% of species on FA and 28% on AR were in higher constancy classes, i.e., III, IV and V (Table 4). It was found that segetal species achieved much higher cover coefficients on AR plantations (D = 7776) compared to FA (D = 4007) (Table 5).
The species that were in higher constancy classes and had cover coefficients above 600 were perennial plants that produced numerous rhizomes and stolons: Achillea millefolium L. s.str. on FA (sum of D = 1375) and Elymus repens (L.) Gould on AR (sum of D = 4201). In accordance with the ages of the plantations, all the species except Achillea millefolium L. s.str. were lowered in their constancy classes and cover coefficients. Due to the many years of willow cultivation, some species of the Stellarietea mediae class, usually accompanying cultivation on arable land, did not occur on 9–10-year-old plantations, e.g., Matricaria maritima subsp. inodora (L.) Dostál, Setaria pumilla (Poir.) Roem and Schult., and Vicia tetrasperma (L.) Schreb. (Table 3). The coverage with species from this class decreased in both types of plantation (Table 4).
3.2. Changes in the Segetal Flora on Salix viminalis L. Plantations Established on Fallow, Depending on the Age of the Plantation
With the aim of determining changes in the species composition of the segetal flora accompanying the willow with time, the flora of Salix viminalis L. plantations of different ages, i.e., 3–10 years old, and established on fallow land were analysed. The species richnesses estimated by the jackknife method were 66.3, 72.9, 73.6 and 64.0 for 3–4-, 5-, 6–7- and 9–10-year-old plantations, respectively. A total of 91 vascular plants species were recorded on these plantations, including 34 segetal species, which accounted for 37% of the total flora. The species belonged to six phytosociological classes and 14 botanical families. The most numerous were Asteraceae (six species), Fabaceae (six species) and Poaceae (five species). The remaining families consisted of 1–3 species (Table 7). On the youngest (3–5 years old) willow plantations, the proportion of segetal species was the highest (approx. 40.0%) and decreased with the age of the plantation; on 6–7-year-old plantations, it constituted 34.5%, and on 9–10-year-old ones, 27.6%. The species that were not confirmed in the flora of the 9–10-year-old plantations were short-lived anthropophytes: Vicia hirsuta (L.) Gray, Vicia villosa Roth, and Viola arvensis Murray.

Table 7.
Segetal species that occurred on 3–10-year-old Salix viminalis L. plantations established on fallow, and their affiliations to botanical families and phytosociological classes.
The species that occurred in all the age groups of the plantations studied were Achillea millefolium L. s.str., Artemisia vulgaris L., Convolvulus arvensis L., Elymus repens (L.), Rumex acetosella L., Trifolium arvense (L.), Vicia cracca and the invasive species Conyza canadensis (L.) Cronquist. Among these, four occurred in higher constancy classes (IV and III) (Table 5): Achillea millefolium L. s.str, Artemisia vulgaris L., Elymus repens (L.) and Rumex acetosella L.
On all the willow plantations, native species predominated over foreign species. Their share increased with the age of the plantations and ranged from 57% on 3–4-year-old plantations to 77% on 9–10-year-old ones (Table 8, Figure 2). Among the apophytes on 3–4-year-old plantations, species of meadow origin (33.3%) and woodland-shrub origin (25%) predominated. On 9–10-year-old plantations, their share decreased and amounted to 20% of each plantation (Table 8, Figure 3).

Table 8.
Characteristics of segetal flora occurring on different age (3–4-, 5-, 6–7- and 9–10-year-old) Salix viminalis L. plantations established on fallow.
An analysis of the biological stability showed that on 3–7-year-old plantations, short-lived species dominated (60% on average), while on older 9–10-year-old plantations, perennial species did (53.8%). In the biological spectrum, therophytes dominated on the 3–5-year-old plantations (constituting, on average, about 62%). Their share decreased in line with the age of the plantations, and on 9–10-year-old plantations, it was 38.5%. On the other hand, the share of hemicryptophytes increased, on average, from 17 to 30.7% (Table 8, Figure 2).
In all the groups of plantations (from 3 to 10 years old), species from the Stellarietea mediae class made up the highest proportions, with the following classes next, in order: Artemisieta vulgaris, Agropyretea intermedio-repentis and Molinio-Arrhenatheretea. On 9–10-year-old plantations, the proportion of species from the Stellarietea mediae class decreased, and species from the classes Artemisieta vulgaris, Molinio-Arrhenatheretea and Agropyretea intermedio-repentis increased (Table 9).

Table 9.
The share of phytosociological classes (%) and cover coefficients (D) and their shares (D%) on 3–4-year-old, 5-year-old, 6–7-year-old and 9–10-year-old Salix viminalis L. plantations established on fallow.
Most of the segetal species were characterised by low constancy classes (I–II). Only eight species were in higher constancy classes (III–V): Achillea millefolium L. s.str., Artemisia vulgaris L., Convolvulus arvensis L., Elymus repens (L.), Rumex acetosella L., Trifolium arvense (L.), Vicia hirsuta and Viola arvensis (Table 5). In total, in all the study periods, the species from the Molinio-Arrhenatheretea class had the highest cover coefficient (D-2913), and the lowest was seen in the species from the Koelerio glauce-Corynephoretea canescentis class (D-581). On older plantations (over 7 years old), a great reduction in the D index value was found in the Stellarietea mediae and Artemisieta vulgaris classes (Table 9). On 9–10-year-old plantations, the number of species in higher constancy classes visibly decreased, or the species disappeared (Table 5).
3.3. Similarities and Differences between the Species Compositions of Six Groups of Salix viminalis L. Plantations Proved by Statistical Methods
The flora of all six groups of Salix viminalis L. plantations totalled 114 vascular plant species. There were 47 (9–10-year-old FA plantations) to 61 (6–7-year-old AR plantations) species. Among these species, there were 13 to 27 segetal plant species (Figure 7).
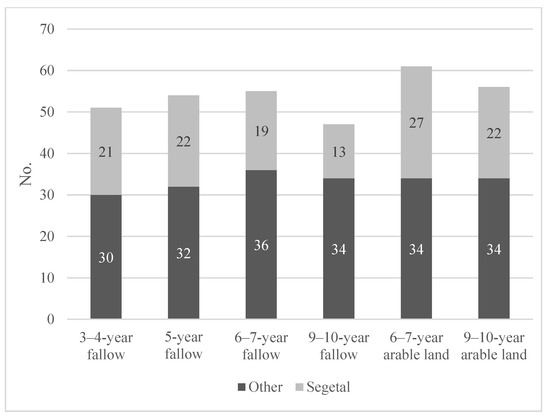
Figure 7.
The numbers of species accompanying Salix viminalis L., including segetal species, recorded on 3–10-year-old plantations established on former arable land and fallow.
Cluster analysis allowed the groups of plantations in question to be connected. Two main groups were distinguished. The first group contains the plantations established on former arable land, and the second one consists of plantations established on formerly fallow land. The abundance of segetal species was similar in each of these groups (Figure 8).
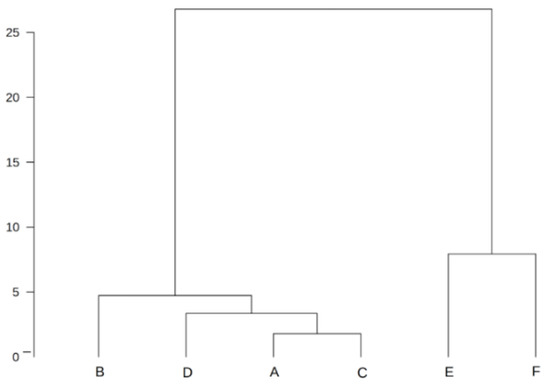
Figure 8.
The dendrogram constructed by the Ward method of cluster analysis for mean segetal species coverage of six plantation groups (A–F). A—3–4-year-old FA plantations; B—5-year-old FA plantations; C—6–7-year-old FA plantations; D—9–10-year-old FA plantations; E—6–7-year-old AR plantations; F—9–10-year-old AR plantations.
The constrained components retained 14.9 percent of the relative species abundance (Figure 9). Of that 14.9 percent, 7.3 percent were related to the first component (CCA 1) and 3.1 percent, to the second one (CCA 2). The permutation test of the significance of the plantation types confirmed the significance of the CCA model (the P p-value statistic was equal to 0.001), and the first two axes (the P p-value statistic was equal to 0.001 and 0.026 for the first axis and for the second axis, respectively, whereas the third axis was not significant at the 0.05 level).
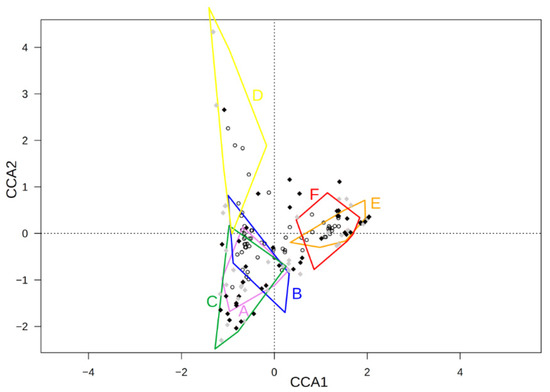
Figure 9.
The biplot for the canonical correspondence analysis for relative species abundance (black rhombus—segetal species; grey rhombus—other species; circle—phytosociological relevés) in the relevés with plantation group (A–F) as the explanatory variable; 7.3 percent of the variability was related to the first CCA axis, and 3.1 percent, to the second CCA axis. A—3–4-year-old FA plantations; B—5-year-old FA plantations; C—6–7-year-old FA plantations; D—9–10-year-old FA plantations; E—6–7-year-old AR plantations; F—9–10-year-old AR plantations.
The similarities between the groups of relevés according to the CCA analysis suggests that the plant communities on the plantations established on the arable lands had similar species abundances. The plantations established on fallow lands also had similar relative species abundance. This is in accordance with the cluster analysis (Figure 8). The CCA analysis also showed that the oldest plantations (9–10 years old) established on fallow areas were different from the rest of the plantations studied but were more similar to younger plantations on FA than those on AR (Figure 9).
3.4. Invasive Species Accompanying Salix viminalis L. Plantations
Among the 114 species of vascular flora of Salix viminalis L. crops, there were nine invasive species (Table 10). These were four segetal species: Anthoxanthum aristatum Boiss., Conyza canadensis (L.) Cronquist., Setaria pumila (Poir.) Roem and Schult., Setaria viridis (L.) P. Beauv. and five other species, i.e., Echinocystis lobata (F. Michx.) Torr. and A. Gray, Erigeron annuus (L.) Pers, Padus serotina (Ehrh.) Borkh, Solidago canadensis L. and Solidago gigantea Aiton.

Table 10.
Constancy classes (S) and cover coefficients (D) of invasive (I), medicinal (L.), poisonous (P) and melliferous (M) species occurring in flora accompanying Salix viminalis L. plantations established on fallow and arable land.
Regardless of the type of land use, before the willow plantations were established (AR and FA), the following species occurred: Conyza canadensis (L.) Cronquist., Erigeron annuus (L.) Pers, Setaria pumila (Poir.) Roem and Schult., and Solidago canadensis L. Two of these species were always present, regardless of the age of the plantation: Conyza canadensis (L.) Cronquist. and Solidago canadensis L.
In line with the age of the plantations, Padus serotina (Ehrh.) Borkh., a species that grows quickly after being cut down and whose fruits are willingly eaten by birds and spread over long distances, increased its coverage on FA plantations. However, on both types of plantations (AR and FA), with time, the coverage of Solidago canadensis L. increased. It is an expansive, perennial species with a high productivity of seeds dispersed by the wind, thus forming dense canopies, which prevented the development of other plants. On the FA plantations, this species had higher coverage (Table 10).
3.5. Medicinal, Poisonous and Melliferous Species of Flora Accompanying the Salix viminalis L. Plantations
Among the flora accompanying the willow energy crops, 34 medicinal, 9 poisonous and 20 melliferous plant species were found (Table 10). These groups of plants accounted for 50% of all the plant species occurring on the willow plantations. In the groups of medicinal and mellifluous plants, segetal species made up considerable proportions, i.e., 29% and 25%, respectively.
The vast majority of the plants from the groups discussed here were perennial, native species that had low degrees of stability and low coverage (Table 10). Only Achillea millefolium L. s.str. had higher degrees of constancy and higher coverage. Of the remaining species, Artemisia vulgaris L. occurred with higher degrees of constancy but with low coverage.
Among the plants in this group, it is worth noting those species providing valuable raw materials for medicines: Achillea millefolium L. s.str., Hypericum perforatum L., Taraxacum officinale F.H.Wigg. and Urtica dioica L. [63]. Poisonous species included Artemisia absinthium L., Convolvulus arvensis L., Senecio jacobaea L., Sonchus arvensis L. and Sonchus asper (L.) Hill. It should be emphasized that with the development of various fields of science, it becomes increasingly difficult to assess whether a species has healing properties or is poisonous.
Among the melliferous plants on the willow plantations, the following species were reported: Melilotus alba Medik., a species providing large amounts of nectar and pollen with a high nutritional value, and Verbascum densiflorum Bertol., a species valuable for nectar because of its extended flowering. Moreover, Chenopodium album L., Rumex acetosella L., Stachys palustris L. and Vicia cracca L. also have melliferous properties.
4. Discussion
The vascular flora of the Salix viminalis L. plantations established in central Poland, amounting to 114 species, is rich and diverse compared to the willow flora found in other areas [29,64,65]. This large species diversity results from the different geographical location, the soil conditions in which the plantations were established and their locations with regard to diverse ecosystems and different types of landscape. Discussion on segetal flora is difficult because in the literature, the vegetation accompanying the energy willow is usually presented as a whole, without separating out segetal plants, and detailed data are often not included.
The 38% share of segetal species (43 taxa) on the Salix viminalis L. plantations found in this author’s own research is high compared to the results obtained by Krechowski et al. [66], who recorded only 8.2% of typical cereal and root crop species on plantations of Salix viminalis L. of various ages, established on former arable and meadow land. This share is similar to the results obtained by Feledyn-Szewczyk et al. [27] in their research on the flora accompanying 10 species of energy crops, including Salix viminalis L. These authors found the occurrence of segetal species to be 38–39%.
The results of the author’s own research showed that the species composition of segetal plants and their structure depend on the type of land use before the plantations were established. This is consistent with earlier studies by Piórek et al. [67] and Trąba et al. [29]. The lower number of segetal species that were found on FA plantations than on AR may be the result of a longer period of the non-use of cultivation treatments that stimulate plant diasporas. Trzcińska-Tacik [68] and Luzuriaga et al. [69] emphasize the importance of agrotechnical factors in shaping the species composition of arable crops. The cessation of mechanical soil cultivation causes the accumulation of diasporas in the soil. It was found that the soil seed bank is much larger in fallow soil compared to in the soil of arable land [70,71]. However, in the plant canopy, these relationships may be opposite [71]. It was found that over the years, the species similarity (in the plant canopy) of fallow land and arable land decreases [72]. In our research, in line with the age of the plantation, the influence of the land use before the establishment of a Salix viminalis L. plantation on the composition of flora became progressively smaller. This confirms the findings obtained by Baum et al. [73], according to which the history of a field may only have an impact on the phytodiversity of short rotation coppice (SRC) plantations in the initial period after establishing the plantation. Similarly, for energy poplar (Populus sp.) crops, an effect of earlier land use was only found in young plantations [32].
The transformations in the flora on energy willow plantations in the following years indicate that the structure of vegetation is mainly influenced by the nutrient content and light conditions [73]. The growth and development of willow plants causes greater soil shading and intensifies the competition between plants. As a result, with time, there is a decrease in the number of species accompanying the willow plantation, including segetal plants, as shown by our study results. It should be emphasized that among the segetal plants, apart from the common species, species rarely found on willow plantations in other regions of Poland were also noted, such as Anthoxanthum aristatum Boiss., Digitaria ischaemum (Schreb.) H.L. Mühl., and Sonchus oleraceus L. [29,74,75].
The predominance of native species (apophytes) among the segetal plants; their proportion, which increases with the age of the plantation; and, at the same time, the decreasing proportion of anthropophytes, regardless of the type of land use before the plantations were established, all confirmed the directions of flora development in Salix viminalis L. crops shown by other authors [33,74,76,77]. Moreover, these transformations confirmed that with a lack of arable land use (ploughing and soil cultivation treatments), perennial energy willow monoculture does not favour the development of alien species [78].
Many years of willow cultivation resulted in a large proportion of woodland and shrub apophytes in the agrophytocenosis structure. Besides, meadow apophytes (on AR plantations) and sandysite apophytes (on FA plantations) constituted a great proportion of the agrophytocenoses. Similarly, Korniak et al. [74] and Janicka et al. [77], studying willow, and Birmele et al. [79], analysing poplar, found a predominance of meadow, woodland and shrub species.
The results of our studies confirm changes in the biological stability of segetal flora on plantations established on fallow lands. An increase in the proportion of perennial species in the whole flora accompanying energy willow plantations and, at the same time, a decrease in the proportion of short-lived species in line with the age of the plantations were shown previously (in plantations more than 3 years old) by Korniak et al. [74] and Trąba et al. [29]. On the older plantations, mainly perennial, rhizomatous and stolon species developed, which was also found by Korniak et al. [74] and Baum et al. [30]. The frequent occurrence of Elymus repens (L.) Gould., which grows intensively on willow energy plantations, is also consistent with the findings of Rowe et al. [80] and Baum et al. [30].
The phytosociological affiliation of the segetal flora presented in this paper is fundamentally different to the phytosociological structure of the whole flora of Salix viminalis L. presented in the literature. The segetal species of the plantations assessed here mainly belonged to the Stellarietea mediae class, while in the willow flora considered as a whole, species from the Molinio-Arrhenatheretea class predominated, and the proportion of plants from the Stellarietea mediae class was much smaller [66,81]. This fact may be a result of the age of the plantations in question. According to Trąba et al. [29], species from the Stellarietea mediae class on perennial plantations do not have suitable conditions for development, due to the fact that the soil surface is largely covered with plants. The dominance of the Stellarietea mediae class on willow and poplar plantations was found by Ziaja and Wnuk et al. [76], but these were young plantations (2–5 years old).
It was found that most of the segetal species on the Salix viminalis L. plantations occurred in low constancy classes (I and II), which is consistent with the results of research on willow flora conducted by, for example, Korniak [74], Trąba [29] and Krechowski [66]. These authors claim that this indicates a poor balance of Salix viminalis L. flora. This is due to the fact that willow is cut systematically (every 2–3 years), causing a change in light, thermal and humidity conditions, which makes it impossible to balance the flora [29].
An important part of the flora accompanying the Salix viminalis L. plantations is medicinal, poisonous and melliferous species. They are important for nature and enrich the biodiversity of agricultural areas, especially in the era of plant protection products being used intensively in agriculture [37,82]. What is worrying, though, is the presence of invasive species, especially those with high constancy classes and coverage, such as Conyza canadensis (L.) Cronquist., Erigeron annuus (L.) Pers., Solidago canadensis L. and Padus serotina (Ehrh.) Borkh. What needs to be particularly emphasized is that Conyza canadensis (L.) Cronquist. is an invasive species in many different habitats [83], and in Poland, it now has the status of an epoecophyte (a species settled in segetal and ruderal habitats). The presence of this species on energy willow plantations was previously confirmed by Wróbel et al. [84], Duer and Feledyn-Szewczyk [85], Feledyn-Szewczyk et al. [27] and Janicka et al. [31], and in Sida hermaphrodita (L.) Rusby by Bacieczko and Borcz [86]. Feledyn-Szewczyk [87] also recorded a considerable proportion of this species in the willow harvested each year. A particularly undesirable element of the flora is Solidago canadensis L., which was found on FA plantations. Solidago spp. is known to be a species typical for fallow lands [88]. The presence of these and other non-native and invasive plant species on willow plantations should be monitored. In conclusion, it should be emphasized that research on the biodiversity of energy willow and other plants used for bioenergy should be continued in the future, as these crops are important in the context of climate change and the increase in greenhouse gas emissions to the atmosphere.
5. Conclusions
Segetal species, occurring mainly in cereal and root crops, constitute a considerable proportion of the flora accompanying Salix viminalis L. (38%). It was found that their number depends on the type of land use before the establishment of the plantations. The plantations established on arable land were richer in segetal species than plantations established on fallow. However, both types of plantations were dominated by the same plant species (with the highest constancy classes and coverage), such as Achillea millefolium L. s.str, Artemisia vulgaris L. and Elymus repens (L.).
Regardless of the type of former land use, before the willow plantations were established, species of local origin were predominant among the segetal plants. In line with the age of the plantations, there was a decrease in the number of segetal species, especially from the Stellarietea mediae class. The proportion of anthropophytes also decreased, and apophytes increased. The dominant phytosociological classes were Molinio-Arrhenatheretea and Agropyretea intermedio-repentis.
Among the flora accompanying the willow, medicinal and melliferous plants important for humanity and biodiversity were noted, as well as invasive alien species, whose presence threatens native flora and should be monitored. A considerable part of the medicinal species was made up of segetal species.
To sum up, Salix viminalis L. plantations promote the preservation of many arable land plant species and contribute to maintaining the mosaic of the agricultural landscape and also the biodiversity of agroecosystems.
Author Contributions
Conceptualization, M.J. and A.K.; methodology, M.J. and A.K.; validation, M.J., A.K. and J.P.; formal analysis, J.P. and A.K.; investigation, A.K. and M.J.; data curation, A.K.; writing—original draft preparation, A.K. and M.J.; writing—review and editing, M.J., A.K. and J.P.; visualization, A.K. and M.J.; supervision, M.J.; project administration, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available by contacting the authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eurostat. Available online: https://ec.europa.eu/eurostat (accessed on 20 December 2019).
- Renewables Global Status Report. Available online: https://www.energia.org/renewables-2018-global-status-report-ren21/ (accessed on 20 December 2019).
- Faber, A. Natural effects of cultivation of energy crops. Studia i Raporty IUNG-PIB 2008, 11, 43–53. [Google Scholar]
- Scioe-Murg, O.M.; Ciolac, R.; Tonea, E.; Maria, C.C.; Martin, S. The impact of willow growings in energy forestry systems upon the environment. Lucrări Științifice Manag. Agric. 2015, 17, 268–272. [Google Scholar]
- Kyoto Protocol. Available online: http://prawo.sejm.gov.pl/isap.nsf/download.xsp/WDU20052031684/O/D20051684.pdf (accessed on 5 January 2020).
- Polish Energy Policy Until 2030. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WMP20100020011/O/M20100011.pdf (accessed on 2 October 2020). (In Polish)
- Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009. Available online: http://data.europa.eu/eli/dir/2001/77/oj (accessed on 1 October 2020).
- Regulation (EC) No 1099/2008 of the European Parliament and of the Council of 22 October 2008. Available online: https://eur-lex.europa.eu/eli/reg/2008/1099/oj (accessed on 2 October 2020).
- GUS. Energy from Renewable Energy Sources in 2018. 2019. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/energia/energia-ze-zrodel-odnawialnych-w-2018-roku,10,2.html (accessed on 28 September 2020).
- Stolarski, M.J. Plantations of Fast-Growing Trees and Shrubs as an Alternative to Wood from the Forest—Private Resource Bases. 2015. Available online: http://www.npl.ibles.pl/sites/default/files/referat/mariusz_stolarski_uwm_plantacje_drzew_i_krzewow_szybko_rosnacych_jako.pdf (accessed on 28 January 2019). (In Polish).
- Stolarski, M.J.; Niksa, D.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Willow productivity from small- and large scale experimental plantations in Poland form 2000 to 2017. Renew. Sustain. Energy Rev. 2019, 101, 461–475. [Google Scholar] [CrossRef]
- Grzybek, A. Bioenergy in Poland. Energy Crops in Poland—The Current State. 2015. Available online: http://www.pimot.eu/attachments/article/753/Anna%20Grzybek%20-%20ITP%20POLBIOM.%20Bioenergia%20w%20Polsce.%20Uprawy%20energetyczne%20w%20Polsce%20-%20stan%20obecny.pdf (accessed on 6 April 2019). (In Polish).
- Jadczyszyn, J.; Faber, A.; Zaliwski, A. Designation of Areas Potentially Suitable for the Cultivation of Willow and Virginia Mallow for Energy Purposes in Poland. Studia Rap. 2008, 11, 55–66. (In Polish) [Google Scholar]
- Błażej, J. Status and prospects of willow energy plantations in the Podkarpackie Voivodeship. Pamiet. Pulawski 2009, 150, 56–63, (Summary In English). [Google Scholar]
- Fry, D.A.; Slater, F.M. Early rotation short rotation willow coppice as a winter food resource for birds. Biomass Bioener. 2011, 35, 2545–2553. [Google Scholar] [CrossRef]
- Chauvat, M.; Perez, G.; Hedde, M.; Lamy, I. Establishment of bioenergy crops on metal contaminated soils stimulates belowground fauna. Biomass Bioener. 2014, 62, 207–211. [Google Scholar] [CrossRef]
- Brunbjerg, A.K.; Høye, T.T.; Eskildsen, A.; Nygaard, B.; Damgaard, C.F.; Ejrnæs, R. The collapse of marsh fritillary (Euphydryas aurinia) populations associated with declining host plant abundance. Biol. Conserv. 2017, 211, 117–124. [Google Scholar] [CrossRef]
- Ledin, S. Environmental consequences when growing short rotation forests in Sweden. Biomass Bioenergy 1998, 15, 49–55. [Google Scholar] [CrossRef]
- Grelle, A.; Aronsson, P.; Weilien, P.; Klemedtsson, L.; Lindroth, A. Large carbon-sink potential by Kyoto forests in Sweden-a case study on willow plantations. Tellus 2007, 59, 910–918. [Google Scholar] [CrossRef]
- Holzner, W. Weed species and weed communities. Vegetatio 1978, 38, 13–20. [Google Scholar] [CrossRef]
- Fanarillo, E.; Petit, S.; Dessaint, F.; Rosati, L.; Abbate, G. Species composition, richness, and diversity of weed communities of winter arable land in relation to geo-environmental factors: A gradient analysis in mainland Italy. Botany 2020, 98, 381–392. [Google Scholar] [CrossRef]
- Kutkowska, A.; Janicka, M.; Paderewski, J. The characteristics of Salix viminalis L. crop flora established on soils with different phosphorous contents. Soil Sci. Ann. 2020, 71, 252–264. [Google Scholar] [CrossRef]
- Lossová, Z.; Chytrý, M.; Cimalová, S.; Kropáč, Z.; Otýpková, Z.; Pyšek, P.; Tichý, L. Weed vegetation of arable land in Central Europe: Gradient of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Fried, G.; Norton, R.L.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Dobrzański, A.; Adamczewski, K. The influence of weed control on agrophytocenosis biodiversity. Prog. Plant Prot. 2009, 49, 982–995, (Summary In English). [Google Scholar]
- Wanic, M.; Parzonka, M.; Załuski, D. Biodiversity of weed communities in common wheat and spelt following various forecrops. Acta Agrobot. 2018, 71, 1751. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Matyka, M.; Staniak, M. Comparision ot the effect of perennial energy crops and agricultural crops on weed flora diversity. Agronomy 2019, 9, 695. [Google Scholar] [CrossRef]
- Kędziora, A.; Karg, J. Risks to biological diversity. Nauka 2010, 4, 107–114, (Summary In English). [Google Scholar]
- Trąba, Cz.; Majda, J.; Wolański, P. Plant communities accompanying plantations of Salix viminalis L. in podkarpackie voievodeship. Pamiet. Pulawski 2009, 150, 323–336, (Summary In English). [Google Scholar]
- Baum, S.; Weih, M.; Bolte, A. Stand age characteristics and soil properties affect species composition of vascular plants in short rotation coppice plantations. BioRisk 2012, 7, 51–71. [Google Scholar] [CrossRef]
- Janicka, M.; Kutkowska, A.; Paderewski, J. Diversity of vascular flora accompanying Salix viminalis L. crops depending on soil conditions. Glob. Ecol. Conserv. 2020, 23, e01068. [Google Scholar] [CrossRef]
- Archaux, F.; Chevalier, R.; Berthelot, A. Towards practices favourable to plant diversity in hybrid poplar plantations. For. Ecol. Manag. 2010, 259, 2410–2417. [Google Scholar] [CrossRef]
- Wojciechowski, W.; Sowiński, J.; Zawieja, J. The effect of age of willow plantation on weed infestation in the Sudety Mountains. Pamiet. Pulawski 2009, 150, 351–358, (Summary In English). [Google Scholar]
- Fehér, A.; Halmová, D.; Končeková, L. Gradient analysis of importance of spontaneously occurring vascular plant species in energy tree and grass stands. Acta Reg. Environ. 2013, 2, 31–33. [Google Scholar] [CrossRef][Green Version]
- Pučka, I.; Lazdiņa, D.; Bebre, I. Ground flora in plantations of three years old short rotation willow coppice. Agron. Res. 2016, 14, 1450–1466. [Google Scholar]
- Hochół, T. Weeds or plants accompanying crops? Pamiet. Pulawski 2003, 134, 89–96, (Summary In English). [Google Scholar]
- Grimau, L.; Gómez, M.; Figueroa, R.; Pizarro, R.; Gabriel Núñez, G.; Montenegro, G. The importance of weeds as melliferous flora in central Chile. Environ. Ecol. 2014, 41, 387–394. [Google Scholar] [CrossRef][Green Version]
- Kabała, C.; Bednarek, R.; Białousz, S.; Bielska, A.; Charzyński, P.; Chodorowski, J.; Chojnicki, J.; Czępińska-Kamińska, D.; Drewnik, M.; Glina, B.; et al. Systematic of Polish Soil, 6th ed.; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu, Instytut Nauk o Glebie i Ochrony Środowiska Uniwersytetu Przyrodniczego we Wrocławiu, Polskie Towarzystwo Gleboznawcze. Komisja Genezy, Klasyfikacji i Kartografii Gleb: Wrocław-Warszawa, Poland, 2019; pp. 1–292. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Re-sources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Polish Society of Soil Science. Particle Size Distribution and Textural Classes of Soils and Mineral Materials-Classification of Polish Society of Soil Science 2008. Soil Sci. Ann. 2009, 60, 5–16, (In Polish with English abstract). [Google Scholar]
- Łódź Voivodeship Geoportal. Available online: http://www.geoportal.lodzkie.pl (accessed on 24 January 2020).
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Vienna, Austria; New York, NY, USA, 1964. [Google Scholar]
- Pawłowski, B.; Pawłowski, B. Composition and structure of plant communities and methods of their research. In Polish Vegetation, 2nd ed.; Szafer, W., Zarzycki, K., Eds.; PWN: Warszawa, Poland, 1972; pp. 237–269. (In Polish) [Google Scholar]
- Dzwonko, Z. Guidebook to Phytosociological Studies, 2nd ed.; Sorus, S.C., Ed.; Instytut Botaniki Uniwersytetu Jagiellońskiego: Poznań- Kraków, Poland, 2008; pp. 1–304. (In Polish) [Google Scholar]
- Matuszkiewicz, W. Guide to the Determination of Polish Plant Communities; Wydawnictwo Naukowe PWN: Warszawa, Polska, 2012; pp. 1–537. (In Polish) [Google Scholar]
- Anioł-Kwiatkowska, J. Flora and synanthropic communities of Legnica, Lubin, Polkowice. Acta Univ. Wratislav. Prace Bot. 1974, 229, 1–151. (In Polish) [Google Scholar]
- Korniak, T. Segetal flora of north-eastern Poland, its spatial differentation and current changes. Acta Acad. Agric. Tech. Olst. Agric. 1992, 53 (Suppl. A), 1–77, (Summary In English). [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering plants and pteridophytes of Poland—A checklist. In Biodiversity of Poland; Mirek, Z., Szafer, W., Eds.; Institute of Botany, Polish Adacemy of Sciences: Kraków, Poland, 2002; Volume 1, pp. 1–442. [Google Scholar]
- Rutkowski, L. Key for the Determination of Lowland Poland Vascular Plants, 2nd ed.; PWN: Warszawa, Polska, 2008; pp. 1–814. (In Polish) [Google Scholar]
- Sowa, R.; Warcholińska, U. Synanthropic flora of Sulejów and Podklasztorze. Acta Univ. Lodz. Folia Bot. 1981, 1, 77–131, (Summary In English). [Google Scholar]
- Szafer, W.; Kulczyński, S.; Pawłowski, B. Polish Vegetation, 3rd ed.; PWN: Warszawa, Poland, 1969; pp. 1–1020. (In Polish) [Google Scholar]
- Zając, E.U.; Zając, A. The list of archeophytes occurring in Poland. Zesz. Nauk. Uniw. Jagiellońskiego Prace Bot. 1975, 395, 7–16, (Summary In English). [Google Scholar]
- Zając, M.; Zajac, A. A tentative list of segetal and ruderal apophytes in Poland. Zesz. Nauk. Uniw. Jagiellońskiego Prace Bot. 1992, 24, 7–23. [Google Scholar]
- Zając, A. The origin of the archaeophytes occurring in Poland. Rozpr. Habilitacyjne Uniw. Jagiellońskiego 1979, 29, 1–218. (In Polish) [Google Scholar]
- Tokarska-Guzik, B.; Dajdok, Z.; Zajac, M.; Zajac, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Plants of Alien Origin in Poland with Particular Emphasis on Invasive Species; Generalna Dyrekcja Ochrony Środowiska: Warszawa, Poland, 2012; pp. 1–197. [Google Scholar]
- Sulborska, A. Melliferous Plants; Bee & Honey Sp. z o.o.: Klecza Dolna, Poland, 2019; pp. 1–754. [Google Scholar]
- Ter Braak, C.J.F. Cannonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Angolini, C.; Nucci, A.; Frignani, F.; Landi, M. Using multivariate analyses to assess effects of fluvial type one plant, species distribution in Mediterranean river. Wetlands 2011, 31, 167–177. [Google Scholar] [CrossRef]
- Legendre, P.; Oksanen, J.; ter Braak, C.J. Testing the significance of canonical axes in redundancy analysis. Methods Ecol. Evol. 2011, 2, 269–277. [Google Scholar] [CrossRef]
- Walther, B.A.; Morand, S. Comparative performance of species richness estimation methods. Parasitology 1998, 116, 395–405. [Google Scholar] [CrossRef]
- Petersen, F.T.; Meier, R.; Larsen, M.N. Testing species richness estimation methods using museum label data on the Danish Asilidae. Biodivers. Conserv. 2003, 12, 687–701. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Podlech, D. Pocket Encyclopedia, 3rd ed.; Wydawnictwo MUZA SA: Warszawa, Poland, 1997; pp. 1–253. [Google Scholar]
- Cunningham, M.D.; Bishop, J.D.; McKay, H.V.; Sage, R.B. ABRE Monitoring-Ecology of Short Rotation Coppice; URN04/961; Department of Trade and Industry: London, UK, 2004; pp. 1–157. [Google Scholar]
- Welc, M.; Lundkvist, A.; Nordh, N.-E.; Verwijst, T. Weed community trajectories in cereal and willow cultivations after termination of a willow short rotation coppice. Agron. Res. 2017, 15, 1795–1814. [Google Scholar]
- Krechowski, J.; Piórek, K.; Ciosek, M.T. Vegetation accompanying energetic willow (Salix viminalis L.) plantations in Radzyń Podlaski. Pamiet. Pulawski 2009, 150, 195–205, (Summary In English). [Google Scholar]
- Piórek, K.; Krechowski, M.T.; Ciosek, M.T.; Sikorski, R. Effect of the selected factors on floristic composition of communities developing in energetic willow. Pamiet. Pulawski 2009, 150, 219–232, (Summary In English). [Google Scholar]
- Trzcińska-Tacik, H. Importance of field weeds species diversity. Pamiet. Pulawski 2003, 134, 253–262, (Summary In English). [Google Scholar]
- Luzuriaga, A.L.; Escudero, A.; Olano, J.M.; Loidi, J. Regenerative role of seed bank following an intense soil disturbance. Acta Oecologica 2005, 27, 57–66. [Google Scholar] [CrossRef]
- Zawieja, J. Increashe of potential weed infestation threat on fields temporarily eliminated from cultivation. Fragm. Agron. 2006, 23, 126–138. [Google Scholar]
- Sekutowski, T.R.; Włodek, S.; Biskupski, A.; Sienkiewicz-Cholewa, U. Comparision of the content of seeds and plants of the goldenrod (Solidago Sp.) in the fallow and adjacent field. Zesz. Nauk. Uniw. Przyr. we Wrocławiu-Rol. 2012, 584, 99–112. [Google Scholar]
- Kurus, J. Weed infestation of the fields neighbouring with multiyear fallow lands on two types of soil. Fragm. Agron. 2010, 27, 84–93. [Google Scholar]
- Baum, S.; Weih, M.; Bolte, A. Floristic diversity in Short Rotation Coppice (SRC) plantations: Comparison between soil seed bank and recent vegetation. Appl. Agric. For. Res. 2013, 63, 221–228. [Google Scholar] [CrossRef]
- Korniak, T.; Hołdyński, Cz.; Wąsowicz, K. Changes in the weed flora of willow plantations in north-eastern Poland. Pamiet. Pulawski 2009, 150, 159–170, (Summary In English). [Google Scholar]
- Skrajna, T.; Skrzyczyńska, J.; Rzymowska, Z.; Affek-Starczewska, A. Composition and structure of communities infestin of Salix sp. in northern part of Południowopodlaska Lowland. Pamiet. Pulawski 2009, 150, 255–264. [Google Scholar]
- Ziaja, M.; Wnuk, Z. Weed infestation of energetic crop in Leszczawa Dolna (podkarpackie voivodeship). Pamiet. Pulawski 2009, 150, 367–375, (Summary In English). [Google Scholar]
- Janicka, M.; Kutkowska, A.; Paderewski, J. Diversity of vascular flora in Salix viminalis L. crops depending on the harvest cycle. Annu. Set Environ. Prot. 2019, 21, 1175–1201. [Google Scholar]
- Anioł-Kwiatkowska, J.; Kącki, Z.; Śliwiński, M. A comparision of species composition of three energy willow crops. Pamiet. Pulawski 2009, 150, 19–33, (Summary In English). [Google Scholar]
- Birmele, J.; Kopp, G.; Brodbeck, F.; Konold, W.; Sauter, U.H. Successional changes of phytodiversity on a short rotation coppice plantation in Oberschwaben, Germany. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Rowe, R.L.; Hanley, M.E.; Goulson, D.; Clarke. D.J.; Doncaster, C.P.; Taylor, G. Potential benefits of commercial willow Short Rotation Coppice (SRC) for farm-scale plant and invertebrate communities in the agri-environment. Biomass Bioener. 2011, 25, 325–336. [Google Scholar] [CrossRef]
- Jezierska-Domaradzka, A.; Domaradzki, K. Accompanying vegetation of Salix viminalis L. on meadow habitat in Muchów (Kaczawskie Highland). Pamiet. Pulawski 2009, 150, 129–135, (Summary In English). [Google Scholar]
- Kwiatkowski, C.A.; Haliniarz, M.; Harasim, E. Weed infestation and health of organically grown Chamomile (Chamomilla recutita (L.) Rausch.) depending on selected foliar sprays and row spacing. Agriculture 2020, 10, 168. [Google Scholar] [CrossRef]
- Djudjević, L.; Mitrović, M.; Gajić, G.; Jarić, S.; Kostić, O.; Oberan, L.; Pavlović, P. An allelopathic investigation of the domination of the introduced invasive Conyza canadensis L. Flora 2011, 206, 921–927. [Google Scholar] [CrossRef]
- Wróbel, M.; Wróbel, J.; Gregorczyk, A. The effect of chemical soil properties on weed infestation structure in willow (Salix L.) Short-Rotation Coppice. Pol. J. Environ. Stud. 2012, 21, 1893–1899. [Google Scholar]
- Duer, I.; Feledyn-Szewczyk, B. Botanical monitoring in perennial bioenergy crops. Pamiet. Pulawski 2009, 150, 105–119, (Summary In English). [Google Scholar]
- Bacieczko, W.; Borcz, A. Segetal flora of the plantations of Virginia fanpetals Sida hermaphrodita (L.) Rusby in Łobez commune (West Pomerania). Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2016, 38, 17–36. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B. The influence of agricultural land use on weed flora diversity. Monogr. Rozpr. Nauk. 2013, 36, 1–184, (Summary In English). [Google Scholar]
- Rola, J.; Rola, H. Solidago spp. as bioindicator of fallow occurence on arable area. Fragm. Agron. 2010, 27, 122–131, (Summary In English). [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).