Effects of Site, Genotype and Subsequent Harvest Rotation on Willow Productivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiments
2.2. Willow Survival Rate, Biometric Features and Biomass Yield
2.3. Statistical Analysis
2.4. Soil and Weather Conditions during the Experiments
3. Results
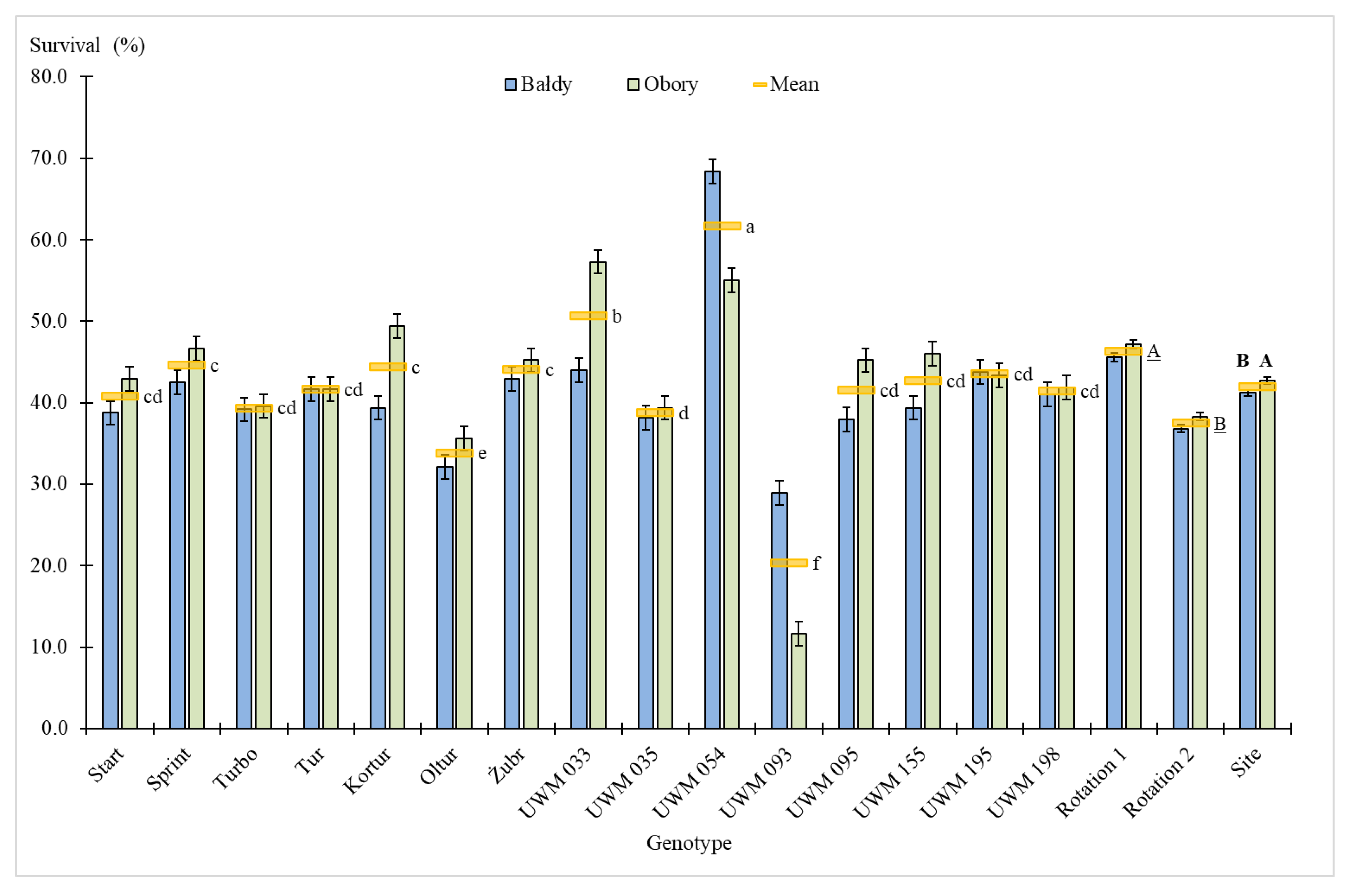
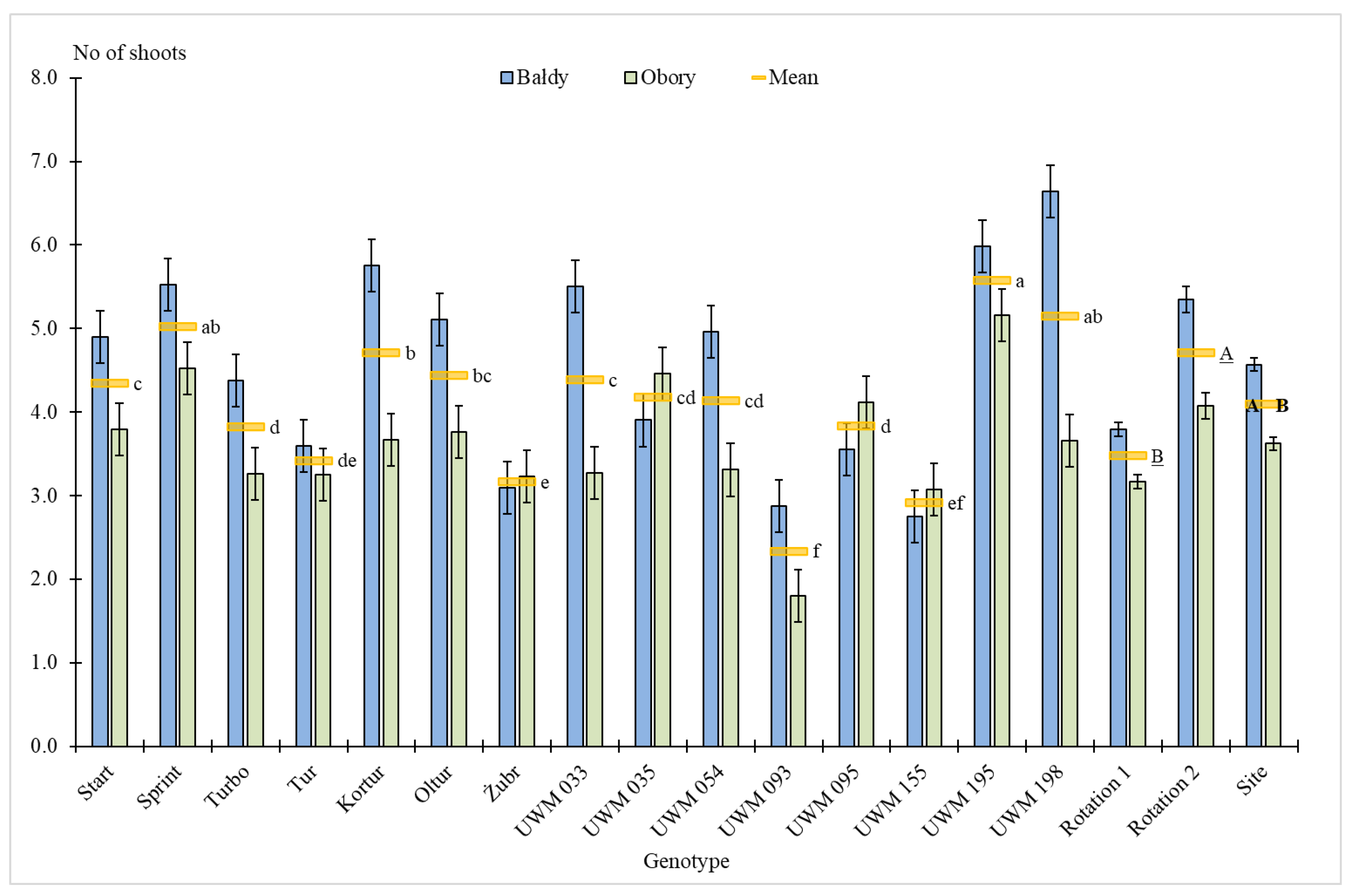
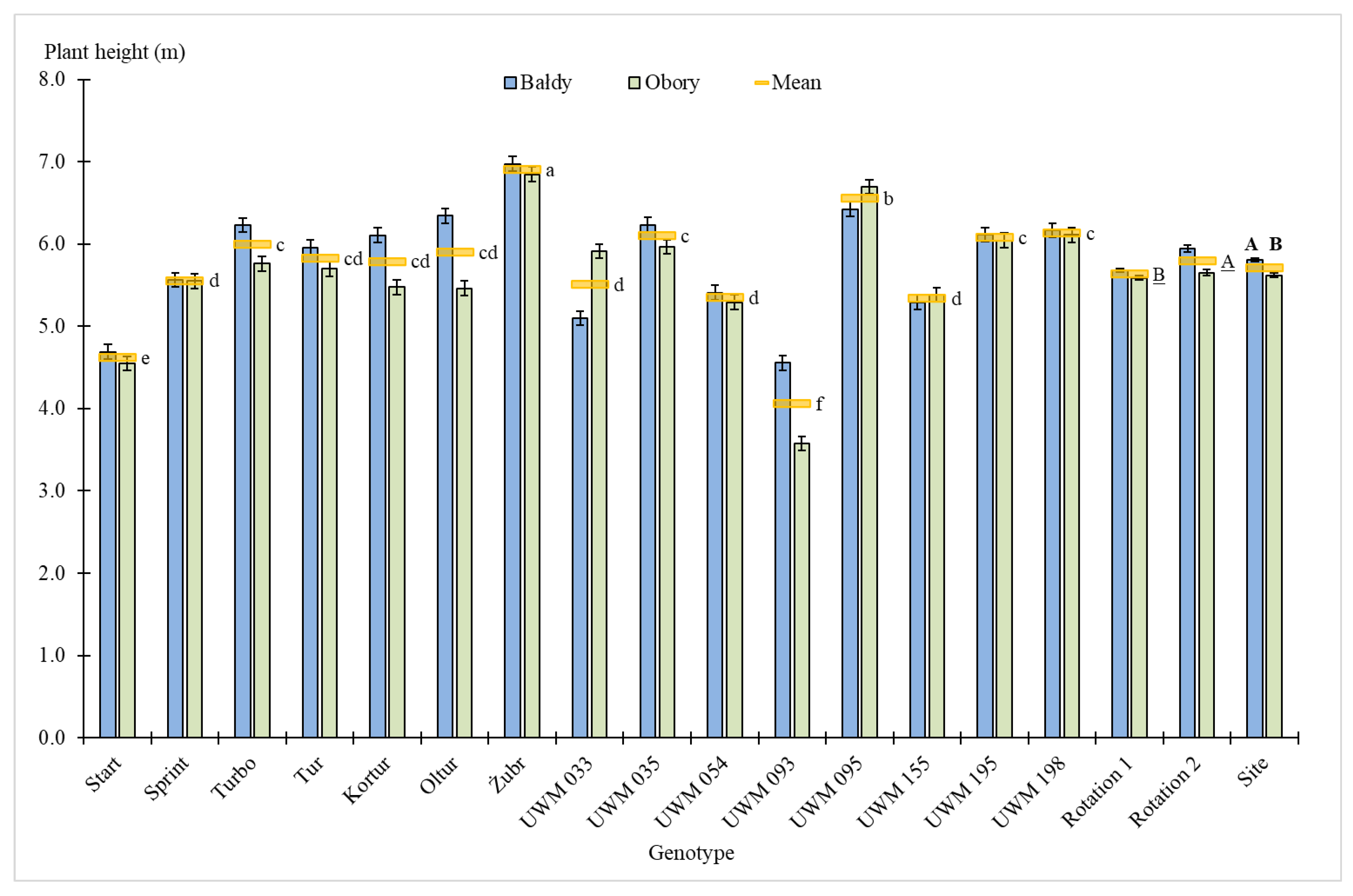
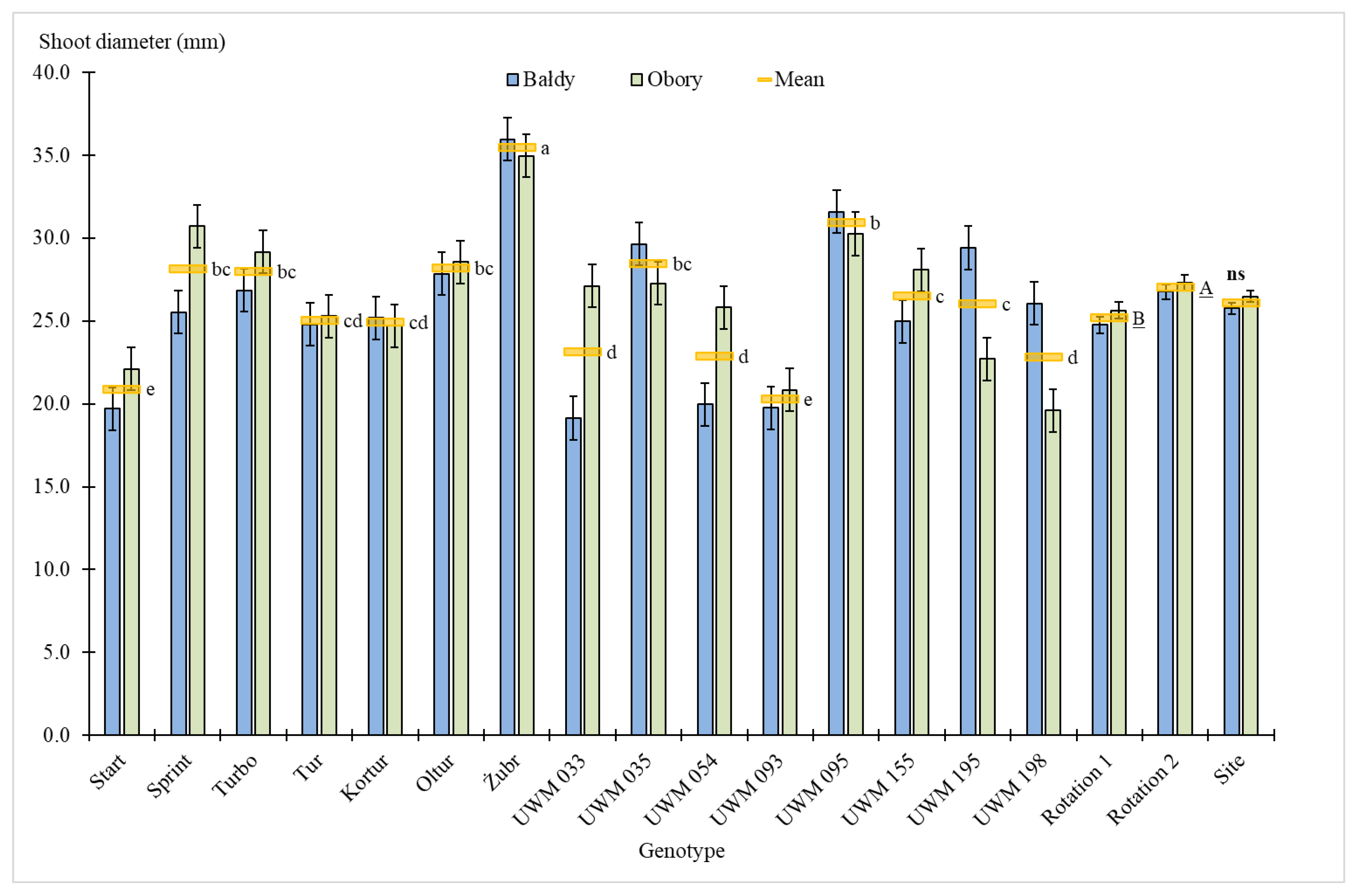
3.1. Plant Survival Rate, Number of Shoots, Plant Height and Shoot Diameter
3.2. Biomass Yield
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tharakan, P.J.; Volk, T.A.; Abrahamson, L.P.; White, E.H. Energy feedstock characteristics of willow and hybrid poplar clones at harvest age. Biomass Bioenergy 2003, 25, 571–580. [Google Scholar] [CrossRef]
- US. Department of Energy. US Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry; U.S. Department of Energy: Oak Ridge, TN, USA, 2011; p. 227.
- Nordborg, M.; Berndes, G.; Dimitriou, I.; Henriksson, A.; Mola-Yudego, B.; Rosenqvist, H. Energy analysis of willow production for bioenergy in Sweden. Renew. Sustain. Energy Rev. 2018, 93, 473–482. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Niksa, D.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Willow productivity from small- and large-scale experimental plantations in Poland from 2000 to 2017. Renew. Sustain. Energy Rev. 2019, 101, 461–475. [Google Scholar] [CrossRef]
- Gonçalves, F.A.; Sanjinez-Argandona, E.J.; Fonseca, G.G. Cellulosic ethanol and its co-products from different substrates, pretreatments, microorganisms and bioprocesses: A review. Nat. Sci. 2013, 5, 624–630. [Google Scholar] [CrossRef]
- Monedero, E.; Portero, H.; Lapuerta, M. Pellet blends of poplar and pine sawdust: Effects of material composition, additive, moisture content and compression die on pellet quality. Fuel Process. Technol. 2015, 132, 15–23. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Łuczyński, M.; Załuski, D.; Szczukowski, S.; Tworkowski, J.; Gołaszewski, J. Lignocellulosic biomass from short rotation woody crops as a feedstock for second-generation bioethanol production. Ind. Crops Prod. 2015, 75, 66–75. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Kowalski, R.; Rój, E.; Warmiński, K.; Krzyżaniak, M.; Gil, Ł.; Stolarski, M.J. Characterization of bioactive compounds in the biomass of black locust, poplar and willow. Trees 2019, 33, 1235–1263. [Google Scholar] [CrossRef]
- Sleight, N.J.; Volk, T.A.; Johnson, G.A.; Eisenbies, M.H.; Shi, S.; Fabio, E.S.; Pooler, P.S. Change in yield between first and second rotations in willow (Salix spp.) biomass crops is strongly related to the level of first rotation yield. BioEnergy Res. 2016, 9, 270–287. [Google Scholar] [CrossRef]
- PocienĖ, L.; KadŽIulienĖ, Ž. Biomass yield and fibre components in reed canary grass and tall fescue grown as feedstock for combustion. Zemdirb. Agric. 2016, 103, 297–304. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Śnieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S.; Graban, Ł.; Lajszner, W. Short rotation coppices, grasses and other herbaceous crops: Biomass properties versus 26 genotypes and harvest time. Ind. Crops Prod. 2018, 119, 22–32. [Google Scholar] [CrossRef]
- Monti, A.; Zanetti, F.; Scordia, D.; Testa, G.; Cosentino, S.L. What to harvest when? Autumn, winter, annual and biennial harvesting of giant reed, miscanthus and switchgrass in northern and southern Mediterranean area. Ind. Crops Prod. 2015, 75, 129–134. [Google Scholar] [CrossRef]
- Sleight, N.J.; Volk, T.A. Recently bred willow (Salix spp.) biomass crops show stable yield trends over three rotations at two sites. BioEnergy Res. 2016, 9, 782–797. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Śnieg, M.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S. Short rotation coppices, grasses and other herbaceous crops: Productivity and yield energy value versus 26 genotypes. Biomass Bioenergy 2018, 119, 109–120. [Google Scholar] [CrossRef]
- Volk, T.A.; Verwijst, T.; Tharakan, P.J.; Abrahamson, L.P.; White, E.H. Growing fuel: A sustainability assessment of willow biomass crops. Front. Ecol. Environ. 2004, 2, 411–418. [Google Scholar] [CrossRef]
- Djomo, S.N.; Kasmioui, O.E.; Ceulemans, R. Energy and greenhouse gas balance of bioenergy production from poplar and willow: A review. GCB Bioenergy 2011, 3, 181–197. [Google Scholar] [CrossRef]
- Aronsson, P.; Rosenqvist, H.; Dimitriou, I. Impact of nitrogen fertilization to short-rotation willow coppice plantations grown in sweden on yield and economy. BioEnergy Res. 2014, 7, 993–1001. [Google Scholar] [CrossRef]
- Larsen, S.U.; Jørgensen, U.; Lærke, P.E. Willow yield is highly dependent on clone and site. BioEnergy Res. 2014, 7, 1280–1292. [Google Scholar] [CrossRef]
- Weger, J.; Hutla, P.; Bubeník, J. Yield and fuel characteristics of willows tested for biomass production on agricultural soil. Res. Agric. Eng. 2016, 62, 155–161. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Field performance and biomass production of 12 willow and poplar clones in short-rotation coppice in southern Quebec (Canada). Biomass Bioenergy 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Guidi Nissim, W.; Pitre, F.E.; Teodorescu, T.I.; Labrecque, M. Long-term biomass productivity of willow bioenergy plantations maintained in southern Quebec, Canada. Biomass Bioenergy 2013, 56, 361–369. [Google Scholar] [CrossRef]
- Volk, T.A.; Abrahamson, L.P.; Nowak, C.A.; Smart, L.B.; Tharakan, P.J.; White, E.H. The development of short-rotation willow in the northeastern United States for bioenergy and bioproducts, agroforestry and phytoremediation. Biomass Bioenergy 2006, 30, 715–727. [Google Scholar] [CrossRef]
- Serapiglia, M.; Cameron, K.; Stipanovic, A.; Abrahamson, L.; Volk, T.; Smart, L. Yield and woody biomass traits of novel shrub willow hybrids at two contrasting sites. BioEnergy Res. 2012, 6, 1–14. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Rosenqvist, H.; Krzyżaniak, M.; Szczukowski, S.; Tworkowski, J.; Gołaszewski, J.; Olba-Zięty, E. Economic comparison of growing different willow cultivars. Biomass Bioenergy 2015, 81, 210–215. [Google Scholar] [CrossRef]
- Styles, D.; Thorne, F.; Jones, M.B. Energy crops in Ireland: An economic comparison of willow and Miscanthus production with conventional farming systems. Biomass Bioenergy 2008, 32, 407–421. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Tworkowski, J.; Szczukowski, S.; Niksa, D. Analysis of the energy efficiency of short rotation woody crops biomass as affected by different methods of soil enrichment. Energy 2016, 113, 748–761. [Google Scholar] [CrossRef]
- Heller, M.C.; Keoleian, G.A.; Volk, T.A. Life cycle assessment of a willow bioenergy cropping system. Biomass Bioenergy 2003, 25, 147–165. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Stolarski, M.J.; Szczukowski, S.; Tworkowski, J. Life cycle assessment of new willow cultivars grown as feedstock for integrated biorefineries. BioEnergy Res. 2016, 9, 224–238. [Google Scholar] [CrossRef]
- Londo, M.; Dekker, J.; ter Keurs, W. Willow short-rotation coppice for energy and breeding birds: An exploration of potentials in relation to management. Biomass Bioenergy 2005, 28, 281–293. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Matyka, M.; Staniak, M. Comparison of the effect of perennial energy crops and agricultural crops on weed flora diversity. Agronomy 2019, 9, 695. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Szczukowski, S.; Tworkowski, J.; Krzyżaniak, M.; Załuski, D. Willow production during 12 consecutive years—The effects of harvest rotation, planting density and cultivar on biomass yield. GCB Bioenergy 2019, 11, 635–656. [Google Scholar] [CrossRef]
- Tharakan, P.J.; Volk, T.A.; Nowak, C.A.; Abrahamson, L.P. Morphological traits of 30 willow clones and their relationship to biomass production. Can. J. For. Res. 2005, 35, 421–431. [Google Scholar] [CrossRef]
- Weih, M. Intensive short rotation forestry in boreal climates: Present and future perspectives. Can. J. For. Res. 2004, 34, 1369–1378. [Google Scholar] [CrossRef]
- Sevel, L.; Nord-Larsen, T.; Ingerslev, M.; Jørgensen, U.; Raulund-Rasmussen, K. Fertilization of SRC willow, I: Biomass production response. BioEnergy Res. 2014, 7, 319–328. [Google Scholar] [CrossRef]
- Amichev, B.Y.; Volk, T.A.; Hangs, R.D.; Bélanger, N.; Vujanovic, V.; Van Rees, K.C.J. Growth, survival, and yields of 30 short-rotation willow cultivars on the Canadian Prairies: 2nd rotation implications. New For. 2018, 49, 649–665. [Google Scholar] [CrossRef]
- Wang, Z.; MacFarlane, D.W. Evaluating the biomass production of coppiced willow and poplar clones in Michigan, USA, over multiple rotations and different growing conditions. Biomass Bioenergy 2012, 46, 380–388. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Szczukowski, S.; Tworkowski, J.; Śnieg, M. Willow productivity on a commercial plantation in triennial harvest cycle. Bulg. J. Agric. Sci. 2016, 22, 65–72. [Google Scholar]
- Nord-Larsen, T.; Sevel, L.; Raulund-Rasmussen, K. Commercially grown short rotation coppice willow in Denmark: Biomass production and factors affecting production. BioEnergy Res. 2015, 8, 325–339. [Google Scholar] [CrossRef]
- Castaño-Díaz, M.; Barrio-Anta, M.; Afif-Khouri, E.; Cámara-Obregón, A. Willow short rotation coppice trial in a former mining area in northern Spain: Effects of clone, fertilization and planting density on yield after five years. Forests 2018, 9, 154. [Google Scholar] [CrossRef]
- Weger, J.; Havlíčková, K.; Bubeník, J. Results of testing of native willows and poplars for short rotation coppice after three harvests. Asp. Appl. Biol. 2011, 112, 335–340. [Google Scholar]
- Lindegaard, K.N.; Carter, M.M.; McCracken, A.; Shield, I.; MacAlpine, W.; Jones, M.H.; Valentine, J.; Larsson, S. Comparative trials of elite Swedish and UK biomass willow varieties 2001–2010. Asp. Appl. Biol. 2011, 112, 57–66. [Google Scholar]
- Bullard, M.J.; Mustill, S.J.; Nixon, P.M.I.; McMillan, S.D.; Britt, C.P. The effect on yield of initial planting density of short rotation coppice. Asp. Appl. Biol. 2001, 65, 167–171. [Google Scholar]
- Otepka, P.; Habán, M. Biomass yield of basket willow (Salix viminalis L.) cultivated as energy plant in a long-term experiment. Acta Fytotech. Zootech. 2006, 9, 70–74. [Google Scholar]
- Labrecque, M.; Teodorescu, T.I. High biomass yield achieved by Salix clones in SRIC following two 3-year coppice rotations on abandoned farmland in southern Quebec, Canada. Biomass Bioenergy 2003, 25, 135–146. [Google Scholar] [CrossRef]
- Hytönen, J. Ten-year biomass production and stand structure of Salix ‘aquatica’ energy forest plantation in Southern Finland. Biomass Bioenergy 1995, 8, 63–71. [Google Scholar] [CrossRef]
- Otepka, P.; Habán, M.; Habánová, M. Cultivation of fast-growing woody plant basket willow (Salix viminalis L.) and their bioremedial abilities while fertilized with wood ash. Res. J. Agric. Sci. 2011, 43, 218–222. [Google Scholar]
- Nordh, N.E. Long Term Changes in Stand Structure and Biomass Production in Short Rotation Willow Coppice. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2005. [Google Scholar]
- McElroy, G.H.; Dawson, W.M. Biomass from short-rotation coppice willow on marginal land. Biomass 1986, 10, 225–240. [Google Scholar] [CrossRef]
- McCracken, A.R.; Dawson, W.M. Short rotation coppice willow in Northern Ireland since 1973: Development of the use of mixtures in the control of foliar rust (Melampsora spp.). Eur. J. For. Pathol. 1998, 28, 241–250. [Google Scholar] [CrossRef]
- Lindegaard, K.N.; Parfitt, R.I.; Donaldson, G.; Hunter, T.; Dawson, W.M.; Forbes, E.G.A.; Carter, M.M.; Whinney, C.C.; Whinney, J.E.; Larsson, S. Comparative trials of elite Swedish and UK biomass willow varieties. Asp. Appl. Biol. 2001, 65, 183–192. [Google Scholar]
- Volk, T.A.; Abrahamson, L.P.; Cameron, K.D.; Castellano, P.; Corbin, T.; Fabio, E.; Johnson, G.; Kuzovkina-Eischen, Y.; Labrecque, M.; Miller, R. Yields of willow biomass crops across a range of sites in North America. Asp. Appl. Biol. 2011, 112, 67–74. [Google Scholar]
- Larsson, S. Commercial varieties from the Swedish willow breeding programme. Asp. Appl. Biol. 2001, 65, 193–198. [Google Scholar]
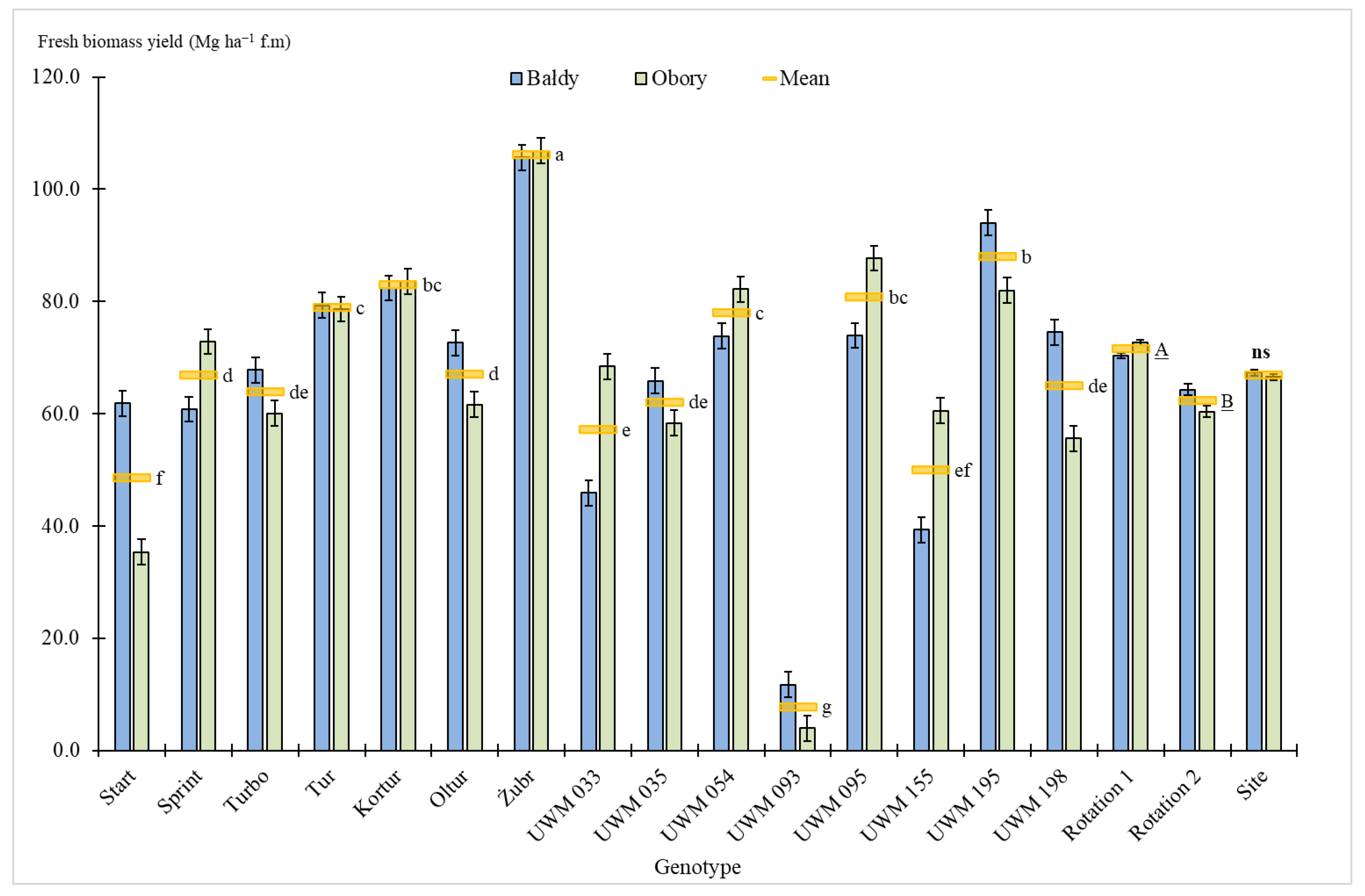
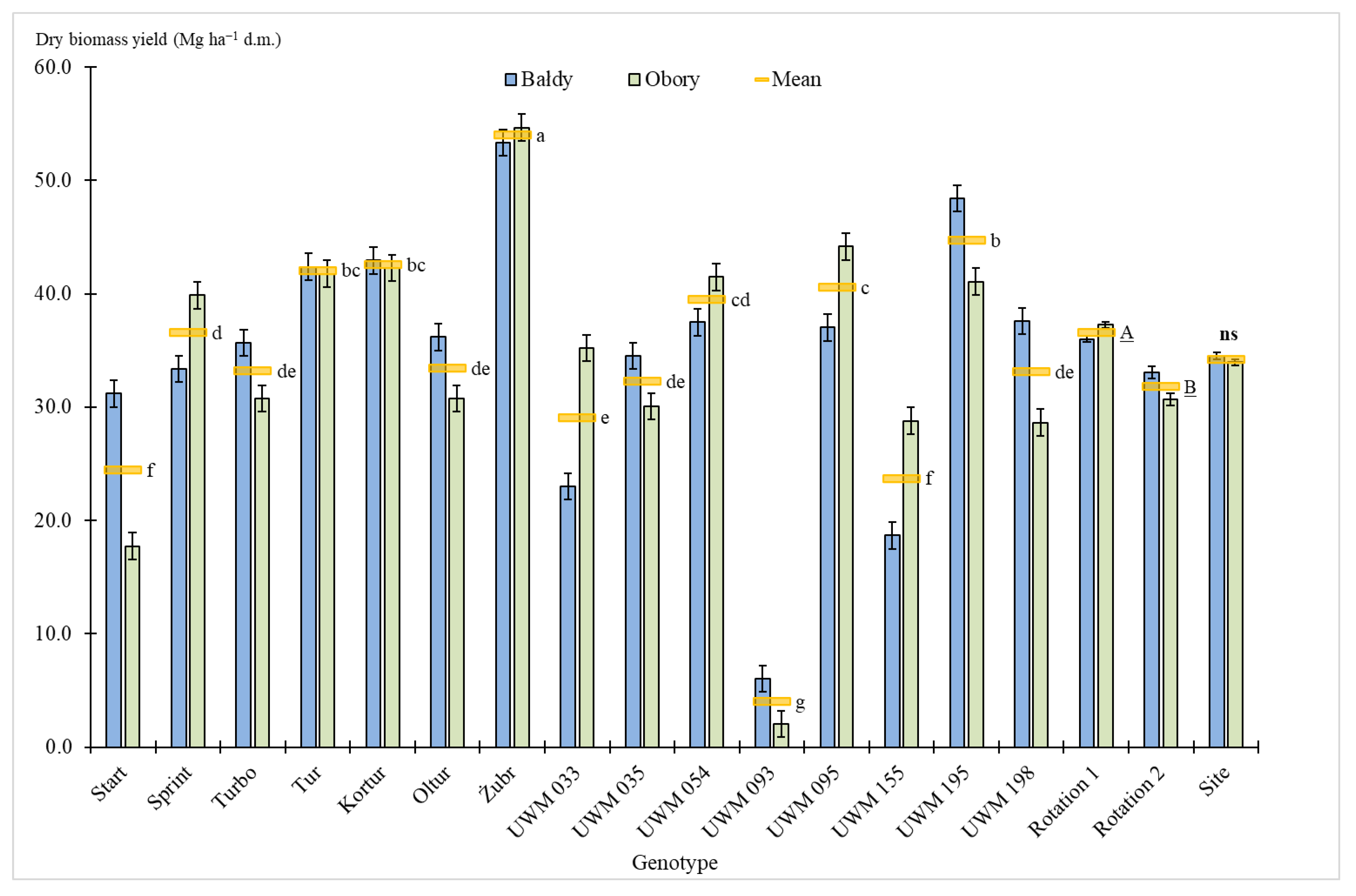
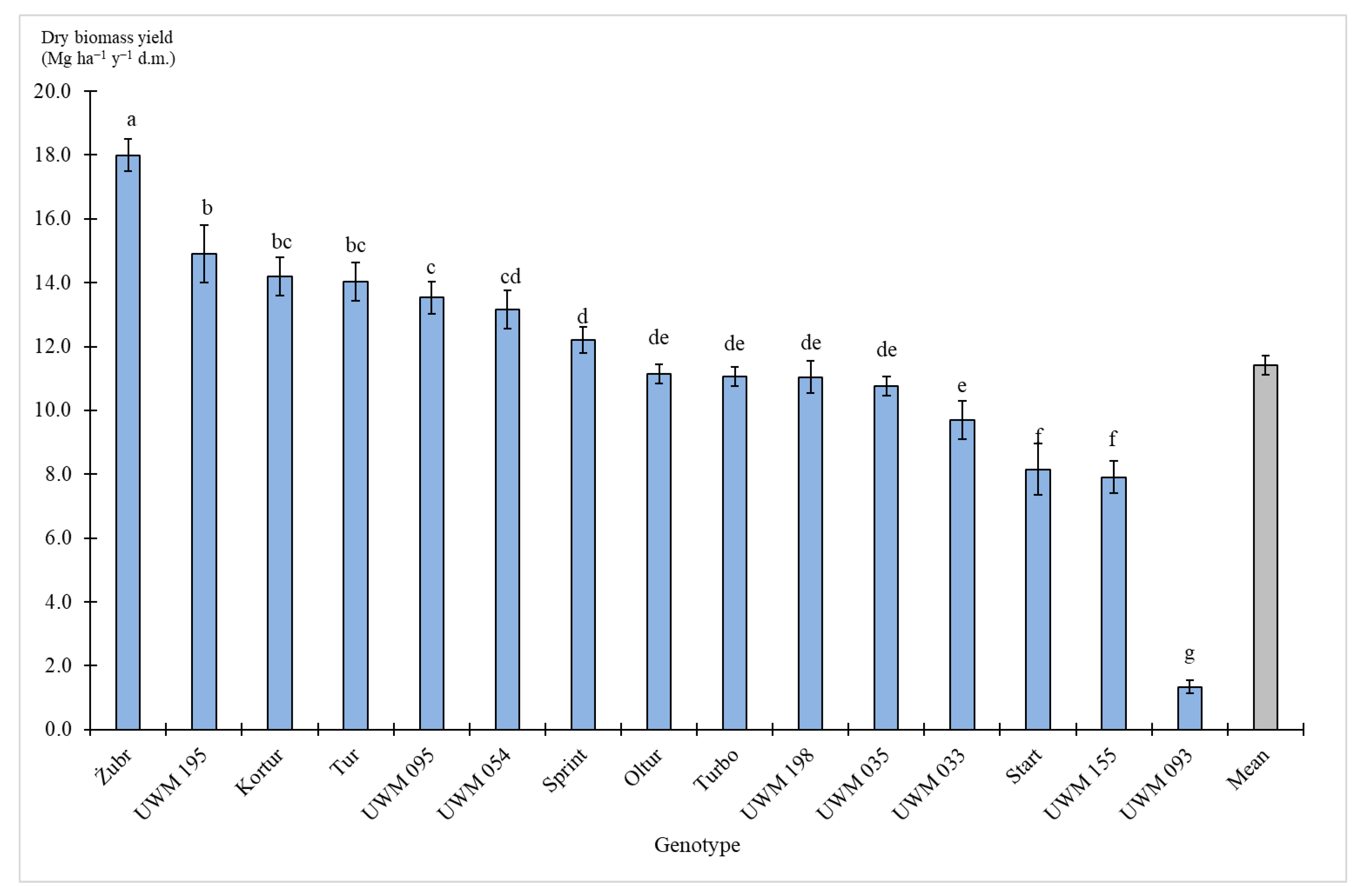
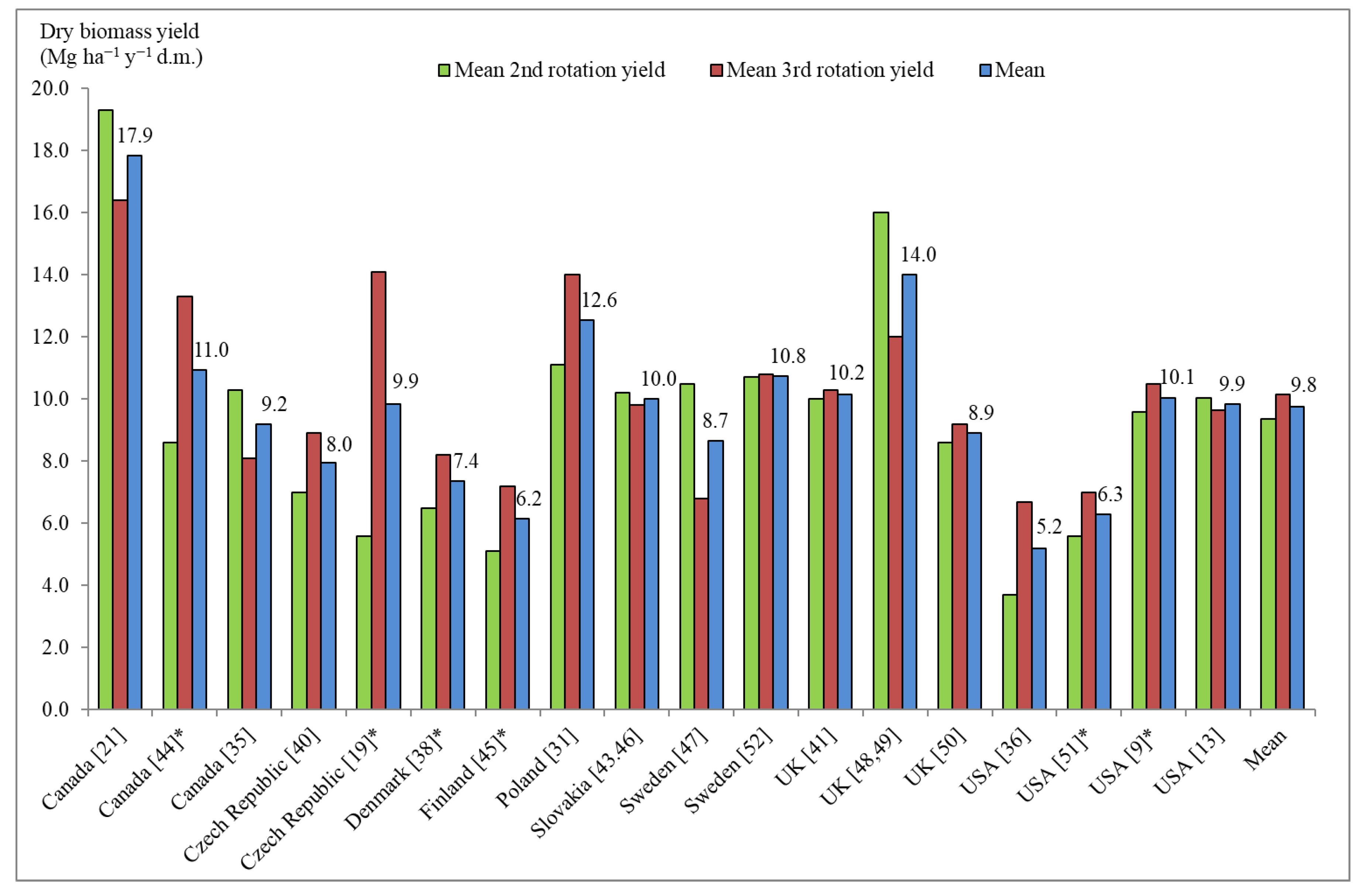
| Species | Genotype Status (Variety or Clone) | Name |
|---|---|---|
| Salix viminalis | variety | Start |
| S. viminalis | variety | Sprint |
| S. viminalis | variety | Turbo |
| S. viminalis | variety | Tur |
| S. viminalis | variety | Kortur |
| S. viminalis | variety | Oltur |
| S. viminalis | variety | Żubr |
| S. acutifolia | clone | UWM 093 |
| S. alba | clone | UWM 095 |
| S. dasyclados | clone | UWM 155 |
| S. fragilis | clone | UWM 195 |
| S. pentandra | clone | UWM 035 |
| S. triandra | clone | UWM 198 |
| S. viminalis × S. amygdalina | clone | UWM 054 |
| S. viminalis × S. purpurea | clone | UWM 033 |
| Source of Variation | df | Survival | No. of Shoots | Height | Shoot Diameter | Fresh Matter Yield | Dry Matter Yield | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Share (%) | F | Share (%) | F | Share (%) | F | Share (%) | F | Share (%) | F | Share (%) | ||
| Site | 1 | 8.1 ** | 0.5 | 68.4 *** | 9.2 | 35.3 *** | 1.6 | 2.3 | 0.5 | 0.9 | 0.0 | 1.8 | 0.1 |
| Rep (Site) | 4 | 1.70 | 0.4 | 3.3 * | 1.8 | 0.7 | 0.1 | 0.3 | 0.2 | 0.4 | 0.0 | 0.4 | 0.0 |
| Genotype (Gen) | 14 | 68.4 *** | 60.7 | 15.7 *** | 29.3 | 131.3 *** | 80.9 | 18.9 *** | 53.3 | 196.5 *** | 81.2 | 194.4 *** | 81.1 |
| Site × Gen | 14 | 14.4 *** | 12.8 | 5.6*** | 10.5 | 13.0 *** | 8.0 | 5.0 *** | 14.1 | 20.2 *** | 8.3 | 20.0 *** | 8.3 |
| Error 1 | 56 | 3.6 | 7.5 | 2.5 | 11.3 | 1.7 | 1.7 | ||||||
| Rotation (Rot) | 1 | 425.1 *** | 17.0 | 88.8 *** | 15.5 | 26.2 *** | 1.2 | 14.7 *** | 3.0 | 136.3 *** | 3.7 | 133.9 *** | 3.7 |
| Rot × Site | 1 | 0.00 | 0.0 | 6.1 * | 1.1 | 9.9 ** | 0.4 | 0.1 | 0.0 | 15.5 *** | 0.4 | 19.5 *** | 0.5 |
| Rot × Rep (Site) | 4 | 1.20 | 0.2 | 0.9 | 0.6 | 1.4 | 0.3 | 0.5 | 0.4 | 0.4 | 0.0 | 0.4 | 0.0 |
| Rot × Gen | 14 | 1.70 | 0.9 | 3.6 *** | 8.8 | 3.2 *** | 2.0 | 0.8 | 2.3 | 4.6 *** | 1.8 | 3.8 *** | 1.5 |
| Rot × Site × Gen | 14 | 2.8 ** | 1.6 | 2.5 ** | 6.1 | 1.0 | 0.6 | 1.1 | 3.3 | 3.4 *** | 1.3 | 3.7 *** | 1.4 |
| Error 2 | 56 | 2.2 | 9.7 | 2.5 | 11.6 | 1.5 | 1.6 | ||||||
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||||||
| Variable | Survival | No. of Shoots | Height | Shoot Diameter |
|---|---|---|---|---|
| Fresh matter yield | 0.57 ** | 0.15 | 0.76 ** | 0.55 ** |
| Dry matter yield | 0.56 ** | 0.17 | 0.75 ** | 0.54 ** |
| Parameter | Parameter Value | p-Value |
|---|---|---|
| Fresh matter yield | ||
| Intercept | −98.95 | <0.0001 |
| Survival | 2.18 | <0.0001 |
| No. of shoots | 0.85 | 0.4826 |
| Height | 15.12 | <0.0001 |
| Shoot diameter | 1.21 | 0.0186 |
| R2 | 0.76 | |
| R2adj. | 0.74 | |
| Dry matter yield | ||
| Intercept | −51.27 | <0.0001 |
| Survival | 1.11 | <0.0001 |
| No. of shoots | 0.58 | 0.3615 |
| Height | 7.64 | 0.0001 |
| Shoot diameter | 0.64 | 0.0180 |
| R2 | 0.75 | |
| R2adj. | 0.73 | |
| Variable | Site | Genotype | Rotation | Total |
|---|---|---|---|---|
| Survival | 25.42 | 17.43 | 23.59 | 25.50 |
| No. of shoots | 34.95 | 30.73 | 33.10 | 38.31 |
| Height | 13.24 | 5.90 | 13.27 | 13.32 |
| Shoot diameter | 20.25 | 14.25 | 20.01 | 20.24 |
| Fresh matter yield | 35.89 | 18.61 | 35.19 | 35.82 |
| Dry matter yield | 36.27 | 18.97 | 35.59 | 36.19 |
| Genotype | Bałdy | Obory | Mean for Two Sites | |
|---|---|---|---|---|
| Top 1 | 1st rotation yield | 18.0 | 20.3 | 19.2 |
| 2nd rotation yield | 17.6 | 16.1 | 16.8 | |
| yield difference | −0.4 | −4.3 | −2.3 | |
| % of yield decrease | 2.1 | 20.9 | 12.1 | |
| mean yield from two rotations | 17.8 | 18.2 | 18.0 | |
| % of mean yield compared to the highest-yielding genotype | 100.0 | 100.0 | 100.0 | |
| Top 3 | 1st rotation yield | 16.7 | 17.6 | 17.1 |
| 2nd rotation yield | 15.4 | 13.1 | 14.3 | |
| yield difference | −1.3 | −4.4 | −2.9 | |
| % of yield decrease | 7.8 | 25.3 | 16.8 | |
| mean yield from two rotations | 16.1 | 15.3 | 15.7 | |
| % of mean yield compared to the highest-yielding genotype | 90.4 | 84.2 | 87.3 | |
| Top 5 | 1st rotation yield | 15.6 | 17.0 | 16.3 |
| 2nd rotation yield | 14.3 | 12.9 | 13.6 | |
| yield difference | −1.3 | −4.1 | −2.7 | |
| % of yield decrease | 8.5 | 24.0 | 16.5 | |
| mean yield from two rotations | 14.9 | 14.9 | 14.9 | |
| % of mean yield compared to the highest-yielding genotype | 84.0 | 81.9 | 83.0 | |
| Mean for all 15 genotypes | 1st rotation yield | 12.0 | 12.4 | 12.2 |
| 2nd rotation yield | 11.0 | 10.2 | 10.6 | |
| yield difference | −1.0 | −2.2 | −1.6 | |
| % of yield decrease | 8.2 | 17.7 | 13.0 | |
| mean yield from two rotations | 11.5 | 11.3 | 11.4 | |
| % of mean yield compared to the highest-yielding genotype | 64.7 | 62.1 | 63.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolarski, M.J.; Krzyżaniak, M.; Załuski, D.; Tworkowski, J.; Szczukowski, S. Effects of Site, Genotype and Subsequent Harvest Rotation on Willow Productivity. Agriculture 2020, 10, 412. https://doi.org/10.3390/agriculture10090412
Stolarski MJ, Krzyżaniak M, Załuski D, Tworkowski J, Szczukowski S. Effects of Site, Genotype and Subsequent Harvest Rotation on Willow Productivity. Agriculture. 2020; 10(9):412. https://doi.org/10.3390/agriculture10090412
Chicago/Turabian StyleStolarski, Mariusz Jerzy, Michał Krzyżaniak, Dariusz Załuski, Józef Tworkowski, and Stefan Szczukowski. 2020. "Effects of Site, Genotype and Subsequent Harvest Rotation on Willow Productivity" Agriculture 10, no. 9: 412. https://doi.org/10.3390/agriculture10090412
APA StyleStolarski, M. J., Krzyżaniak, M., Załuski, D., Tworkowski, J., & Szczukowski, S. (2020). Effects of Site, Genotype and Subsequent Harvest Rotation on Willow Productivity. Agriculture, 10(9), 412. https://doi.org/10.3390/agriculture10090412






