A Meta-Analytical Approach on Arbuscular Mycorrhizal Fungi Inoculation Efficiency on Plant Growth and Nutrient Uptake
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Data Collection
2.2. Selection Criteria
2.3. Data Acquisition
2.4. Meta-Analysis
- R = Response ratio
- XE = Treatment mean (with inoculation)
- XC = Control mean (without inoculation)
- SE = Treatment standard deviation
- SC = Control standard deviation
- NC = Control replication number
- NE = Treatment replication number
3. Results
3.1. Overview
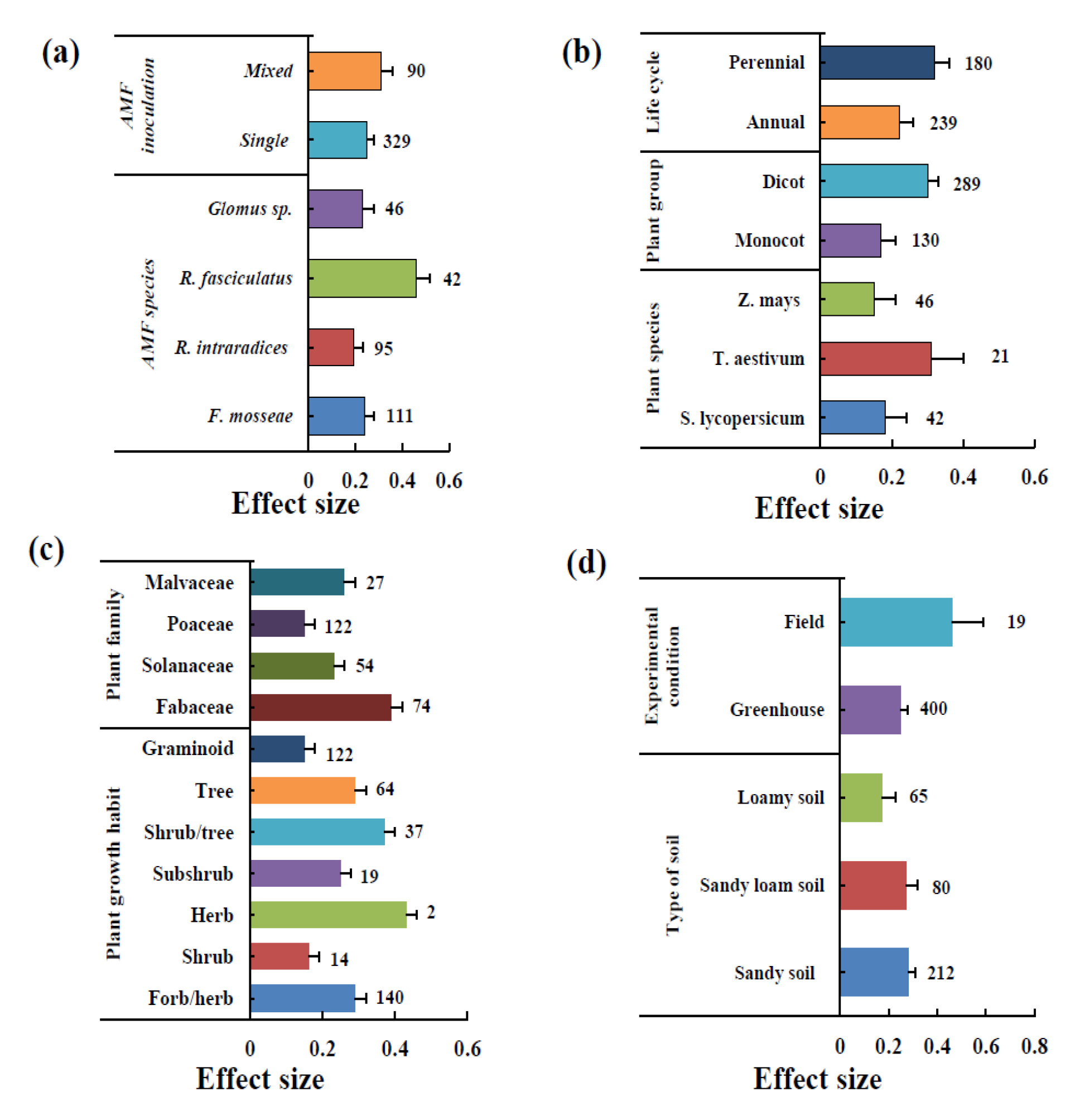
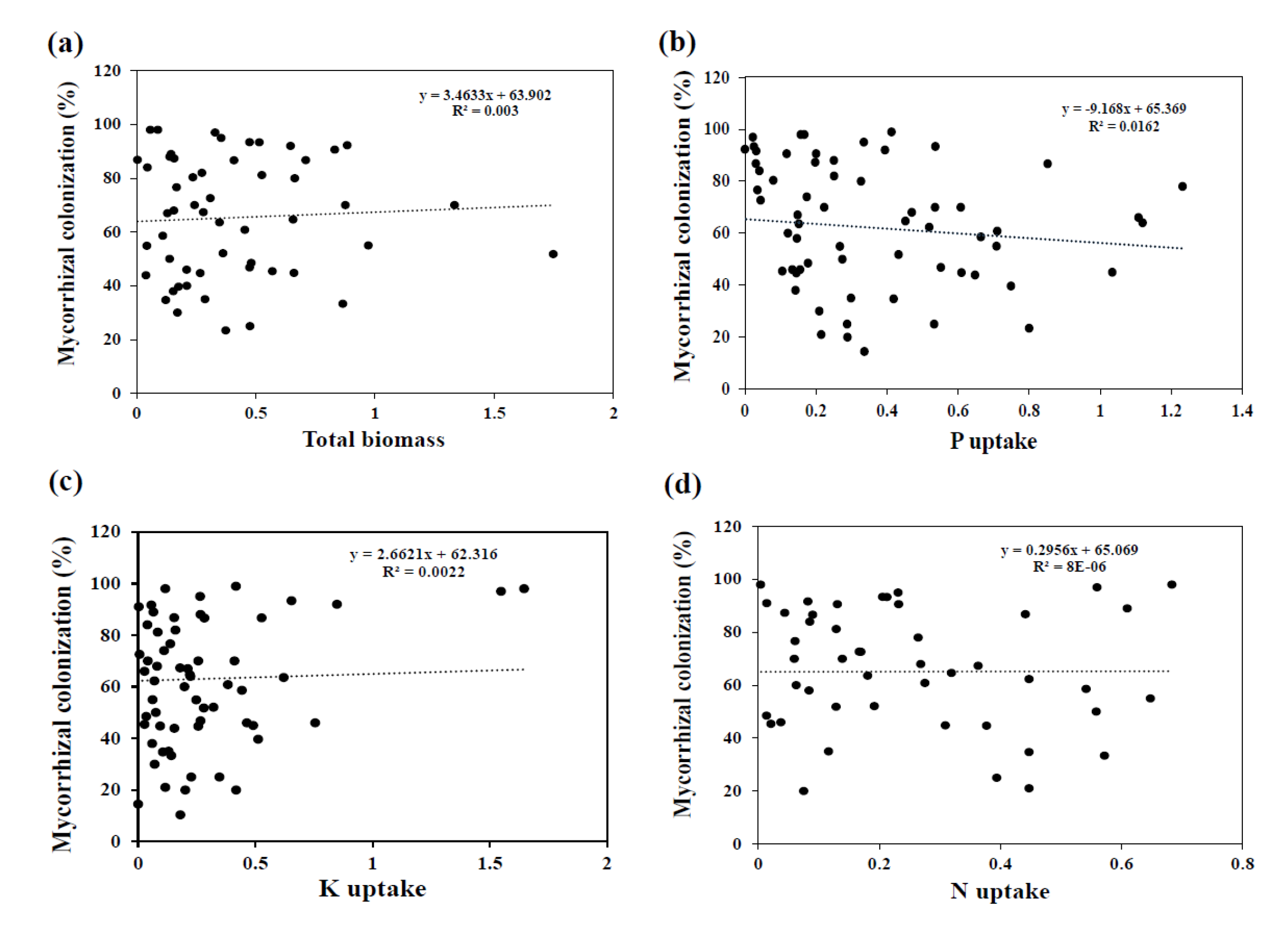
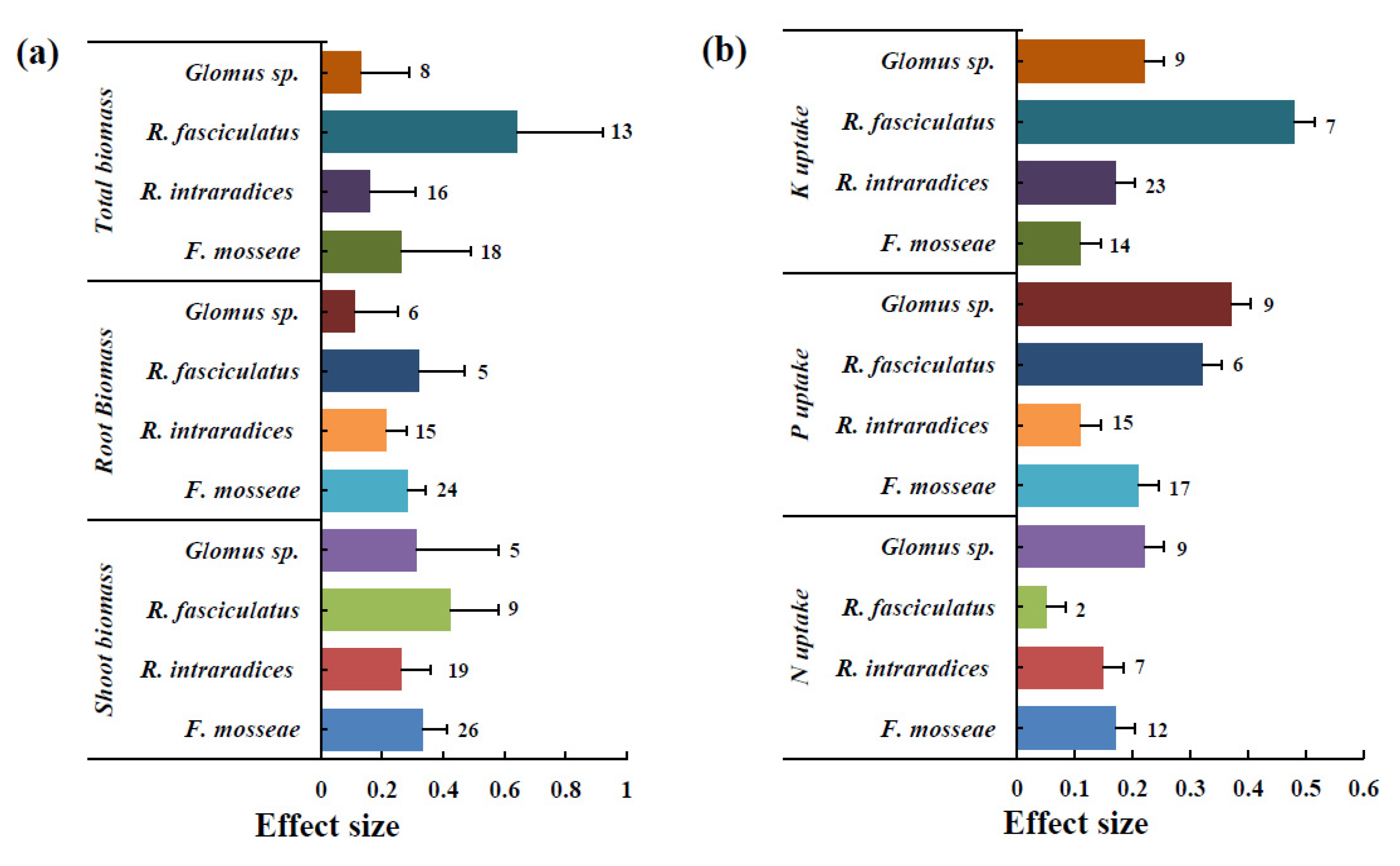
3.2. Effect of AMF Inoculation on Biomass
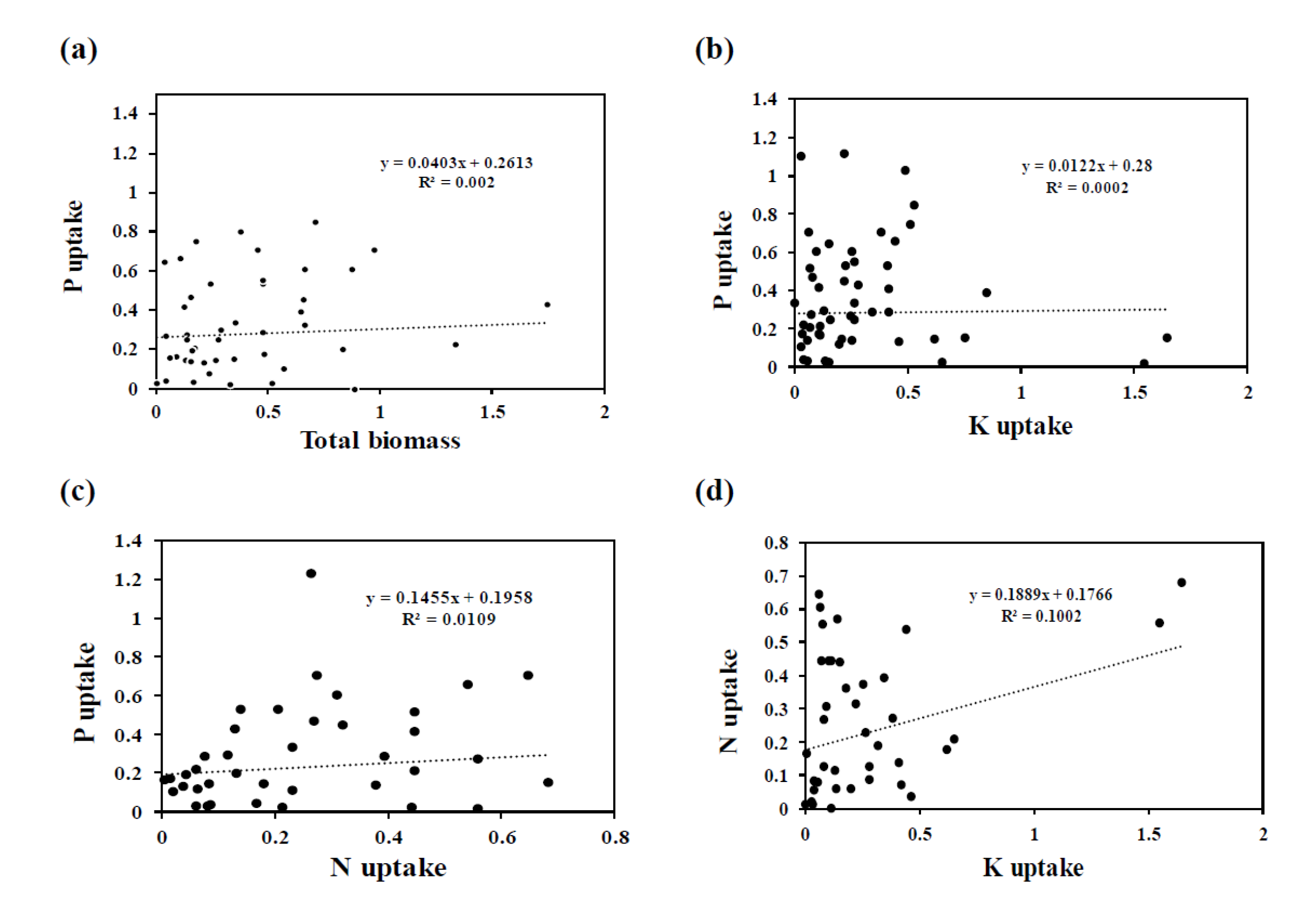
3.3. Effect of AMF Inoculation on P Uptake
3.4. Effect of AMF Inoculation on N Uptake
3.5. Effect of AMF Inoculation on K Uptake
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 1–11. [Google Scholar] [CrossRef]
- Ingraffia, R.; Amato, G.; Frenda, A.S.; Giambalvo, D. Impacts of arbuscular mycorrhizal fungi on nutrient uptake, N2 fixation, N transfer, and growth in a wheat/faba bean intercropping system. PLoS ONE 2019, 14, e0213672. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.; Zhu, L.; Yuan, Y.; Xu, L.; Wang, G.G.; Zhai, L.; Yang, L.; Zhang, J. Arbuscular mycorrhizal fungi effectively enhances the growth of Gleditsia sinensis Lam. seedlings under greenhouse conditions. Forests 2019, 10, 567. [Google Scholar] [CrossRef]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crop. Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress—A meta-analysis. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef]
- Liu, J.; Maldonado-Mendoza, I.; Lopex-Meyer, M.; Cheung, F.; Town, C.D.; Harrison, M.J. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J. 2007, 50, 529–544. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, A.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A.; Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 2003, 133, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Karaki, G.N. Growth of mycorrhizal tomato and mineral acquisition under salt stress. Mycorrhiza 2000, 10, 51–54. [Google Scholar] [CrossRef]
- Echeverria, M.; Sannazzaro, A.I.; Ruiz, O.A.; Menéndez, A.B. Modulatory effects of Mesorhizobium tianshanense and Glomus intraradices on plant proline and polyamine levels during early plant response of Lotus tenuis to salinity. Plant Soil 2013, 364, 69–79. [Google Scholar] [CrossRef]
- Evelin, H.; Kapoor, R.; Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 2009, 104, 1263–1280. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grnlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Treseder, K.K. The extent of mycorrhizal colonization of roots and its influence on plant growth and phosphorus content. Plant Soil 2013, 371, 1–13. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, F.S.; Li, X.L.; Tian, C.Y.; Tang, C.; Rengel, Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza 2002, 12, 185–190. [Google Scholar]
- Gurevitch, J.; Hedges, L.V. Statistical issues in ecological meta-analysis. Ecology 1999, 80, 1142–1149. [Google Scholar] [CrossRef]
- Hedges, L.V.; Gurevitch, J.; Curtis, P.S. The meta-analysis of response ratios in experimental ecology. Ecology 1999, 80, 1150–1156. [Google Scholar] [CrossRef]
- Borowicz, V.A. Do arbuscular mycorrhizal fungi alter plant–pathogen relations? Ecology 2001, 82, 3057–3068. [Google Scholar]
- Alberton, O.; Kuyper, T.W.; Gorissen, A. Taking mycocentrism seriously: Mycorrhizal fungal and plant responses to elevated CO2. New Phytol. 2005, 167, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Morris, W.F.; Hufbauer, R.A.; Agrawal, A.A.; Bever, J.D.; Borowicz, V.A.; Gilbert, G.S.; Maron, J.; Mitchell, C.E.; Parker, I.M.; Power, A.G.; et al. Direct and interactive effects of enemies and mutualists on plant performance: A meta-analysis. Ecology 2007, 88, 1021–1029. [Google Scholar] [CrossRef]
- Koricheva, J.; Gange, A.C.; Jones, T. Effects of mycorrhizal fungi on insect herbivores: A meta-analysis. Ecology 2009, 90, 2088–2097. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Sonia, B.; Hu, S.; Oh, S.H.; Sa, T. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 2014, 24, 611–625. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Menexes, G.; Rillig, M.C. Do arbuscular mycorrhizal fungi affect the allometric partition of host plant biomass to shoots and roots? A meta-analysis of studies from 1990 to 2010. Mycorrhiza 2012, 22, 227–235. [Google Scholar] [CrossRef]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
- Lekberg, Y.; Koide, R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef]
- Hoeksema, J.D.; Chaudhary, V.B.; Gehring, C.A.; Johnson, N.C.; Karst, J.; Koide, R.T.; Pringle, A.; Zabinski, C.; Bever, J.D.; Moore, J.C.; et al. A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 2010, 13, 394–407. [Google Scholar] [CrossRef]
- Jayne, B.; Quigley, M. Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: A meta-analysis. Mycorrhiza 2014, 24, 109–119. [Google Scholar] [CrossRef]
- Rosenberg, N.J.; Adams, D.C.; Gurevitch, J. MetaWin: Statistical Software for Meta-Analysis Version 2.0; Sinauer: Sunderland, MA, USA, 2000. [Google Scholar]
- Gurevitch, J.; Curtis, P.S.; Jones, M.H. Meta-analysis in ecology. Adv. Ecol. Res. 2001, 32, 199–247. [Google Scholar]
- Al-Karaki, G.N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 2006, 109, 1–7. [Google Scholar] [CrossRef]
- Borde, M.; Dudhane, M.; Jite, P.K. Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition. Crop Prot. 2011, 30, 265–271. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. Role of Arbuscular mycorrhizae in the alleviation of ionic, osmotic and oxidative stresses induced by salinity in Cajanus cajan (L.) Millsp. (pigeonpea). J. Agron. Crop Sci. 2009, 195, 110–123. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Sonmez, O.; Aydemir, S.; Tuna, A.L.; Cullu, M.A. The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hortic. 2009, 121, 1–6. [Google Scholar] [CrossRef]
- Munkvold, L.; Kjøller, R.; Vestberg, M.; Rosendahl, S.; Jakobsen, I. High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol. 2004, 164, 357–364. [Google Scholar] [CrossRef]
- Avio, L.; Pellegrino, E.; Bonari, E.; Giovannetti, M. Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelial networks. New Phytol. 2006, 172, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Burleigh, S.H.; Cavagnaro, T.; Jakobsen, I. Functional diversity of arbuscular mycorrhizas extends to the expression of plant genes involved in P nutrition. J. Exp. Bot. 2002, 53, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Croll, D.; Wille, L.; Gamper, H.A.; Mathimaran, N.; Lammers, P.J.; Corradi, N.; Sanders, I.R. Genetic diversity and host plant preferences revealed by simple sequence repeat and mitochondrial markers in a population of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2008, 178, 672–687. [Google Scholar] [CrossRef]
- Angelard, C.; Colard, A.; Niculita-Hirzel, H.; Croll, D.; Sanders, I.R. Segregation in a mycorrhizal fungus alters rice growth and symbiosis-specific gene transcription. Curr. Biol. 2010, 20, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Ronga, D.; Caradonia, F.; Francia, E.; Morcia, C.; Rizza, F.; Badeck, F.-W.; Ghizzoni, R.; Terzi, V. Interaction of tomato genotypes and arbuscular aycorrhizal fungi under reduced Irrigation. Horticulturae 2019, 5, 79. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF Inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Schellenbaum, L.; Berta, G.; Ravolanirina, F.; Tisserant, B.; Gianinazzi, S.; Fitter, A.H. Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.). Ann. Bot. 1991, 68, 135–141. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azco’n, R. Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 2000, 10, 137–143. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, R.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; He, X.H. Contributions of arbuscular mycorrhizal fungi togrowth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol. Plant. 2010, 32, 297–304. [Google Scholar] [CrossRef]
- Giri, B.; Mukerji, K.G. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: Evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 2004, 14, 307–312. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N.; He, X.H. Mycorrhizal symbiosis enhances tolerance to NaCl stress through selective absorption but not selective transport of K+ over Na+ in trifoliate orange. Sci. Hortic. 2013, 160, 366–374. [Google Scholar] [CrossRef]
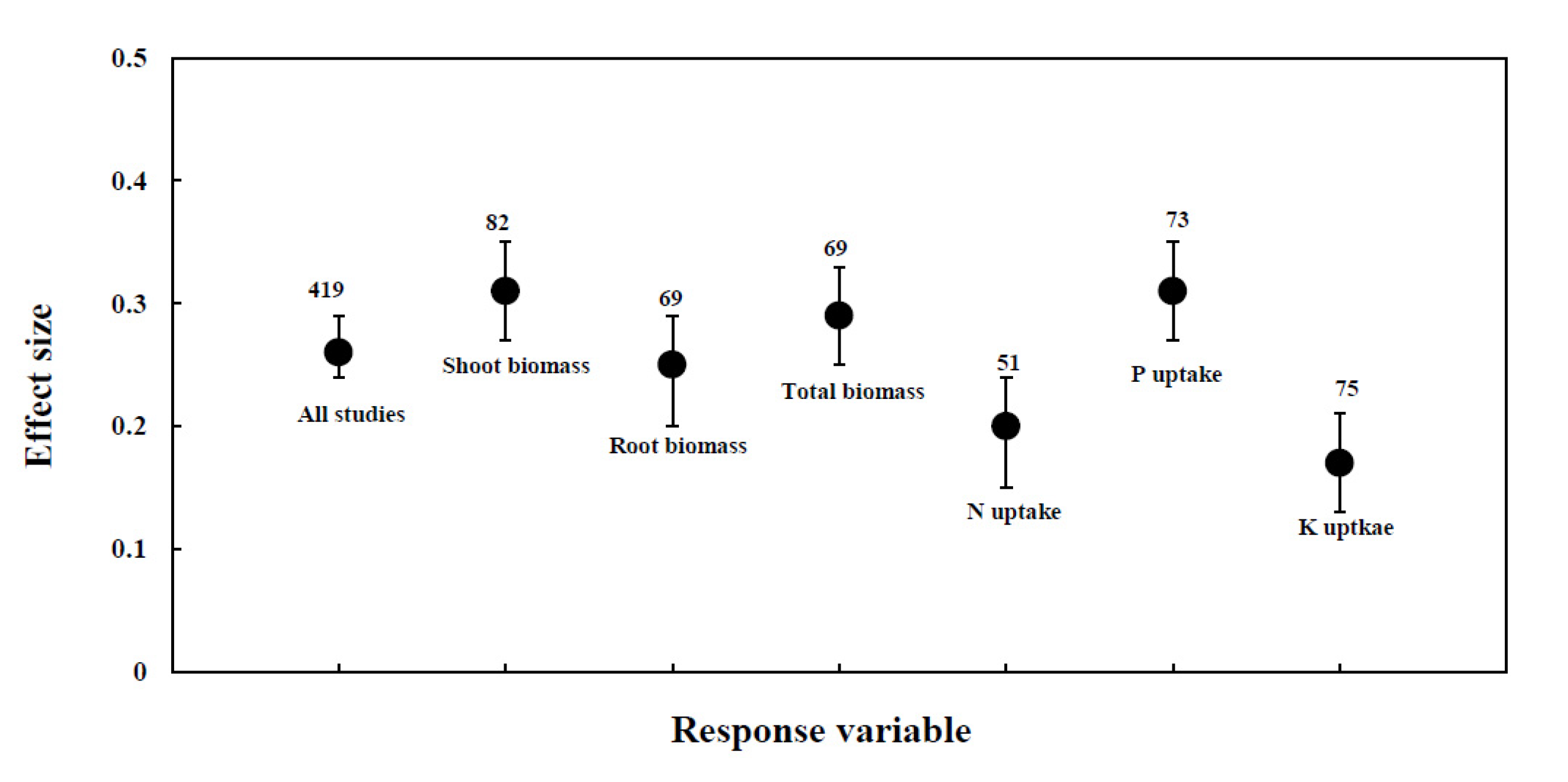

| Response Variable | Effect Size | Number of Trails | 95% BS CI | Q-Total | P (Chi-Square) |
|---|---|---|---|---|---|
| All studies | 0.26 | 419 | 0.22 to 0.29 | 1696.26 | 0.00000 |
| Shoot Biomass | 0.34 | 82 | 0.26 to 0.42 | 221.44 | 0.00000 |
| Root Biomass | 0.27 | 69 | 0.18 to 0.37 | 87.67 | 0.02166 |
| Total Biomass | 0.28 | 69 | 0.20 to 0.35 | 122.65 | 0.00002 |
| P uptake | 0.25 | 73 | 0.18 to 0.31 | 54.83 | 0.75865 |
| N uptake | 0.20 | 51 | 0.13 to 0.27 | 32.45 | 0.82749 |
| K uptake | 0.19 | 75 | 0.08 to 0.29 | 75.56 | 0.17405 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekaran, M. A Meta-Analytical Approach on Arbuscular Mycorrhizal Fungi Inoculation Efficiency on Plant Growth and Nutrient Uptake. Agriculture 2020, 10, 370. https://doi.org/10.3390/agriculture10090370
Chandrasekaran M. A Meta-Analytical Approach on Arbuscular Mycorrhizal Fungi Inoculation Efficiency on Plant Growth and Nutrient Uptake. Agriculture. 2020; 10(9):370. https://doi.org/10.3390/agriculture10090370
Chicago/Turabian StyleChandrasekaran, Murugesan. 2020. "A Meta-Analytical Approach on Arbuscular Mycorrhizal Fungi Inoculation Efficiency on Plant Growth and Nutrient Uptake" Agriculture 10, no. 9: 370. https://doi.org/10.3390/agriculture10090370
APA StyleChandrasekaran, M. (2020). A Meta-Analytical Approach on Arbuscular Mycorrhizal Fungi Inoculation Efficiency on Plant Growth and Nutrient Uptake. Agriculture, 10(9), 370. https://doi.org/10.3390/agriculture10090370





