Form of Silica Improves Yield, Fruit Quality and Antioxidant Defense System of Tomato Plants under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Crop Growth
2.2. Application of Treatments
2.3. Crop Growth Variables
2.4. Sampling
2.5. Fruit Quality
2.6. Biochemical Analysis
2.7. Statistical Analysis
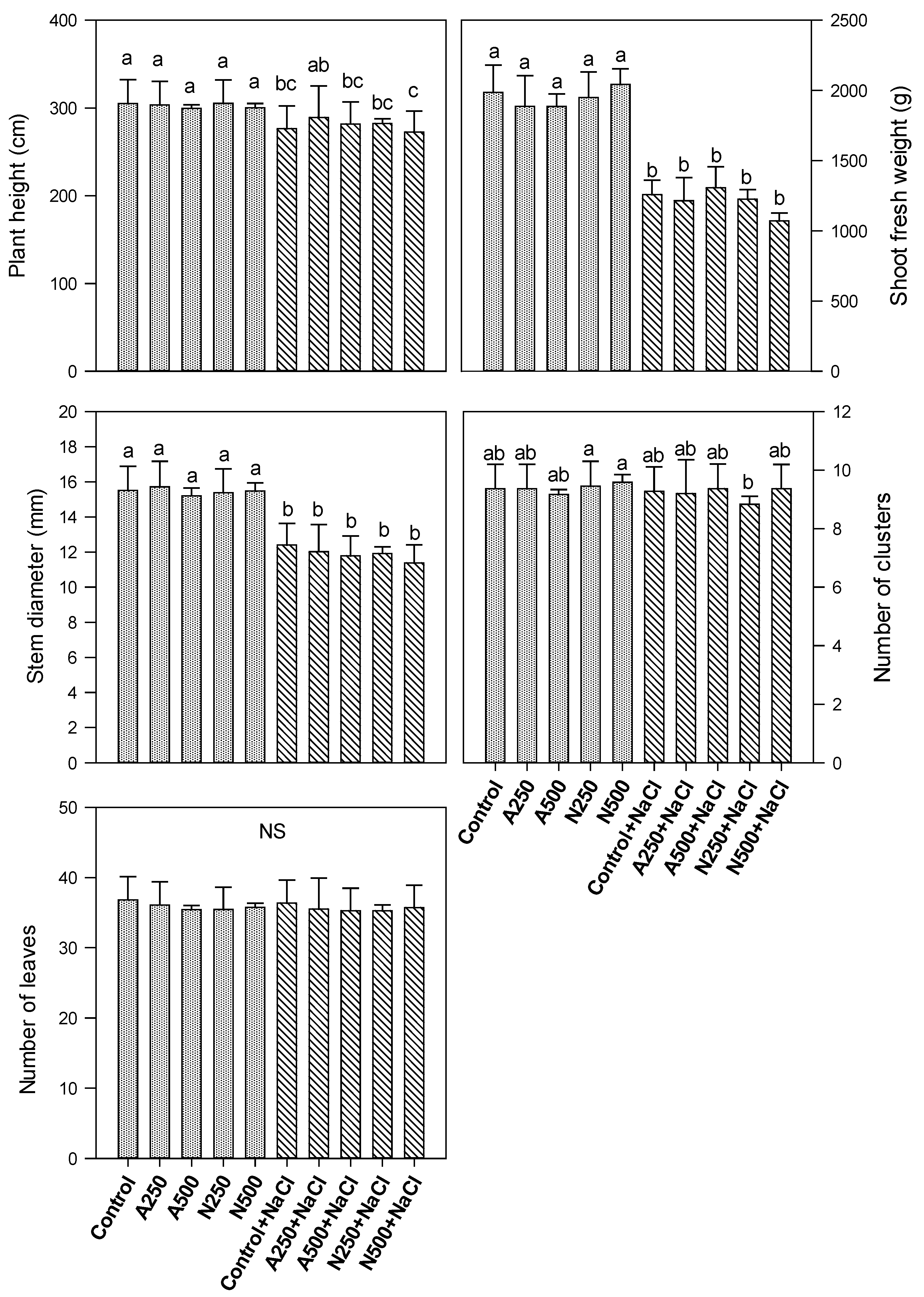
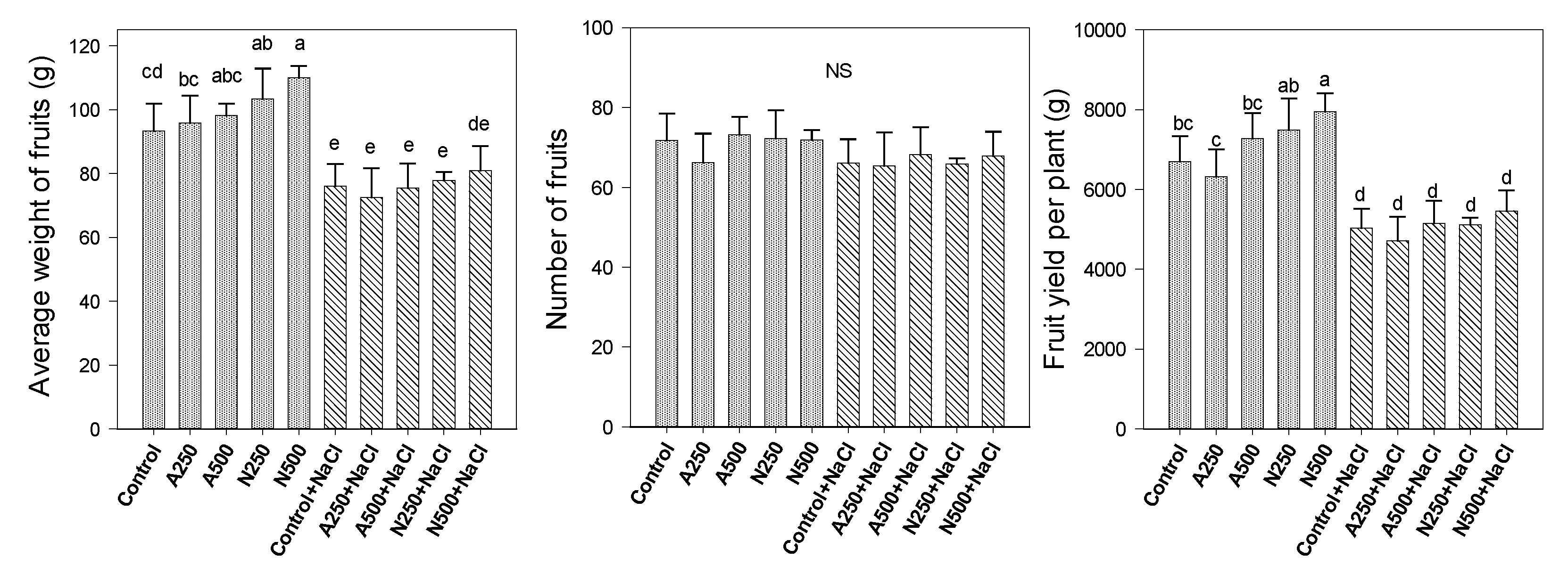
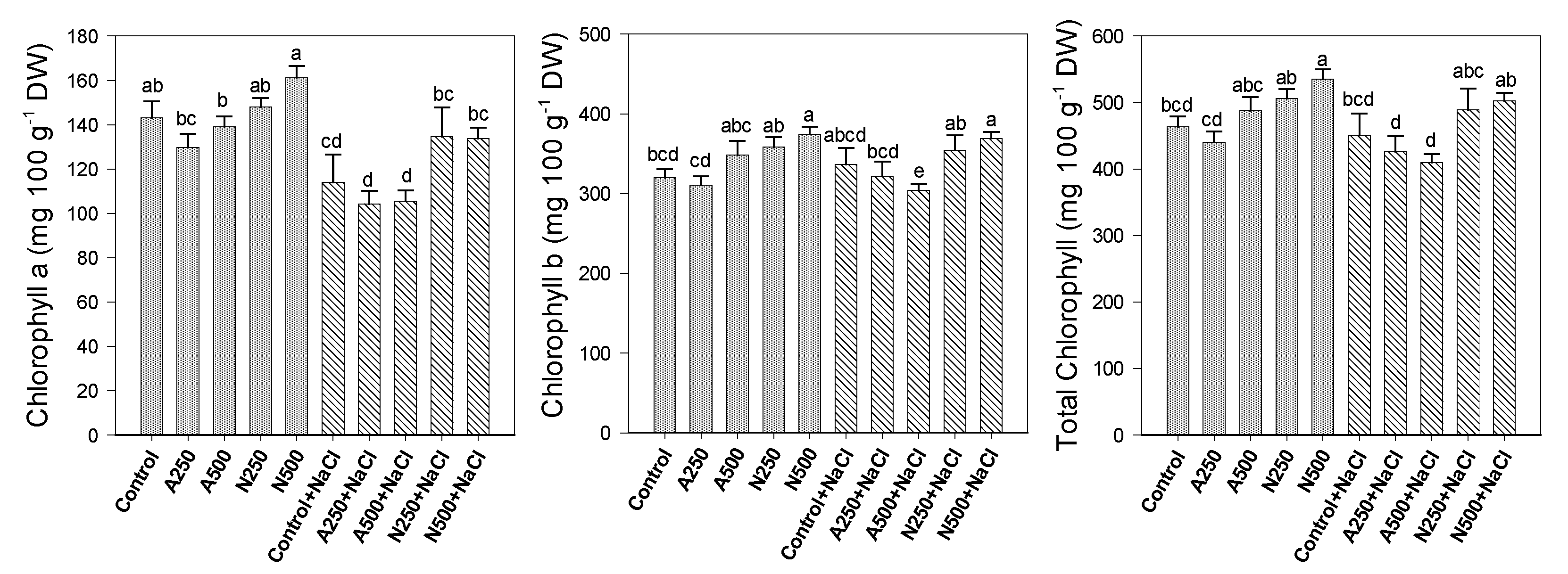
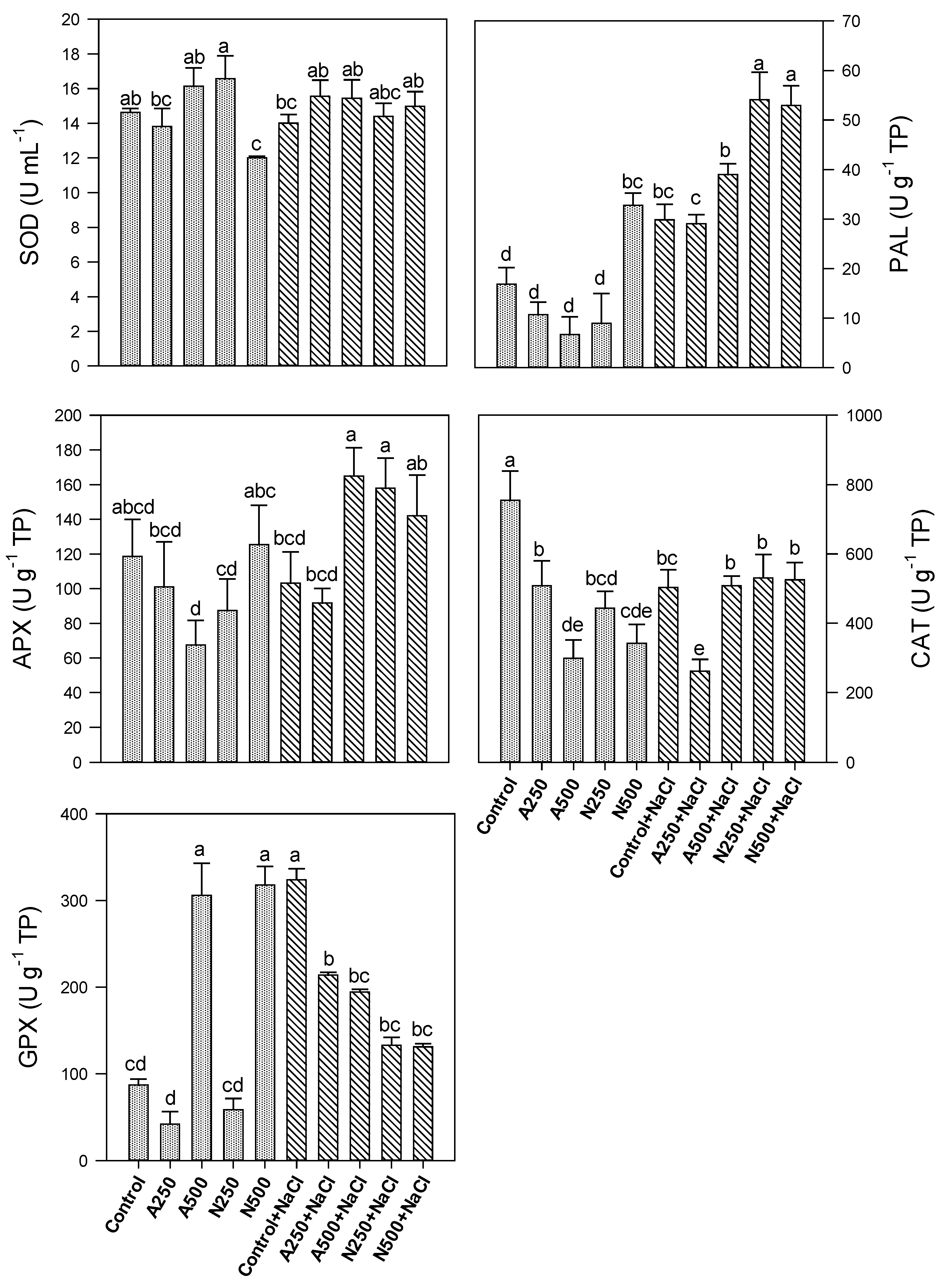
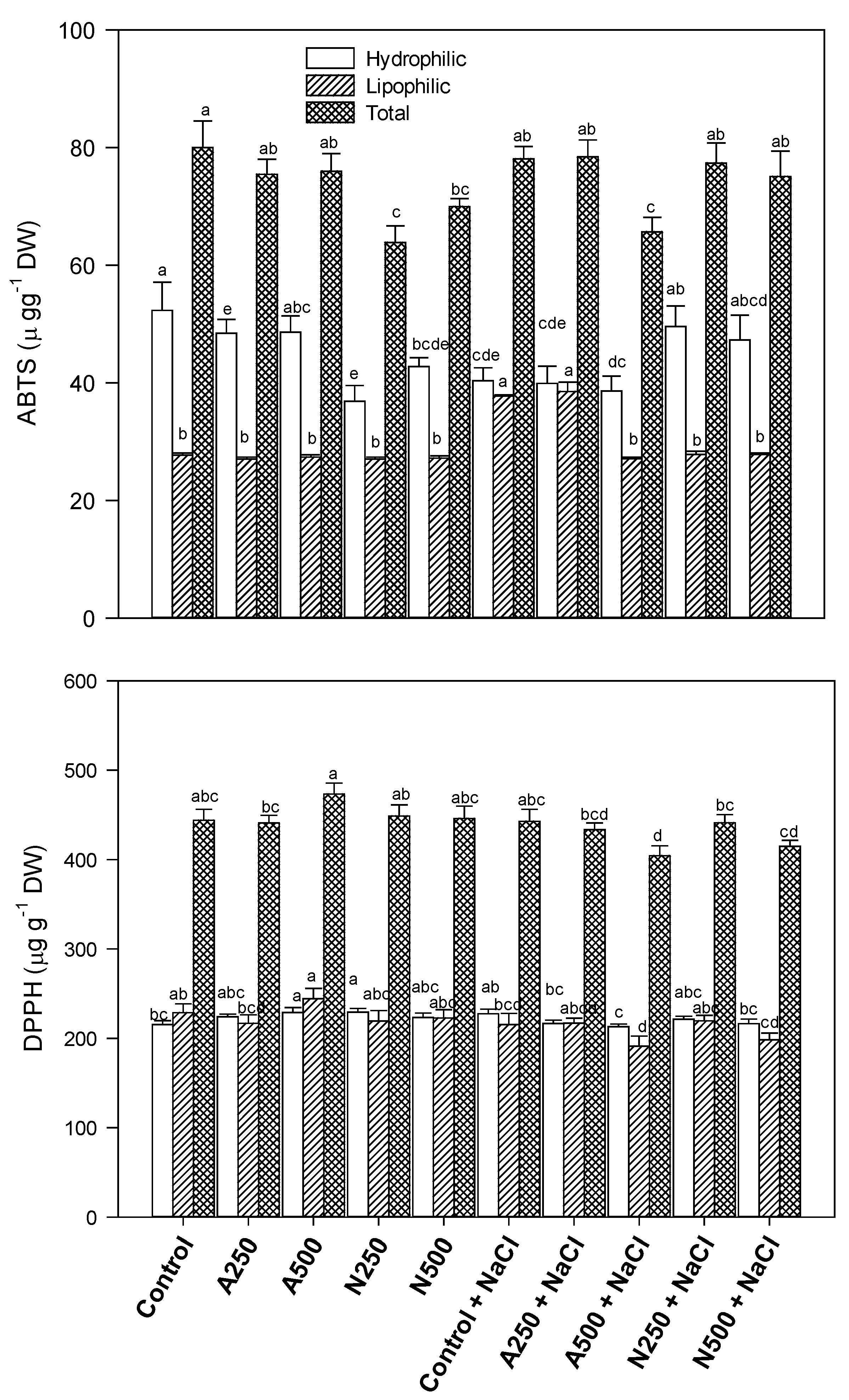
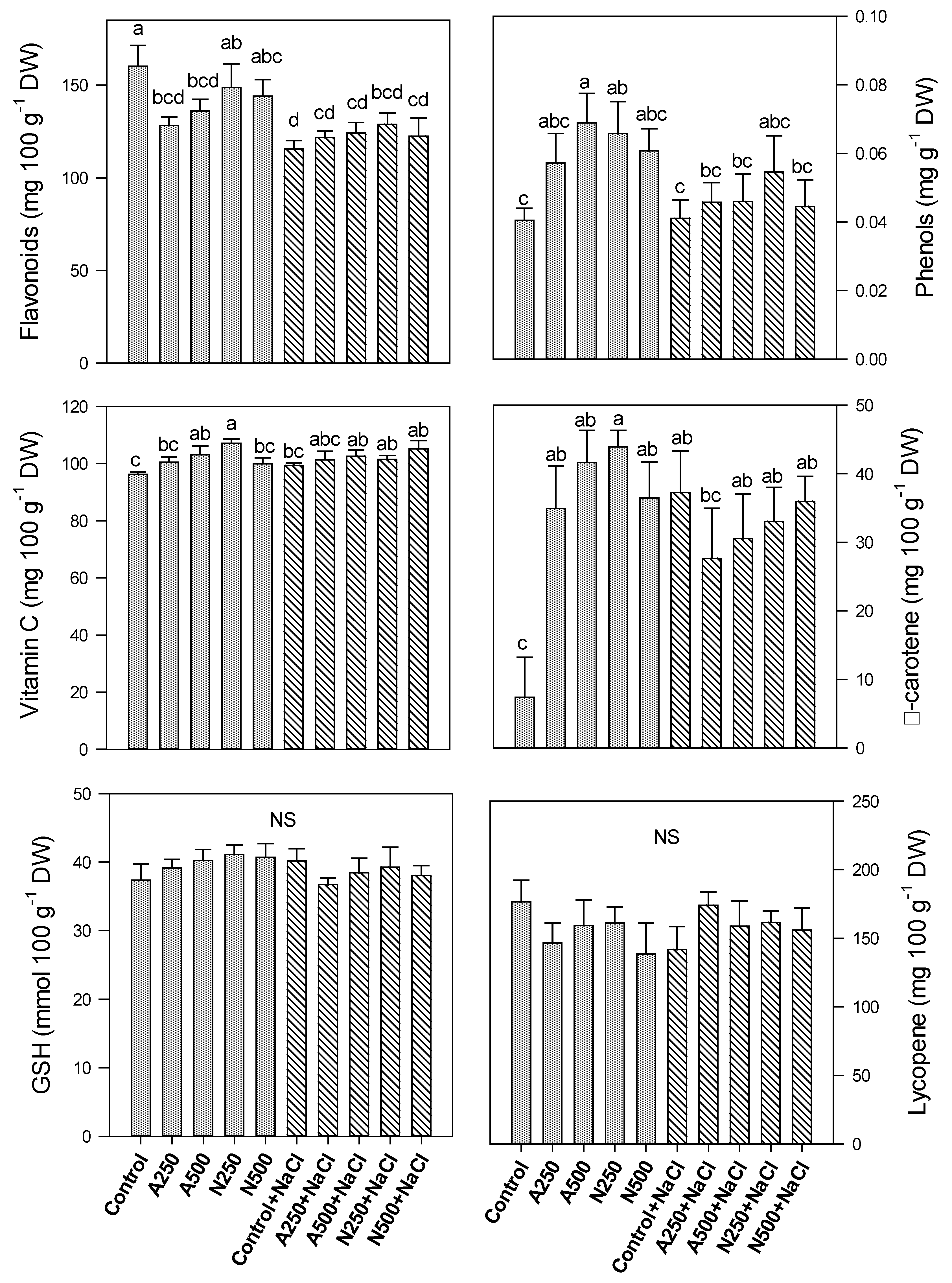
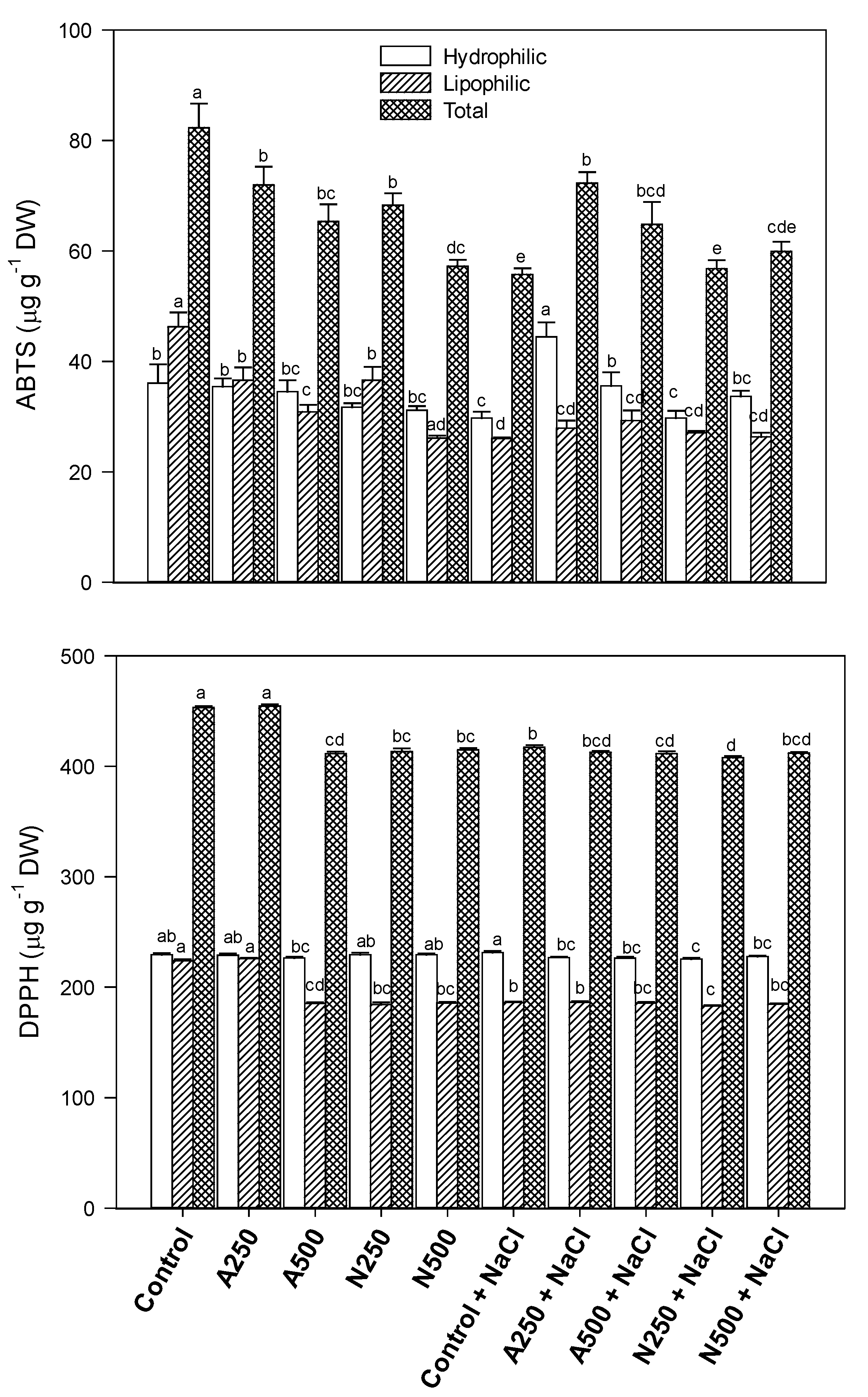
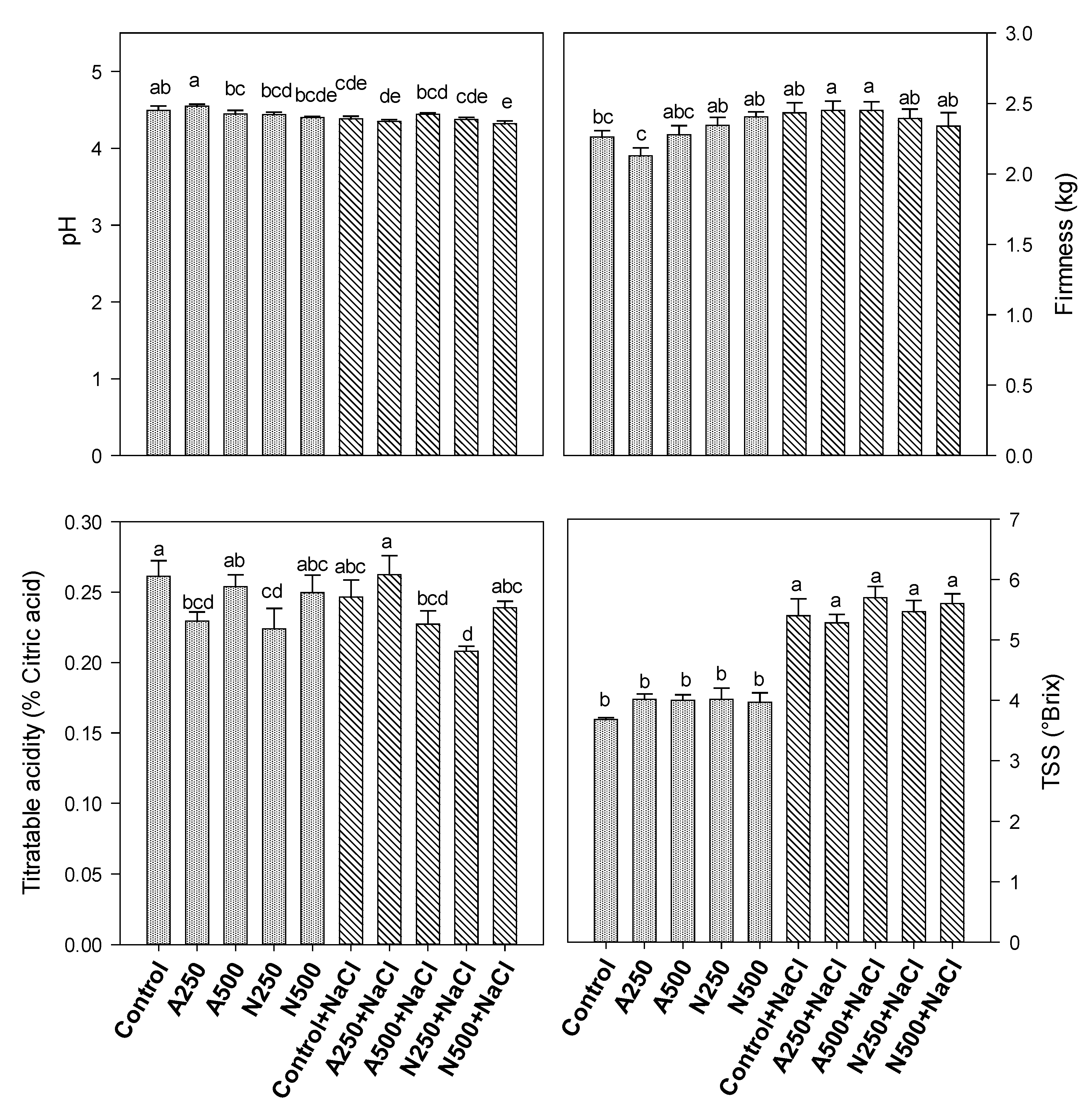
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W. The Tomato as a Functional Food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Amador, B.; Reyes-Pérez, J.J.; Hernández-Montiel, L.G.; Rueda-Puente, E.O.; De Lucia, B.; Beltrán-Morales, F.A.; Ruiz-Espinoza, F.H. Physiological responses to salinity in Solanum lycopersicum L. varieties. Pak. J. Bot. 2017, 49, 809–818. [Google Scholar]
- Hu, Y.; Schmidhalter, U. Limitation of Salt Stress to Plant Growth. In Plant Toxicology; Hock, B., Elstner, E.F., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 205–238. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Exley, C. Silicon in life: A bioinorganic solution to bioorganic essentiality. Inorg. Biochem. 1998, 69, 139–144. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Flowers, T.J.; Gong, H. Silicon decreases chloride transport in rice (Oryza sativa L.) in saline conditions. J. Plant Physiol. 2013, 170, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortiz, H.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-de la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N.S.; Prabu, P. Influence of nanosilica powder on the growth of maize crop (Zea Mays L.). Int. J. Green Nanotechnol. Biomed. 2011, 3, 180–190. [Google Scholar] [CrossRef]
- Sanglard, L.M.V.P.; Martins, S.C.V.; Detmann, K.C.; Silva, P.E.M.; Lavinsky, A.O.; Silva, M.M.; Detmann, E.; Araújo, W.L.; DaMatta, F.M. Silicon nutrition alleviates the negative impacts of arsenic on the photosynthetic apparatus of rice leaves: An analysis of the key limitations of photosynthesis. Physiol. Plant. 2014, 152, 355–366. [Google Scholar] [CrossRef]
- Gowayed, S.M.H.; Al-Zahrani, H.S.M.; Metwali, E.M.R. Improving the Salinity Tolerance in Potato (Solanum tuberosum) by Exogenous Application of Silicon Dioxide Nanoparticles. Int. J. Agric. Biol. 2017, 19, 183–192. [Google Scholar] [CrossRef]
- Almutairi, Z.M. Effect of nano-silicon application on the expression of salt tolerance genes in germinating tomato (Solanum lycopersicum L.) seedlings under salt stress. Plant Omics 2016, 9, 106–114. [Google Scholar]
- Farhangi-Abriz, S.; Torabian, S. Nano-silicon alters antioxidant activities of soybean seedlings under salt toxicity. Protoplasma 2018, 255, 953–962. [Google Scholar] [CrossRef]
- Xie, Y.; Li, B.; Tao, G.; Zhang, Q.; Zhang, C. Effects of nano-silicon dioxide on photosynthetic fluorescence characteristics of Indocalamus barbatus McClure. J. Nanjing For. Univ. Nat. Sci. Ed. 2012, 36, 59–63. [Google Scholar]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Prabu, P.; Rajendran, V.; Kannan, N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanoparticle Res. 2012, 14, 1–14. [Google Scholar] [CrossRef]
- Steiner, A.A. A universal method for preparing nutrient solutions of a certain desired composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- González-Moscoso, M.; Martínez-Villegas, N.V.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Del Carmen Rivera-Cruz, M.; González-Morales, S.; Juárez-Maldonado, A. Impact of silicon nanoparticles on the antioxidant compounds of tomato fruits stressed by arsenic. Foods 2019, 8, 612. [Google Scholar] [CrossRef]
- Lopez-Vargas, E.R.; Ortega-ortiz, H.; Cadenas-pliego, G.; De-Alba-Romenus, K.; Cabrera-De-La-Fuente, M.; Benavides-Mendoza, A.; Juarez-Maldonado, A. Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. J. Jpn. Soc. Food Sci. Technol. Shokuhin Kagaku Kogaku Kaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analisys of total phenols and other oxidation sobstrates and antioxidants by means of Folin Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of a propolis extract and identification of the main constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Patterson, B.D.; MacRae, E.A.; Ferguson, I.B. Estimation of hydrogen peroxide in plant extracts using titanium(IV). Anal. Biochem. 1984, 139, 487–492. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Purification of ascorbate peroxidase in spinach chloroplasts; its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987, 28, 131–140. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Sykłowska-Baranek, K.; Pietrosiuk, A.; Naliwajski, M.R.; Kawiak, A.; Jeziorek, M.; Wyderska, S.; Łojkowska, E.; Chinou, I. Effect of l-phenylalanine on PAL activity and production of naphthoquinone pigments in suspension cultures of Arnebia euchroma (Royle) Johnst. Vitr. Cell. Dev. Biol. Plant 2012, 48, 555–564. [Google Scholar] [CrossRef]
- Hossain, Z.; Mustafa, G.; Komatsu, S. Plant Responses to Nanoparticle Stress. Int. J. Mol. Sci. 2015, 16, 26644–26653. [Google Scholar] [CrossRef]
- Zribi, L.; Fatma, G.; Fatma, R.; Salwa, R.; Hassan, N.; Néjib, R.M. Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato “Solanum lycopersicum (variety Rio Grande)”. Sci. Hortic. 2009, 120, 367–372. [Google Scholar] [CrossRef]
- Giuffrida, F.; Scuderi, D.; Giurato, R.; Leonardi, C. Physiological response of broccoli and cauliflower as affected by NaCl salinity. Acta Hortic. 2013, 435–441. [Google Scholar] [CrossRef]
- Kalteh, M.; Alipour, Z.T.; Ashraf, S.; Aliabadi, M.M.; Nosratabadi, A.F. Effect of silica nanoparticles on basil (Ocimum basilicum) under salinity stress. J. Chem. Health Risks 2014, 4, 49–55. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Watanabe, S.; Nakashima, T.; Okano, K. Effects of salinity at two ripening stages on the fruit quality of single-truss tomato grown in hydroponics. J. Hortic. Sci. Biotechnol. 1999, 74, 690–693. [Google Scholar] [CrossRef]
- Rawson, H.; Long, M.; Munns, R. Growth and Development in NaCl-Treated Plants. I. Leaf Na+ and Cl- Concentrations Do Not Determine Gas Exchange of Leaf Blades in Barley. Funct. Plant Biol. 1988, 15, 519. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Aust. J. Plant Physiol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Cao, K.F. Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant. 2008, 30, 769–777. [Google Scholar] [CrossRef]
- Matichenkov, V.V.; Kosobrukhov, A.A. Si effect on the plant resistance to salt toxicity. Conserv. Soil Water Soc. Shar. Solut. 2004, 2, 1–4. [Google Scholar]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Soundararajan, P.; Manivannan, A.; Ko, C.H.; Jeong, B.R. Silicon Enhanced Redox Homeostasis and Protein Expression to Mitigate the Salinity Stress in Rosa hybrida ‘Rock Fire’. J. Plant Growth Regul. 2018, 37, 16–34. [Google Scholar] [CrossRef]
- Valentine, I.K.; Maria, V.K.; Bruno, B. Phenolic Cycle in Plants and Environment. In Mechanisms of Landscape Rehabilitation and Sustainability; Palavan-Unsal, N., Kefeli, V., Blum, W., Eds.; Bentham Science Publishers: Sharjah, UAE, 2012; Volume 13, pp. 75–78. [Google Scholar]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I.C.F.R. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.M.; Cao, F.; Han, Y.; Nadira, U.A.; Zhang, G.; Wu, F. Differential changes in grain ultrastructure, amylase, protein and amino acid profiles between Tibetan wild and cultivated barleys under drought and salinity alone and combined stress. Food Chem. 2013, 141, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Telesiński, A.; Nowak, J.; Smolik, B.; Dubowska, A.; Skrzypiec, N. Effect of soil salinity on activity of antioxidant enzymes and content of ascorbic acid and phenols in bean (Phaseolus vulgaris L.) plants. J. Elem. 2008, 13, 401–409. [Google Scholar]
- Smirnoff, N. Ascorbic acid: Metabolism and functions of a multi-facetted molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Yamamoto, A.; Bhuiyan, M.N.H.; Waditee, R.; Tanaka, Y.; Esaka, M.; Oba, K.; Jagendorf, A.T.; Takabe, T. Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. J. Exp. Bot. 2005, 56, 1785–1796. [Google Scholar] [CrossRef]
- Athar, H.R.; Khan, A.; Ashraf, M. Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ. Exp. Bot. 2008, 63, 224–231. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2019, 9, 1–21. [Google Scholar] [CrossRef]
- Leiva-Brondo, M.; Valcárcel, M.; Cortés-Olmos, C.; Roselló, S.; Cebolla-Cornejo, J.; Nuez, F. Exploring alternative germplasm for the development of stable high vitamin C content in tomato varieties. Sci. Hortic. 2012, 133, 84–88. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rohman, M.M.; Anee, T.I.; Huang, Y.; Fujita, M. Exogenous Silicon Protects Brassica napus Plants from Salinity-Induced Oxidative Stress Through the Modulation of AsA-GSH Pathway, Thiol-Dependent Antioxidant Enzymes and Glyoxalase Systems. Gesunde Pflanz. 2018, 70, 185–194. [Google Scholar] [CrossRef]
- Cai, J.; Wang, P.; Tian, S.; Qin, G. Quantitative proteomic analysis reveals the involvement of mitochondrial proteins in tomato fruit ripening. Postharvest Biol. Technol. 2018, 145, 213–221. [Google Scholar] [CrossRef]
- Qin, G.; Wang, Q.; Liu, J.; Li, B.; Tian, S. Proteomic analysis of changes in mitochondrial protein expression during fruit senescence. Proteomics 2009, 9, 4241–4253. [Google Scholar] [CrossRef] [PubMed]
- Van Bockhaven, J.; De Vleesschauwer, D.; Höfte, M. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Nwugo, C.C.; Huerta, A.J. The effect of silicon on the leaf proteome of rice (Oryza sativa L.) plants under cadmium-stress. J. Proteome Res. 2011, 10, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Ibrahim, E.; Hani, H. Copper Nanoparticles Induced Genotoxicty, Oxidative Stress, and Changes in Superoxide Dismutase (SOD) Gene Expression in Cucumber (Cucumis sativus) Plants. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nazaralian, S.; Majd, A.; Irian, S.; Najafi, F.; Ghahremaninejad, F.; Landberg, T.; Greger, M. Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiol. Biochem. 2017, 115, 25–33. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Horemans, N.; Foyer, C.H.; Asard, H. Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci. 2000, 5, 263–267. [Google Scholar] [CrossRef]
- May, M.J.; Vernoux, T.; Leaver, C.; Montagu, M.V.; Inze, D. Glutathione homeostasis in plants: Implications for environmental sensing and plant development. J. Exp. Bot. 1998, 49, 649–667. [Google Scholar] [CrossRef]
- Tausz, M. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of Tomato Plants under Saline Stress to Foliar Application of Copper Nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haliem, M.E.F.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- Rao, A.; Rao, L. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B. Antioxidants in Photosynthesis and Human Nutrition. Science 2002, 298, 2149–2153. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Ascorbate and glutathione: The heart of the redox hub. Plant Physiol. 2011, 155, 2–18. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Sharma, S. Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russ. J. Plant Physiol. 2010, 57, 509–517. [Google Scholar] [CrossRef]
- Torabian, S.; Farhangi-Abriz, S.; Zahedi, M. Efficacy of FeSO4 nano formulations on osmolytes and antioxidative enzymes of sunflower under salt stress. Ind. J. Plant Physiol. 2018, 23, 305–315. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Che, Y.; Zhang, N.; Zhu, X.; Li, S.; Wang, S.; Si, H. Enhanced tolerance of the transgenic potato plants overexpressing Cu/Zn superoxide dismutase to low temperature. Sci. Hortic. 2020, 261, 108949. [Google Scholar] [CrossRef]
- Asada, K. The water–water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2000, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Dietz, K.-J. Tuning of Redox Regulatory Mechanisms, Reactive Oxygen Species and Redox Homeostasis under Salinity Stress. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Shekari, F.; Abbasi, A.; Mustafavi, S.H. Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J. Saudi Soc. Agric. Sci. 2017, 16, 367–374. [Google Scholar] [CrossRef]
- Al-aghabary, K.; Zhu, Z.; Shi, Q. Influence of silicon supply on chlorophyll content, chlorophyll fluorescence, and antioxidative enzyme activities in tomato plants under salt stress. J. Plant Nutr. 2004, 27, 2101–2115. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and Plant Phenylalanine Ammonia-lyase. Mycobiology 2011, 39, 257–265. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Wanner, L.A.; Li, G.; Ware, D.; Somssich, I.E.; Davis, K.R. The phenylalanine ammonia-lyase gene family in Arabidopsis thaliana. Plant Mol. Biol. 1995, 27, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.-K.; Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Bilal, S.; Khan, A.L.; Imran, M.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.J. Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 2020, 188, 109885. [Google Scholar] [CrossRef]
- Vinha, A.F.; Alves, R.C.; Barreira, S.V.P.; Castro, A.; Costa, A.S.G.; Oliveira, M.B.P.P. Effect of peel and seed removal on the nutritional value and antioxidant activity of tomato (Lycopersicon esculentum L.) fruits. LWT Food Sci. Technol. 2014, 55, 197–202. [Google Scholar] [CrossRef]
- Kotíková, Z.; Lachman, J.; Hejtmánková, A.; Hejtmánková, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Costan, A.; Stamatakis, A.; Chrysargyris, A.; Petropoulos, S.A.; Tzortzakis, N. Interactive effects of salinity and silicon application on Solanum lycopersicum growth, physiology and shelf-life of fruit produced hydroponically. J. Sci. Food Agric. 2020, 100, 732–743. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinedo-Guerrero, Z.H.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; González-Morales, S.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Form of Silica Improves Yield, Fruit Quality and Antioxidant Defense System of Tomato Plants under Salt Stress. Agriculture 2020, 10, 367. https://doi.org/10.3390/agriculture10090367
Pinedo-Guerrero ZH, Cadenas-Pliego G, Ortega-Ortiz H, González-Morales S, Benavides-Mendoza A, Valdés-Reyna J, Juárez-Maldonado A. Form of Silica Improves Yield, Fruit Quality and Antioxidant Defense System of Tomato Plants under Salt Stress. Agriculture. 2020; 10(9):367. https://doi.org/10.3390/agriculture10090367
Chicago/Turabian StylePinedo-Guerrero, Zeus H., Gregorio Cadenas-Pliego, Hortensia Ortega-Ortiz, Susana González-Morales, Adalberto Benavides-Mendoza, Jesús Valdés-Reyna, and Antonio Juárez-Maldonado. 2020. "Form of Silica Improves Yield, Fruit Quality and Antioxidant Defense System of Tomato Plants under Salt Stress" Agriculture 10, no. 9: 367. https://doi.org/10.3390/agriculture10090367
APA StylePinedo-Guerrero, Z. H., Cadenas-Pliego, G., Ortega-Ortiz, H., González-Morales, S., Benavides-Mendoza, A., Valdés-Reyna, J., & Juárez-Maldonado, A. (2020). Form of Silica Improves Yield, Fruit Quality and Antioxidant Defense System of Tomato Plants under Salt Stress. Agriculture, 10(9), 367. https://doi.org/10.3390/agriculture10090367








