Evaluation of Pyrenophora tritici-repentis Infection of Wheat Heads
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Inoculation
2.2. Re-Isolation of Ptr from Inoculated Wheat Heads
2.3. PCR Detection of Ptr from Inoculated Wheat Heads
2.4. Germination of Seedlings from Recovered Grain
3. Results
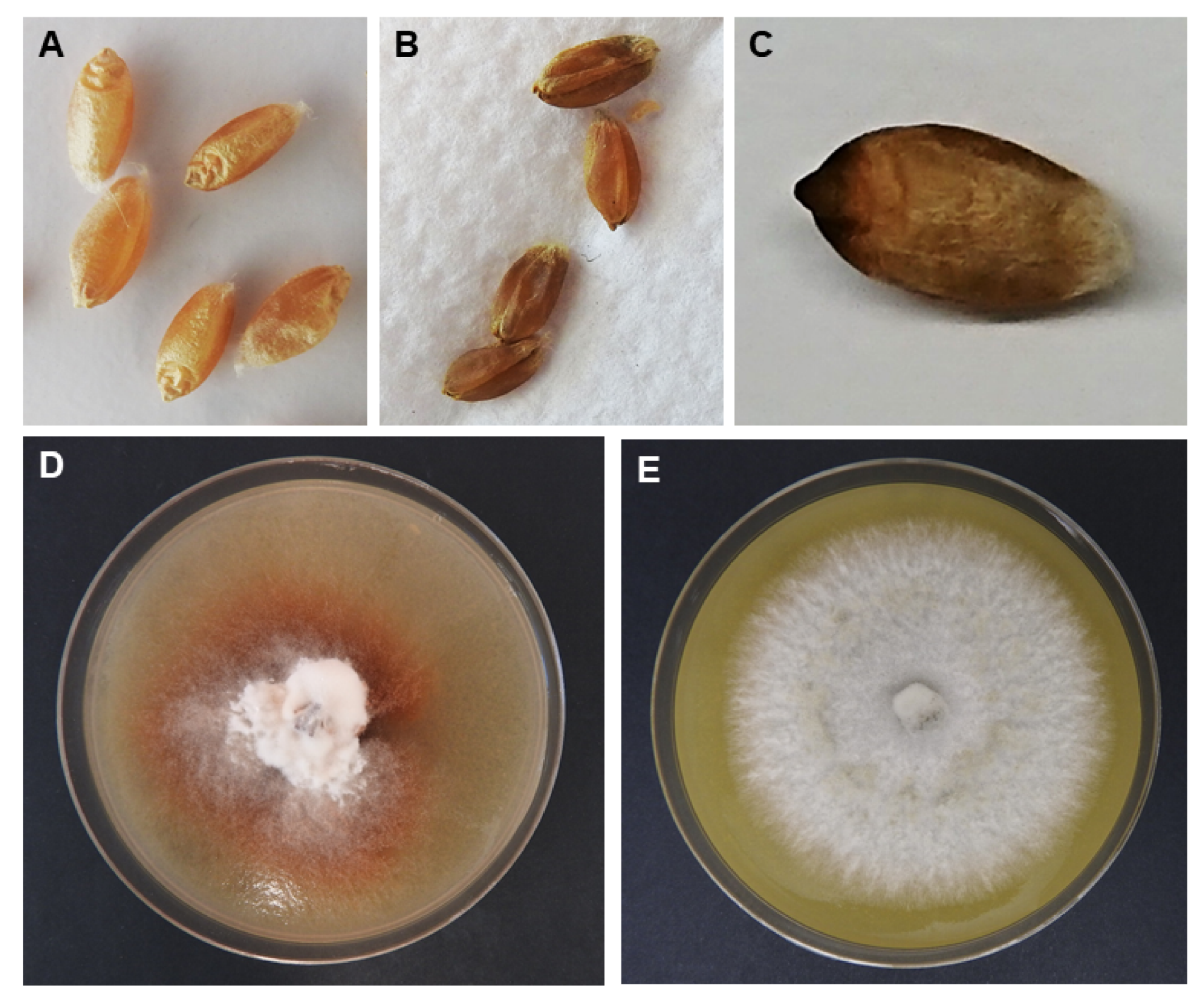
3.1. Wheat Head Symptoms and Seed Discoloration
3.2. Re-Isolation of Ptr Colonies from Inoculated Wheat Heads
3.3. PCR Detection of Ptr from Inoculated Wheat Heads
3.4. Systemic Transmission of Tan Spot Disease via Seeds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fernandez, M.R.; Clarke, J.M.; Depauw, R.; Irvine, R.B.; Knox, R. Black point and red smudge in irrigated durum wheat in southern Saskatchewan in 1990–1992. Can. J. Plan Pathol. 1994, 16, 221–227. [Google Scholar] [CrossRef]
- Fernadez, M.R.; Clarke, J.M.; de Pauw, R.M.; Lefkovitch, L.P. Emergence and growth of durum wheat derived from red smudge-infected seed. Crop Sci. 1997, 37, 510–514. [Google Scholar] [CrossRef]
- Schilder, A.M.C.; Bergstrom, G.C. Pyrenophora-tritici-repentis as a component of the fungal flora of winter-wheat seed in New-York. Seed Sci. Technol. 1994, 22, 285–297. [Google Scholar]
- Carmona, M.; Ferrazini, M.; Barreto, D.E. Tan spot of wheat caused by Drechslera tritici-repentis: Detection, transmission, and control in wheat seed. Cereal Res. Commun. 2006, 34, 1043–1049. [Google Scholar] [CrossRef]
- Fernandez, M.R.; de Pauw, R.M.; Clarke, J.M.; Fox, S.L. Discoloration of wheat kernels by Pyrenophora tritici-repentis. Can. J. Plant Pathol. Rev. Can. Phytopathol. 1998, 20, 380–383. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Clarke, J.M.; de Pauw, R.M. Effect of environmental variables on the development of kernel discoloration by Pyrenophora tritici-repentis in durum wheat. Can. J. Plant Pathol. Rev. Can. Phytopathol. 1998, 20, 104–110. [Google Scholar] [CrossRef]
- Rees, R.G.; Martin, D.J.; Law, D.P. Black point in bread wheat: Effects on quality and germination, and fungal associations. Aust. J. Exp. Agric. 1984, 24, 601–605. [Google Scholar] [CrossRef]
- Fernandez, W.; Singh, A. Impact of seed discolouration on emergence and early plant growth of durum wheat at different soil gravimetric water contents. Can. J. Plant Pathol. 2014, 36, 509–516. [Google Scholar] [CrossRef]
- Fernandez, M.R.; de Pauw, R.M.; Clarke, J.M. Reaction of common and durum wheat cultivars to infection of kernels by Pyrenophora tritici-repentis. Can. J. Plant Pathol. 2001, 23, 158–162. [Google Scholar] [CrossRef]
- Grain Trade Australia: Wheat Standards 2020/2021—Statement of Standards. Available online: www.graintrade.org.au (accessed on 24 August 2020).
- Fox, S.L.; Fernandez, M.R.; de Pauw, R.M. Red smudge infection modifies sprouting response in four wheat lines. Can. J. Plant Sci. 2003, 83, 163–169. [Google Scholar] [CrossRef]
- Fernandez, M.R.; de Pauw, R.M.; Clarke, J.M.; Lefkovitch, L.P. Red smudge in durum wheat reduces seedling vigour. Can. J. Plant Sci. 1996, 76, 321–324. [Google Scholar] [CrossRef]
- Schilder, A.M.C.; Bergstrom, G.C. Seed transmission of Pyrenophora tritici-repentis, causal fungus of tan spot of wheat. Eur. J. Plant Pathol. 1995, 101, 81–91. [Google Scholar] [CrossRef]
- Wilson, J.M. Analysis of Black Point in Wheat; Technical Bulletin 88; Department of Agriculture and Food: Perth, Australia, 1993. [Google Scholar]
- Klein, T.A. Fungi associated with discoloured wheat grain in northern New South Wales. Australas. Plant Pathol. 1987, 16, 69–71. [Google Scholar] [CrossRef]
- Shackley, B.; Paynter, B.; Troup, G.; Bucat, J.; Seymour, M.; Blake, A. 2020 Western Australia Crop Sowing Guide; Grains Research and Development Corporation: Perth, Australia, 2020. [Google Scholar]
- Moffat, C.S.; See, P.T.; Oliver, R.P. Generation of a ToxA knockout strain of the wheat tan spot pathogen Pyrenophora tritici-repentis. Mol. Plant Pathol. 2014, 15, 918–926. [Google Scholar] [CrossRef]
- Moffat, C.S.; See, P.T.; Oliver, R.P. Leaf yellowing of the wheat cultivar mace in the absence of yellow spot disease. Australas. Plant Pathol. 2015, 44, 161–166. [Google Scholar] [CrossRef]
- Moolhuijzen, P.; See, P.T.; Hane, J.K.; Shi, G.; Liu, Z.; Oliver, R.P.; Moffat, C.S. Comparative genomics of the wheat fungal pathogen Pyrenophora tritici-repentis reveals chromosomal variations and genome plasticity. BMC Genom. 2018, 19, 279. [Google Scholar] [CrossRef]
- See, P.T.; Moffat, C.S.; Morina, J.; Oliver, R.P. Evaluation of a multilocus indel DNA region for the detection of the wheat tan spot pathogen Pyrenophora tritici-repentis. Plant Dis. 2016, 100, 2215–2225. [Google Scholar] [CrossRef]
- Lachman, J.; Martinek, P.; Kotíková, Z.; Orsák, M.; Šulc, M. Genetics and chemistry of pigments in wheat grain—A review. J. Cereal Sci. 2017, 74, 145–154. [Google Scholar] [CrossRef]
- Böhmdorfer, S.; Oberlerchner, J.T.; Fuchs, C.; Rosenau, T.; Grausgruber, H. Profiling and quantification of grain anthocyanins in purple pericarp × blue aleurone wheat crosses by high-performance thin-layer chromatography and densitometry. Plant Methods 2018, 14, 29. [Google Scholar] [CrossRef]
- Fernandez, M.R.; Pearse, P.G.; Holzgang, G.; Basnyat, P.; Zentner, R.P. Impacts of agronomic practices on the leaf spotting complex of common wheat in eastern Saskatchewan. Can. J. Plant Sci. 2009, 89, 717–730. [Google Scholar] [CrossRef]
- Bouras, N.; Kim, Y.M.; Strelkov, S.E. Influence of water activity and temperature on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Int. J. Food Microbiol. 2009, 131, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Bouras, N.; Strelkov, S.E. Influence of carbon source on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Can. J. Microbiol. 2010, 56, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Bouras, N.; Holtz, M.D.; Aboukhaddour, R.; Strelkov, S.E. Influence of nitrogen sources on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Crop J. 2016, 4, 119–128. [Google Scholar] [CrossRef]
- Bouras, N.; Strelkov, S.E. The anthraquinone catenarin is phytotoxic and produced in leaves and kernels of wheat infected by Pyrenophora tritici-repentis. Physiol. Mol. Plant Pathol. 2008, 72, 87–95. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Susca, A.; Ghionna, V.; Logrieco, A.F.; Franzoni, M.; Ravaglia, S.; Meca, G.; Moretti, A. Molecular identification and mycotoxin production by alternaria species occurring on durum wheat, showing black point symptoms. Toxins 2020, 12, 275. [Google Scholar] [CrossRef]
- Lehmensiek, A.; Campbell, A.W.; Sutherland, M.W.; Williamson, P.M.; Michalowitz, M.; Daggard, G.E. QTLs for black-point resistance in wheat and the identification of potential markers for use in breeding programmes. Plant Breed. 2004, 123, 410–416. [Google Scholar] [CrossRef]
- Li, Q.; Niu, H.; Xu, K.; Xu, Q.; Wang, S.; Liang, X.; Jiang, Y.; Niu, J. GWAS for resistance against black point caused by Bipolaris sorokiniana in wheat. J. Cereal Sci. 2020, 91, 102859. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Wu, L.; Bai, B.; Wen, W.; Xie, C.; Xia, X. Genome-wide linkage mapping of QTL for black point reaction in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2016, 129, 2179–2190. [Google Scholar] [CrossRef]
- Liu, J.; He, Z.; Rasheed, A.; Wen, W.; Yan, J.; Zhang, P.; Wan, Y.; Zhang, Y.; Xie, C.; Xia, X. Genome-wide association mapping of black point reaction in common wheat (Triticum aestivum L.). BMC Plant Biol. 2017, 17, 220. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Li, T.; Zhang, G.; Bai, G. Multiple minor QTLs are responsible for fusarium head blight resistance in chinese wheat landrace haiyanzhong. PLoS ONE 2016, 11, e0163292. [Google Scholar] [CrossRef]
- Czembor, P.C.; Arseniuk, E.; Radecka-Janusik, M.; Piechota, U.; Słowacki, P. Quantitative trait loci analysis of adult plant resistance to Parastagonospora nodorum blotch in winter wheat cv. Liwilla (Triticum aestivum L.). Eur. J. Plant Pathol. 2019, 155, 1001–1016. [Google Scholar] [CrossRef]
- Shatalina, M.; Messmer, M.; Feuillet, C.; Mascher, F.; Paux, E.; Choulet, F.; Wicker, T.; Keller, B. High-resolution analysis of a QTL for resistance to Stagonospora nodorum glume blotch in wheat reveals presence of two distinct resistance loci in the target interval. Theor. Appl. Genet. 2014, 127, 573–586. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, X.; Cheng, J.; Jiang, Z.; Hu, W.; Bie, T.; Gao, D.; Li, D.; Wu, R.; Li, Y.; Chen, S.; et al. Genetic analysis of fusarium head blight resistance in CIMMYT bread wheat line C615 using traditional and conditional QTL mapping. Front. Plant Sci. 2018, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Chen, L.; Zhang, W.; Yang, L.; Zhu, W.; Li, J.; Liu, Y.; Tong, H.; Fu, L.; Liu, J.; et al. Genome-wide association analysis of fusarium head blight resistance in Chinese elite wheat lines. Front. Plant Sci. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Gala, A.; Yen, Y. Identification of functional genic components of major fusarium head blight resistance quantitative trait loci in wheat cultivar Sumai 3. Mol. Plant Microbe Interact. 2013, 26, 442–450. [Google Scholar] [CrossRef]
- Schilder, A.M.C.; Bergstrom, G.C. Infection of wheat seed by Pyrenophora tritici-repentis. Can. J. Bot. 1994, 72, 510–519. [Google Scholar] [CrossRef]
- Campbell, M.A.; Medd, R.W.; Brown, J.B. Optimizing conditions for growth and sporulation of Pyrenophora semeniperda. Plant Pathol. 2003, 52, 448–454. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
See, P.T.; Schultz, N.; Moffat, C.S. Evaluation of Pyrenophora tritici-repentis Infection of Wheat Heads. Agriculture 2020, 10, 417. https://doi.org/10.3390/agriculture10090417
See PT, Schultz N, Moffat CS. Evaluation of Pyrenophora tritici-repentis Infection of Wheat Heads. Agriculture. 2020; 10(9):417. https://doi.org/10.3390/agriculture10090417
Chicago/Turabian StyleSee, Pao Theen, Nikki Schultz, and Caroline S. Moffat. 2020. "Evaluation of Pyrenophora tritici-repentis Infection of Wheat Heads" Agriculture 10, no. 9: 417. https://doi.org/10.3390/agriculture10090417
APA StyleSee, P. T., Schultz, N., & Moffat, C. S. (2020). Evaluation of Pyrenophora tritici-repentis Infection of Wheat Heads. Agriculture, 10(9), 417. https://doi.org/10.3390/agriculture10090417




