The Influence of Three Years of Supplemental Nitrogen on Above- and Belowground Biomass Partitioning in a Decade-Old Miscanthus × giganteus in the Lower Silesian Voivodeship (Poland)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Fertilization Treatments of Miscanthus × Giganteus
2.2. Plant Growth Measurement
- FM—fresh mass.
- DM—dry mass.
2.3. Soil and Weather Conditions
2.4. Statistical Analysis
3. Results
3.1. Effect of Nitrogen Fertilization on Morphological Features of Miscanthus × Giganteus
3.2. Effect of Nitrogen Fertilization on Water Concentration of Miscanthus × Giganteus
3.3. Effect of Nitrogen Fertilization on Dry Matter Yield of Miscanthus × Giganteus
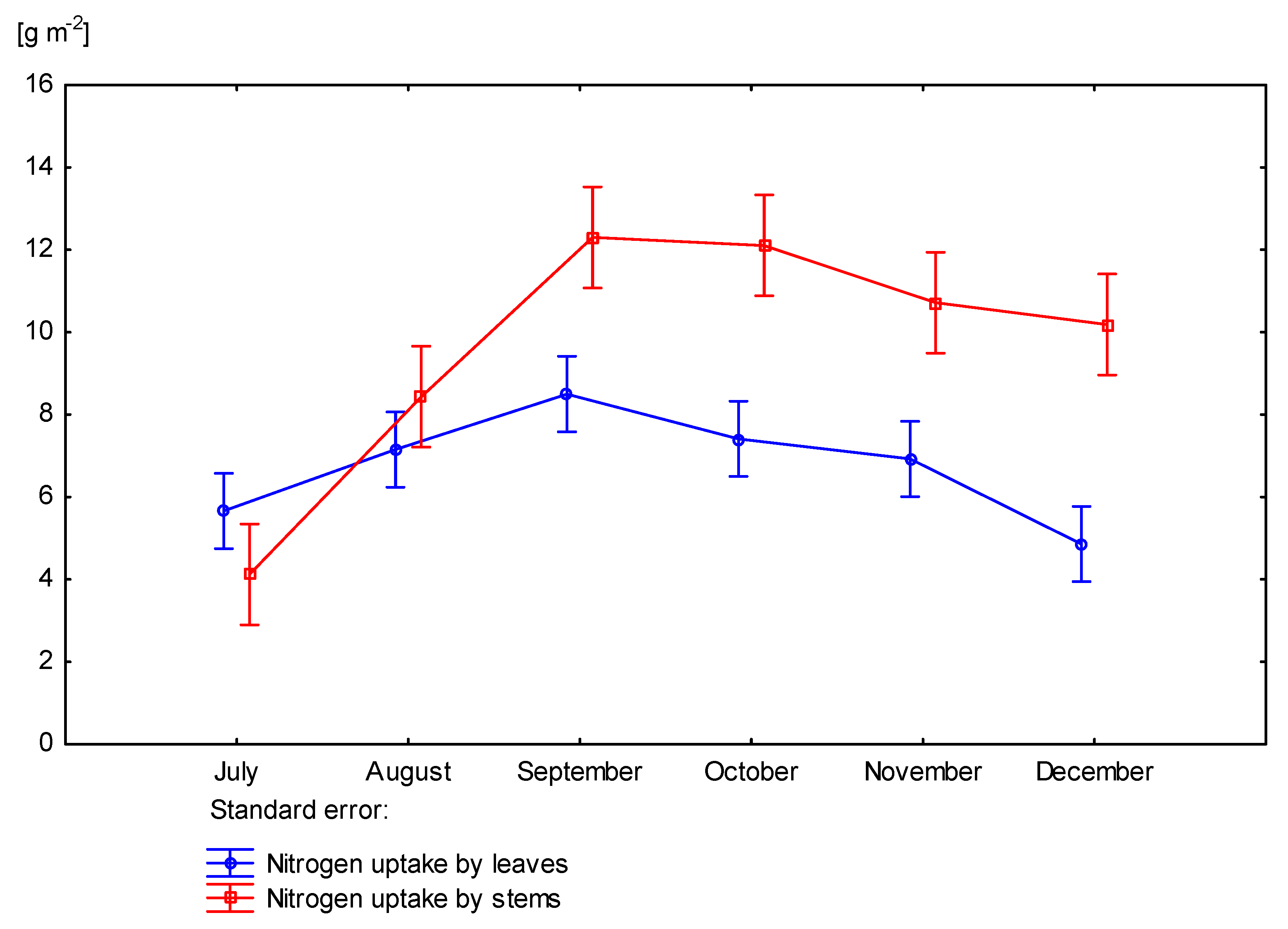
3.4. Nitrogen Uptake by Miscanthus × Giganteus
4. Discussion
- Most research on Miscanthus productivity has been conducted in Europe (different soils, different spatial diversity, and topographic diversity);
- The studies carried out are generally short-term;
- The potential share of nitrogen reserves in rhizomes and soil nitrogen increases the uncertainty of the Miscanthus nitrogen requirements [29].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baxter, X.C.; Darvell, L.I.; Jones, J.M.; Barraclough, T.; Yates, N.E.; Shield, I. Miscanthus combustion properties and variations with Miscanthus agronomy. Fuel 2014, 117, 851–869. [Google Scholar] [CrossRef] [Green Version]
- Karl, T.R.; Trenberth, K.E. Modern global climate change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capellán-Pérez, I.; Mediavilla, M.; de Castro, C.; Carpintero, Ó.; Miguel, L.J. Fossil fuel depletion and socio-economic scenarios: An integrated approach. Energy 2014, 77, 641–666. [Google Scholar]
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Hager, H.A.; Sinasac, S.E.; Gedalof, Z.; Newman, J.A. Predicting potential global distributions of two miscanthus grasses: Implications for horticulture, biofuel production, and biological invasions. PLoS ONE 2014, 9, e100032. [Google Scholar] [CrossRef] [Green Version]
- Hastings, A.; Clifton-brown, J.; Wattenbach, M.; Mitchell, C.P.; Stampfl, P.; Smith, P. Future energy potential of Miscanthus in Europe. GCB Bioenergy 2009, 30, 1058. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; Van Der Linden, G.C.; Schwarz, K.U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.L.; Dolstra, O.; Donnison, I.S.; et al. Progress on optimizing miscanthus biomass production for the european bioeconomy: Results of the EU FP7 Project OPTIMISC. Front. Plant Sci. 2016, 7, 1620. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, I.; Clifton-Brown, J.; Scurlock, J.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal agricultural land low-input systems for biomass production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, B.; Baxter, L.; Miles, T. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Sahoo, K.; Milewski, A.; Mani, S.; Hoghooghi, N.; Panda, S. Assessment of miscanthus yield potential from strip-mined lands (SML) and its impacts on stream water quality. Water 2019, 11, 546. [Google Scholar] [CrossRef] [Green Version]
- Kering, M.K.; Butler, T.J.; Biermacher, J.T.; Guretzky, J.A. Biomass yield and nutrient removal rates of perennial grasses under nitrogen fertilization. BioEnergy Res. 2011, 5, 61–70. [Google Scholar] [CrossRef]
- Roncucci, N.; Di Nasso, N.N.O.; Tozzini, C.; Bonari, E.; Ragaglini, G. Miscanthus × giganteus nutrient concentrations and uptakes in autumn and winter harvests as influenced by soil texture, irrigation and nitrogen fertilization in the Mediterranean. GCB Bioenergy 2015, 7, 1009–1018. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Long, S.P. Meeting US biofuel goals with less land: The potential of Miscanthus. Glob. Chang. Biol. 2008, 14, 2000–2014. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Miguez, A.F.; Juvik, J.A.; Lozovaya, V.; Widholm, J.; Zabotina, O.A.; McIsaac, G.F.; David, M.B.; Voigt, T.B.; et al. Miscanthus. A promising biomass crop. Adv. Bot. Res. 2010, 56, 75–137. [Google Scholar] [CrossRef]
- Dierking, R.M.; Allen, D.J.; Cunningham, S.M.; Brouder, S.M.; Volenec, J.J. Nitrogen reserve pools in two Miscanthus × giganteus genotypes under contrasting N managements. Front. Plant Sci. 2017, 8, 1618. [Google Scholar] [CrossRef] [Green Version]
- Stavridou, E.; Hastings, A.; Webster, R.; Robson, P. The impact of soil salinity on the yield, composition and physiology of the bioenergy grass Miscanthus × giganteus. GCB Bioenergy 2017, 9, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Stefanovska, T.; Pidlisnyuk, V.; Lewis, E.; Gorbatenko, A. Herbivorous insects diversity at miscanthus × giganteus in Ukraine. Agriculture 2017, 63, 23–32. [Google Scholar] [CrossRef]
- Galatsidas, S.; Gounaris, N.; Vlachaki, D.; Dimitriadis, E.; Kiourtsis, F.; Keramitzis, D.; Gerwin, W.; Repmann, F.; Rettenmaier, N.; Reinhardt, G.; et al. Revealing bioenergy potentials: Mapping marginal lands in Europe—The seemla approach. In Proceedings of the European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–17 May 2018. [Google Scholar]
- Maughan, M.; Bollero, G.; Lee, D.K.; Darmody, R.; Bonos, S.; Cortese, L.; Murphy, J.; Gaussoin, R.; Sousek, M.; Williams, D.; et al. Miscanthus × giganteus productivity: The effects of management in different environments. GCB Bioenergy 2012, 4, 253–265. [Google Scholar] [CrossRef]
- Zub, H.W.; Brancourt-Hulmel, M. Agronomic and physiological performances of different species ofMiscanthus, a major energy crop. A review. Agron. Sustain. Dev. 2010, 30, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Styles, D.; Jones, M.B. Energy crops in Ireland: Quantifying the potential life-cycle greenhouse gas reductions of energy-crop electricity. Biomass Bioenergy 2007, 31, 759–772. [Google Scholar] [CrossRef]
- Di Nasso, N.N.O.; Roncucci, N.; Triana, F.; Tozzini, C.; Bonari, E. Seasonal nutrient dynamics and biomass quality of giant reed (Arundo donax L.) and miscanthus (Miscanthus × giganteus Greef et Deuter) as energy crops. Ital. J. Agron. 2011, 6, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Drazic, G.; Milovanovic, J.; Stefanovic, S.; Petric, I. Potential of Miscanthus × Giganteus for heavy metals removing from industrial deposol. Acta Reg. Environ. 2017, 2, 56–58. [Google Scholar]
- Lee, D.K.; Boe, A. Biomass production of switchgrass in central south Dakota. Crop. Sci. 2005, 45, 2583. [Google Scholar] [CrossRef]
- Dohleman, F.G.; Heaton, E.A.; Arundale, R.A.; Long, S.P. Seasonal dynamics of above- and below-ground biomass and nitrogen partitioning in Miscanthus × giganteus and Panicum virgatum across three growing seasons. GCB Bioenergy 2012, 4, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Clifton-Brown, J.C.; Lewandowski, I. Water use efficiency and biomass partitioning of three different miscanthus genotypes with limited and unlimited water supply. Ann. Bot. 2000, 86, 191–200. [Google Scholar] [CrossRef]
- Beale, C.; Long, S. Seasonal dynamics of nutrient accumulation and partitioning in the perennial C4-grasses Miscanthus × giganteus and Spartina cynosuroides. Biomass Bioenergy 1997, 12, 419–428. [Google Scholar] [CrossRef]
- Strullu, L.; Cadoux, S.; Preudhomme, M.; Jeuffroy, M.H.; Beaudoin, N. Biomass production and nitrogen accumulation and remobilisation by Miscanthus × giganteus as influenced by nitrogen stocks in belowground organs. Field Crop. Res. 2011, 121, 381–391. [Google Scholar] [CrossRef]
- Cadoux, S.; Riche, A.B.; Yates, N.E.; Machet, J.M. Nutrient requirements of Miscanthus × giganteus: Conclusions from a review of published studies. Biomass Bioenergy 2012, 38, 14–22. [Google Scholar] [CrossRef]
- Arundale, R.A.; Dohleman, F.G.; Heaton, E.A.; McGrath, J.M.; Voigt, T.B.; Long, S.P. Yields of Miscanthus × giganteus and panicum virgatum decline with stand age in the Midwestern USA. GCB Bioenergy 2014, 6, 71–87. [Google Scholar] [CrossRef]
- Christian, D.; Riche, A.; Yates, N. Growth, yield and mineral content of Miscanthus × giganteus grown as a biofuel for 14 successive harvests. Ind. Crop. Prod. 2008, 28, 320–327. [Google Scholar] [CrossRef]
- Schwarz, H.; Liebhard, P.; Ehrendorfer, K.; Ruckenbauer, P. The effect of fertilization on yield and quality of Miscanthus sinensis ‘Giganteus’. Ind. Crop. Prod. 1994, 2, 153–159. [Google Scholar] [CrossRef]
- Greef, J.M. Etablierung und Biomassebildung von Miscanthus × Giganteus; Cuvillier Verlag: Gottingen, Germany, 1995; pp. 1–162. [Google Scholar]
- Kahle, P.; Beuch, S.; Boelcke, B.; Leinweber, P.; Schulten, H.R. Cropping of Miscanthus in Central Europe: Biomass production and influence on nutrients and soil organic matter. Eur. J. Agron. 2001, 15, 171–184. [Google Scholar] [CrossRef]
- Lee, M.-S.; Wycislo, A.; Guo, J.; Lee, D.K.; Voigt, T. Nitrogen fertilization effects on biomass production and yield components of Miscanthus × giganteus. Front. Plant Sci. 2017, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yost, M.A.; Randall, B.K.; Kitchen, N.R.; Heaton, E.A.; Myers, R.L. Yield potential and nitrogen requirements of miscanthus × giganteus on eroded soil. Agron. J. 2017, 109, 684–695. [Google Scholar] [CrossRef] [Green Version]
- Finnan, J.; Burke, B. Nitrogen dynamics in a mature Miscanthus × giganteus crop fertilized with nitrogen over a five year period. Irish J. Agric. Food Res. 2014, 53, 171–188. [Google Scholar]
- Wildová, R.; Wild, J.; Herben, T. Fine-scale dynamics of rhizomes in a grassland community. Ecography 2007, 30, 264–276. [Google Scholar] [CrossRef]
- Mann, J.J.; Barney, J.N.; Kyser, G.B.; DiTOMASO, J.M. Root system dynamics of Miscanthus × giganteus and panicum virgatum in response to rainfed and irrigated conditions in California. BioEnergy Res. 2013, 6, 678–687. [Google Scholar] [CrossRef]
- Ameen, A.; Liu, J.; Han, L.P.; Xie, G.H. Effects of nitrogen rate and harvest time on biomass yield and nutrient cycling of switchgrass and soil nitrogen balance in a semiarid sandy wasteland. Ind. Crop. Prod. 2019, 136, 1–10. [Google Scholar] [CrossRef]
- Peixoto, M.; Friesen, P.C.; Sage, R.F. Winter cold-tolerance thresholds in field-grown Miscanthus hybrid rhizomes. J. Exp. Bot. 2015, 66, 4415–4425. [Google Scholar] [CrossRef] [Green Version]
- Clifton-Brown, J.C.; Lewandowski, I. Overwintering problems of newly established Miscanthus plantations can be overcome by identifying genotypes with improved rhizome cold tolerance. New Phytol. 2000, 148, 287–294. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Agharezaee, M.; Kamgar-Haghighi, A.A.; Sepaskhah, A.R. Comparing canopy temperature and leaf water potential as irrigation scheduling criteria of potato in water-saving irrigation strategies. Int. J. Plant Prod. 2017, 11, 333–347. [Google Scholar]
- Heaton, E. A quantitative review comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenergy 2004, 27, 21–30. [Google Scholar] [CrossRef]
- IUNG-PIB. Available online: http://www.iung.pulawy.pl/ (accessed on 13 September 2020).
- Avramidis, P.; Nikolaou, K.; Bekiari, V. Total organic carbon and total nitrogen in sediments and soils: A comparison of the wet oxidation—Titration method with the combustion-infrared method. Agric. Agric. Sci. Procedia 2015, 4, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Kondratowicz-Maciejewska, K.; Kobierski, M. Content of available magnesium, phosphorus and potassium forms in soil exposed to varied crop rotation and fertilisation. J. Elementology 2011, 16, 543–553. [Google Scholar] [CrossRef]
- Slepetiene, A.; Slepetys, J. Status of humus in soil under various long-term tillage systems. Geoderma 2005, 127, 207–215. [Google Scholar] [CrossRef]
- Kilmer, V.J.; Nearpass, D.C. The determination of available sulfur in soils. Soil Sci. Soc. Am. J. 1960, 24, 337–340. [Google Scholar] [CrossRef]
- De Rooij, G.H. Methods of soil analysis. Vadose Zone J. 2004, 24, 337–340. [Google Scholar]
- Finnan, J.; Burke, B. Nitrogen fertilization of Miscanthus × giganteus: Effects on nitrogen uptake, growth, yield and emissions from biomass combustion. Nutr. Cycl. Agroecosyst. 2016, 106, 249–256. [Google Scholar] [CrossRef]
- Cosentino, S.; Patanè, C.; Sanzone, E.; Copani, V.; Foti, S. Effects of soil water content and nitrogen supply on the productivity of Miscanthus×giganteus Greef et Deu. in a Mediterranean environment. Ind. Crop. Prod. 2007, 25, 75–88. [Google Scholar] [CrossRef]
- Wiesler, F.; Dickmann, J.; Horst, W.J. Effects of nitrogen supply on growth and nitrogen uptake byMiscanthus sinensis during establishment. J. Plant Nutr. Soil Sci. 1997, 160, 25–31. [Google Scholar] [CrossRef]
- Chen, Z.; Tao, X.; Khan, A.; Tan, D.K.Y.; Luo, H. Biomass Accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.; Arundale, R.; Maughan, M.; Oladeinde, A.; Wycislo, A.; Voigt, T. Growth and agronomy of Miscanthus × giganteus for biomass production. Biofuels 2011, 2, 71–87. [Google Scholar] [CrossRef]
- Himken, M.; Lammel, J.; Neukirchen, D.; Czypionka-Krause, U.; Olfs, H.W. Cultivation of Miscanthus under West European conditions: Seasonal changes in dry matter production, nutrient uptake and remobilization. Plant Soil 1997, 189, 117–126. [Google Scholar] [CrossRef]
- Miguez, F.E.; Villamil, M.; Long, S.P.; Bollero, G.A. Meta-analysis of the effects of management factors on Miscanthus×giganteus growth and biomass production. Agric. For. Meteorol. 2008, 148, 1280–1292. [Google Scholar] [CrossRef]
- Paruelo, J.; Lauenroth, W.K.; Burke, I.C.; Sala, O.E. Grassland precipitation-use efficiency varies across a resource gradient. Ecosystems 1999, 2, 64–68. [Google Scholar] [CrossRef]





| Year | pH 1 N KCl | C g kg−1 | Humus g kg−1 | N g kg−1 | C:N | P mg kg−1 | K mg kg−1 | Mg mg kg−1 | S mg kg−1 |
|---|---|---|---|---|---|---|---|---|---|
| 2014 | 5.0 | 5.82 | 10.00 | 0.58 | 10.53 | 119.6 | 114.0 | 24.3 | 188.0 |
| 2015 | 5.0 | 5.86 | 10.05 | 0.60 | 10.60 | 119.6 | 115.3 | 27.3 | 192.6 |
| 2016 | 4.8 | 5.86 | 10.05 | 0.59 | 10.63 | 119.7 | 112.6 | 26.0 | 190.0 |
| Year | Fe mg kg−1 | Mn mg kg−1 | Zn mg kg−1 | Cu mg kg−1 |
|---|---|---|---|---|
| 2014 | 428 | 93.4 | 82.3 | 1.82 |
| 2015 | 461 | 97.1 | 79.4 | 1.69 |
| 2016 | 463 | 95.2 | 78.5 | 1.78 |
| Month | Temperature [°C] | Precipitation [mm] | ||||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | Average 1981–2010 | 2014 | 2015 | 2016 | Average 1981–2010 | |
| I | 0.0 | 2.3 | −1.2 | −0.8 | 35.8 | 46.0 | 33.4 | 31.9 |
| II | 3.7 | 1.5 | 3.8 | 0.3 | 1.2 | 15.6 | 56.2 | 26.7 |
| III | 7.0 | 5.4 | 4.3 | 3.8 | 40.1 | 39.5 | 55.9 | 31.7 |
| IV | 10.6 | 8.9 | 8.7 | 8.9 | 55.2 | 15.8 | 46.4 | 30.5 |
| V | 13.3 | 13.5 | 15.3 | 14.4 | 101.4 | 21.0 | 5.3 | 51.3 |
| VI | 16.6 | 16.6 | 18.6 | 17.1 | 40.2 | 73.3 | 44.6 | 59.5 |
| VII | 21.2 | 20.3 | 19.5 | 19.3 | 52.9 | 55.6 | 114.3 | 78.9 |
| VIII | 17.3 | 22.7 | 17.9 | 18.3 | 75.0 | 5.6 | 27.1 | 61.7 |
| IX | 15.5 | 15.1 | 16.4 | 13.6 | 72.2 | 23.2 | 44.7 | 45.3 |
| X | 10.7 | 8.4 | 8.5 | 9.1 | 59.4 | 20.0 | 83.8 | 32.3 |
| XI | 6.6 | 6.2 | 3.4 | 3.9 | 15.5 | 52.4 | 36.3 | 36.6 |
| XII | 2.3 | 5.4 | 1.2 | 0.2 | 17.5 | 24.0 | 36.1 | 37.4 |
| Average annual air temperature and total precipitation | 10.4 | 10.5 | 9.7 | 9.0 | 566.4 | 392.0 | 584.1 | 523.8 |
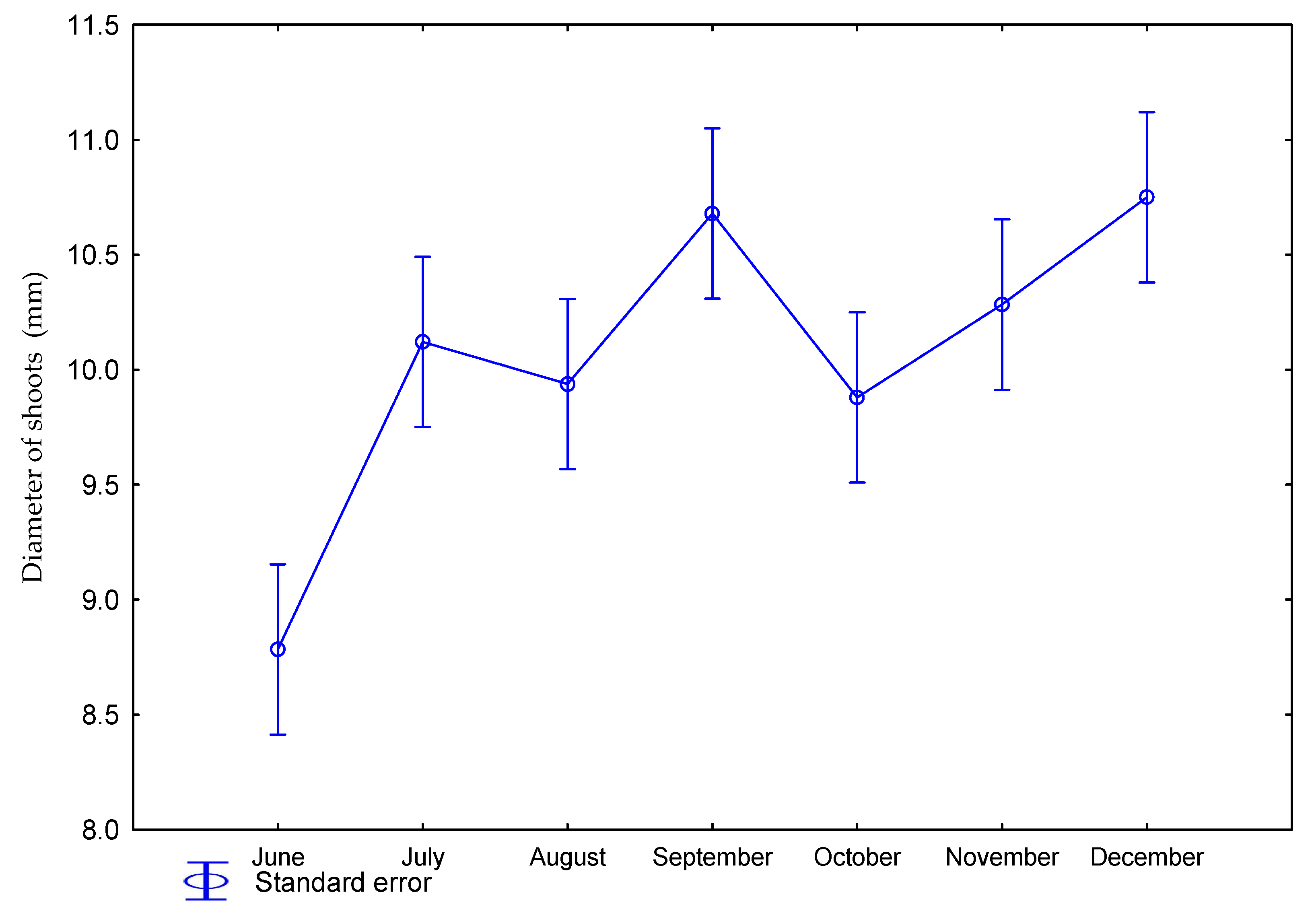
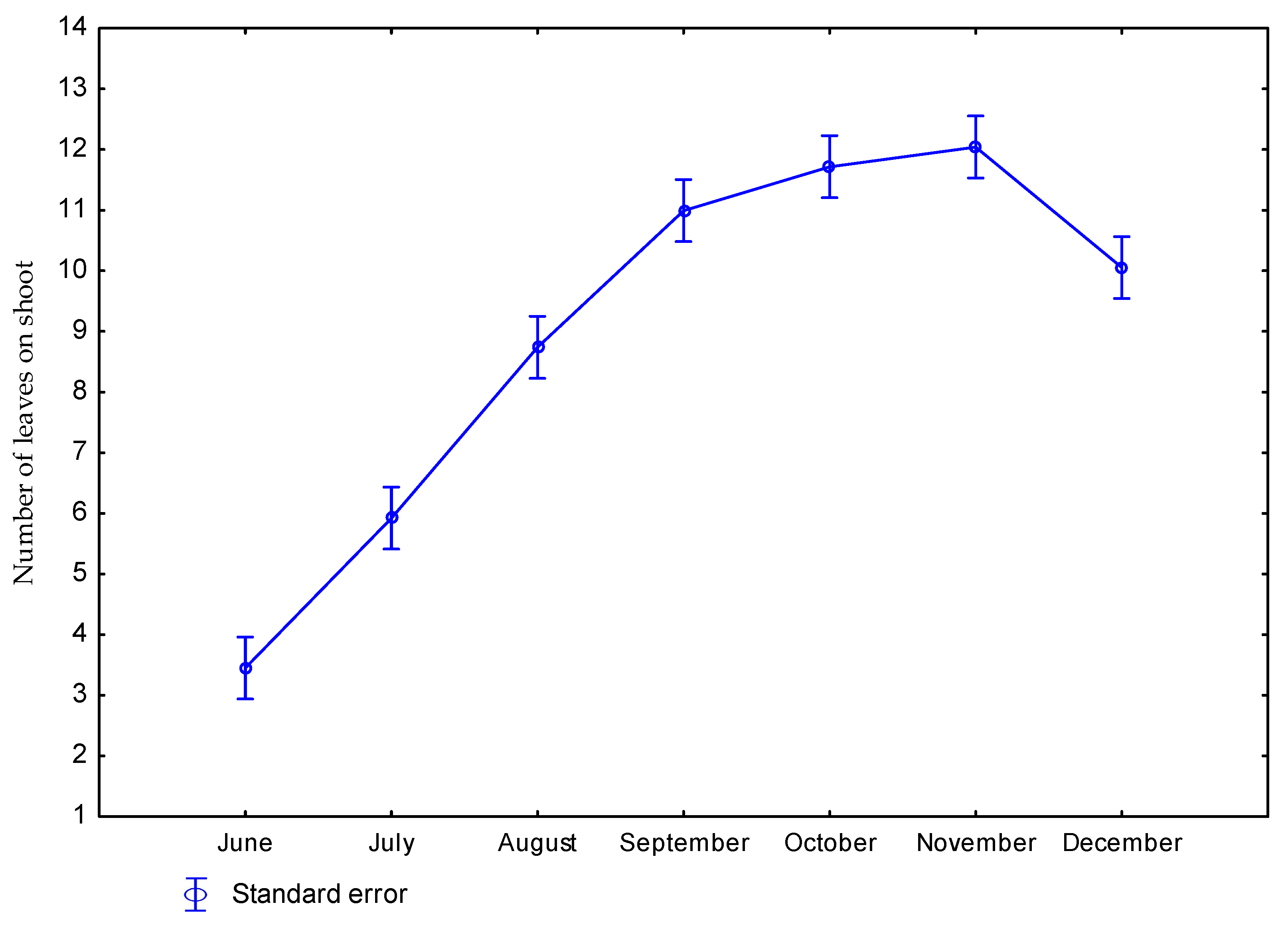
| Dose kg ha−1 N | Number of Days Starting from Beginning of Vegetation Period | Number of Shoots per 1 m2 | Height of Plants (m) | Diameter of Shoots (mm) | Number of Leaves on Shoot |
|---|---|---|---|---|---|
| 0 | June | 52 | 0.18 | 8.5 | 3.1 |
| July | 59 | 1.23 | 10.3 | 5.9 | |
| August | 64 | 2.14 | 9.7 | 8.7 | |
| September | 64 | 2.33 | 10.5 | 10.7 | |
| October | 66 | 2.98 | 9.6 | 11.3 | |
| November | 74 | 3.07 | 10.0 | 11.7 | |
| December | 72 | 3.34 | 10.8 | 8.9 | |
| 60 | June | 53 | 0.21 | 9.1 | 3.8 |
| July | 64 | 1.04 | 9.9 | 5.9 | |
| August | 66 | 2.24 | 10.1 | 8.8 | |
| September | 76 | 2.71 | 10.8 | 11.3 | |
| October | 78 | 3.09 | 10.2 | 12.1 | |
| November | 72 | 3.13 | 10.5 | 12.4 | |
| December | 78 | 3.31 | 10.8 | 11.2 | |
| p value | 0.2884 | 0.0001 | 0.4553 | 0.0322 | |
| Averages for Factors and Years | |||||
| 0 | - | 64 | 2.18 | 9.9 | 8.6 |
| 60 | - | 70 | 2.25 | 10.2 | 9.3 |
| p value | 0.0018 | 0.7020 | 0.1004 | 0.1484 | |
| 2014 | - | 67 | 2.28 | 10.0 | 8.5 |
| 2015 | 66 | 2.20 | 10.0 | 8.6 | |
| 2016 | 68 | 2.16 | 10.2 | 9.9 | |
| p value | 0.4112 | 0.8354 | 0.4200 | 0.4040 | |
| Dose kg ha−1 N | Number of Days Starting from Beginning of Vegetation Period | Rhizomes | Stems | Leaves | Aboveground Part of Plant |
|---|---|---|---|---|---|
| 0 | June | 722 | - | - | 882 |
| July | 689 | 870 | 879 | 875 | |
| August | 709 | 772 | 777 | 775 | |
| September | 684 | 697 | 715 | 702 | |
| October | 663 | 691 | 702 | 694 | |
| November | 663 | 662 | 698 | 672 | |
| December | 670 | 622 | 679 | 635 | |
| 60 | June | 744 | - | - | 883 |
| July | 707 | 867 | 853 | 862 | |
| August | 712 | 827 | 810 | 820 | |
| September | 713 | 764 | 781 | 769 | |
| October | 673 | 720 | 740 | 726 | |
| November | 683 | 676 | 707 | 685 | |
| December | 704 | 661 | 701 | 672 | |
| p value | 0.4958 | 0.0015 | 0.0260 | 0.00120 | |
| Average for Factors and Years | |||||
| 0 | 686 | 719 | 742 | 748 | |
| 60 | 705 | 752 | 765 | 774 | |
| p value | 0.0004 | 0.0218 | 0.0669 | 0.0693 | |
| 2014 | 673 | 714 | 722 | 738 | |
| 2015 | 693 | 722 | 735 | 750 | |
| 2016 | 721 | 771 | 803 | 795 | |
| p value | 0.0000 | 0.0022 | 0.0000 | 0.0025 | |
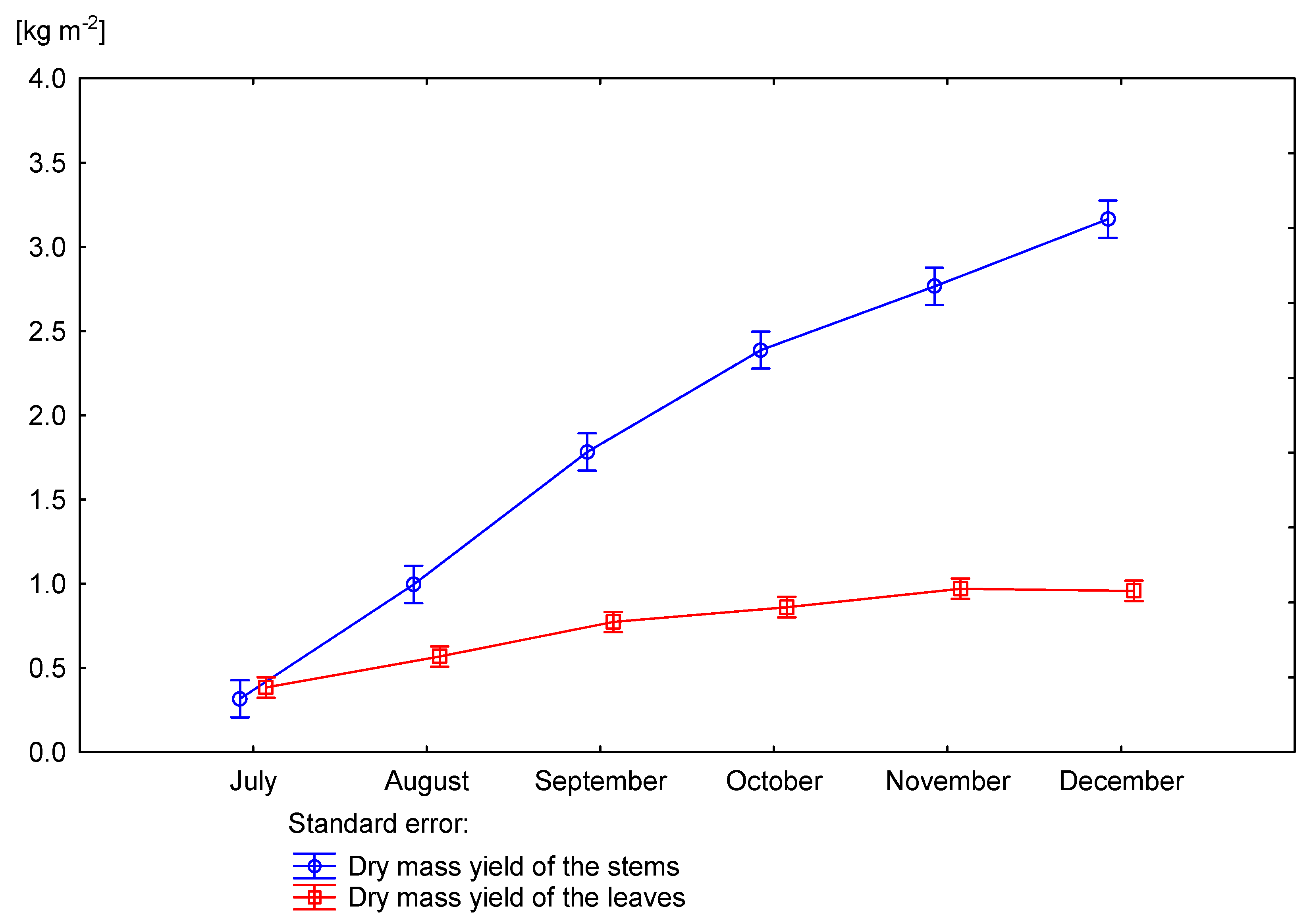
| Dose kg ha−1 N | Number of Days Starting from Beginning of Vegetation Period | Rhizomes | Aboveground Part | Rhizomes and Aboveground Part | ||
|---|---|---|---|---|---|---|
| Stems | Leaves | All Together | ||||
| 0 | June | 0.52 | - | - | 0.34 | 0.86 |
| July | 0.65 | 0.28 | 0.32 | 0.60 | 1.25 | |
| August | 0.77 | 0.94 | 0.45 | 1.39 | 2.16 | |
| September | 1.15 | 1.67 | 0.63 | 2.30 | 3.45 | |
| October | 1.34 | 2.22 | 0.78 | 3.00 | 4.34 | |
| November | 1.67 | 2.81 | 0.89 | 3.70 | 5.37 | |
| December | 1.73 | 3.08 | 0.84 | 3.92 | 5.65 | |
| 60 | June | 0.73 | - | - | 0.44 | 1.17 |
| July | 0.77 | 0.35 | 0.45 | 0.80 | 1.57 | |
| August | 0.97 | 1.06 | 0.68 | 1.74 | 2.71 | |
| September | 1.22 | 1.90 | 0.92 | 2.82 | 4.04 | |
| October | 1.64 | 2.55 | 0.94 | 3.49 | 5.13 | |
| November | 1.64 | 2.72 | 1.06 | 3.78 | 5.42 | |
| December | 1.83 | 3.25 | 1.07 | 4.32 | 6.15 | |
| p value | 0.0125 | 0.1223 | 0.1393 | 0.0153 | 0.0056 | |
| Average for Factors and Years | ||||||
| 0 | 1.12 | 1.83 | 0.65 | 2.18 | 3.29 | |
| 60 | 1.26 | 1.97 | 0.85 | 2.48 | 3.74 | |
| p value | 0.0524 | 0.4310 | 0.0000 | 0.1586 | 0.1181 | |
| 2014 | 1.20 | 2.00 | 0.75 | 2.41 | 3.61 | |
| 2015 | 1.15 | 1.91 | 0.71 | 2.30 | 3.45 | |
| 2016 | 1.21 | 1.79 | 0.79 | 2.27 | 3.48 | |
| p value | 0.7318 | 0.6005 | 0.3165 | 0.8522 | 0.8881 | |
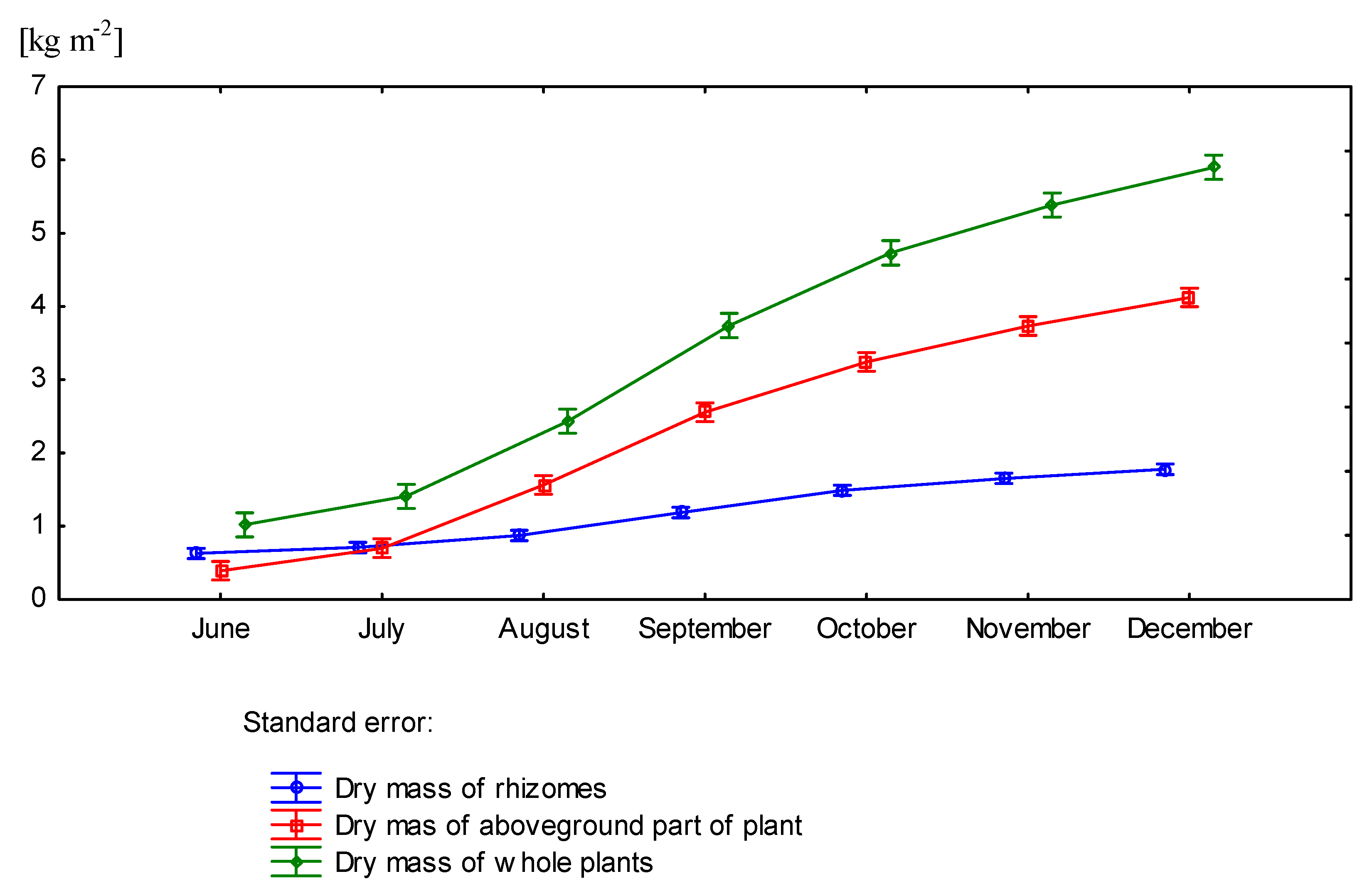
| Dose kg ha−1 N | Number of Days from the Start of the Growing Season | Rhizomes | Aboveground Part of Plants | Rhizomes and Aboveground Part of Plants | ||
|---|---|---|---|---|---|---|
| Stems | Leaves | Together | ||||
| 0 | June | 5.35 | - | - | 4.75 | 10.10 |
| July | 3.25 | 3.16 | 4.34 | 7.50 | 10.75 | |
| August | 3.65 | 8.24 | 4.79 | 13.03 | 16.68 | |
| September | 5.29 | 10.24 | 5.99 | 16.24 | 21.53 | |
| October | 6.62 | 8.50 | 6.04 | 14.54 | 21.16 | |
| November | 10.19 | 8.61 | 6.66 | 15.27 | 25.46 | |
| December | 10.68 | 7.97 | 3.97 | 11.95 | 22.63 | |
| 60 | June | 9.24 | - | - | 7.60 | 16.84 |
| July | 5.79 | 5.08 | 6.98 | 12.05 | 17.84 | |
| August | 5.40 | 8.63 | 9.52 | 18.15 | 23.55 | |
| September | 6.91 | 14.36 | 11.01 | 25.37 | 32.28 | |
| October | 7.72 | 15.71 | 8.78 | 24.51 | 32.23 | |
| November | 14.07 | 12.82 | 7.19 | 20.01 | 34.08 | |
| December | 14.94 | 12.40 | 5.74 | 18.15 | 33.09 | |
| p value | 0.0118 | 0.0000 | 0.000 | 0.0000 | 0.0000 | |
| Means for Factors and Years | ||||||
| 0 | 6.43 | 7.79 | 5.30 | 11.90 | 18.33 | |
| 60 | 9.15 | 11.50 | 8.20 | 17.98 | 27.13 | |
| p value | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | |
| 2014 | 8.18 | 10.07 | 6.53 | 15.06 | 23.24 | |
| 2015 | 7.48 | 10.10 | 7.05 | 15.55 | 23.03 | |
| 2016 | 7.71 | 8.77 | 6.67 | 14.19 | 21.89 | |
| p value | 0.6315 | 0.1925 | 0.5895 | 0.5205 | 0.6493 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołąb-Bogacz, I.; Helios, W.; Kotecki, A.; Kozak, M.; Jama-Rodzeńska, A. The Influence of Three Years of Supplemental Nitrogen on Above- and Belowground Biomass Partitioning in a Decade-Old Miscanthus × giganteus in the Lower Silesian Voivodeship (Poland). Agriculture 2020, 10, 473. https://doi.org/10.3390/agriculture10100473
Gołąb-Bogacz I, Helios W, Kotecki A, Kozak M, Jama-Rodzeńska A. The Influence of Three Years of Supplemental Nitrogen on Above- and Belowground Biomass Partitioning in a Decade-Old Miscanthus × giganteus in the Lower Silesian Voivodeship (Poland). Agriculture. 2020; 10(10):473. https://doi.org/10.3390/agriculture10100473
Chicago/Turabian StyleGołąb-Bogacz, Izabela, Waldemar Helios, Andrzej Kotecki, Marcin Kozak, and Anna Jama-Rodzeńska. 2020. "The Influence of Three Years of Supplemental Nitrogen on Above- and Belowground Biomass Partitioning in a Decade-Old Miscanthus × giganteus in the Lower Silesian Voivodeship (Poland)" Agriculture 10, no. 10: 473. https://doi.org/10.3390/agriculture10100473
APA StyleGołąb-Bogacz, I., Helios, W., Kotecki, A., Kozak, M., & Jama-Rodzeńska, A. (2020). The Influence of Three Years of Supplemental Nitrogen on Above- and Belowground Biomass Partitioning in a Decade-Old Miscanthus × giganteus in the Lower Silesian Voivodeship (Poland). Agriculture, 10(10), 473. https://doi.org/10.3390/agriculture10100473







