Spouted Bed Dried Rosmarinus officinalis Extract: A Novel Approach for Physicochemical Properties and Antioxidant Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Herbal Material and Reagents
2.2. Experimental Procedure
2.2.1. Extraction of the Bioactive Compounds from Rosemary Leaves
2.2.2. Physicochemical Characterization of the Powdered Raw Herbal Material and Extractive Solution
2.2.3. Spouted Bed Drying
Experimental Apparatus
Drying Procedure
2.2.4. Physicochemical Characterization of Dried Rosemary Extract
2.2.5. Antioxidant Activity of the Rosemary Extracts
DPPH• Radical Scavenging Assay
Lipid Peroxidation Method
2.3. Experimental Design
3. Results and Discussion
3.1. Physicochemical Characterization of the Powdered Herbal Material and of Extractive Solution
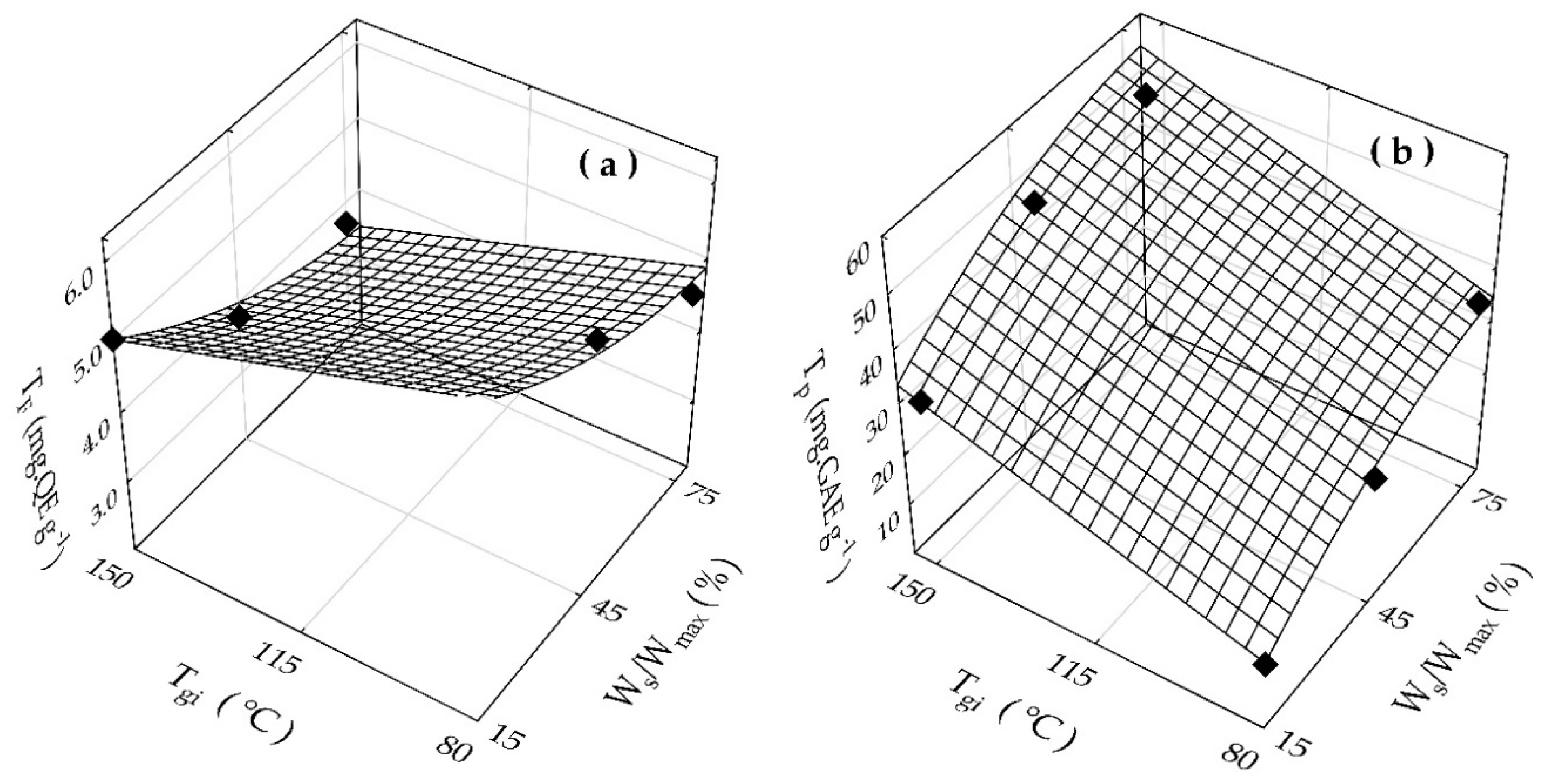
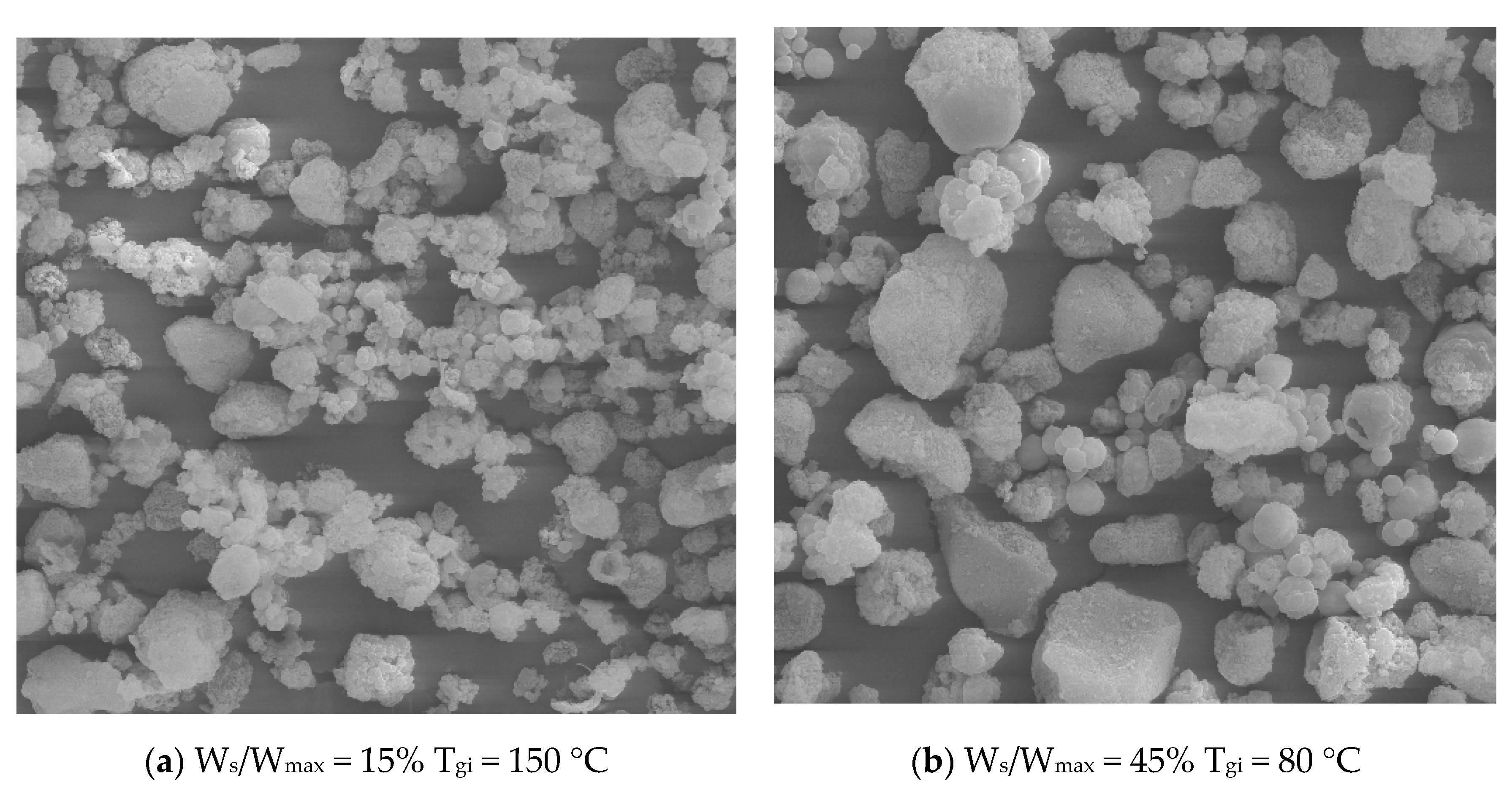
3.2. Spouted Bed Drying and Physicochemical Characterization of the Dried Rosemary Extracts
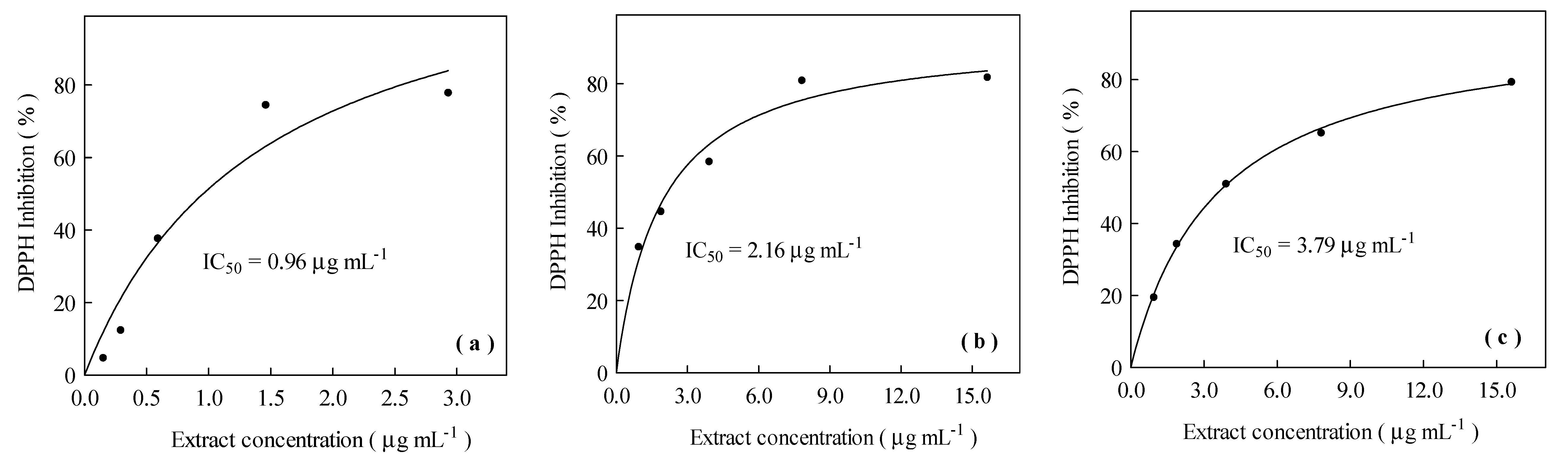
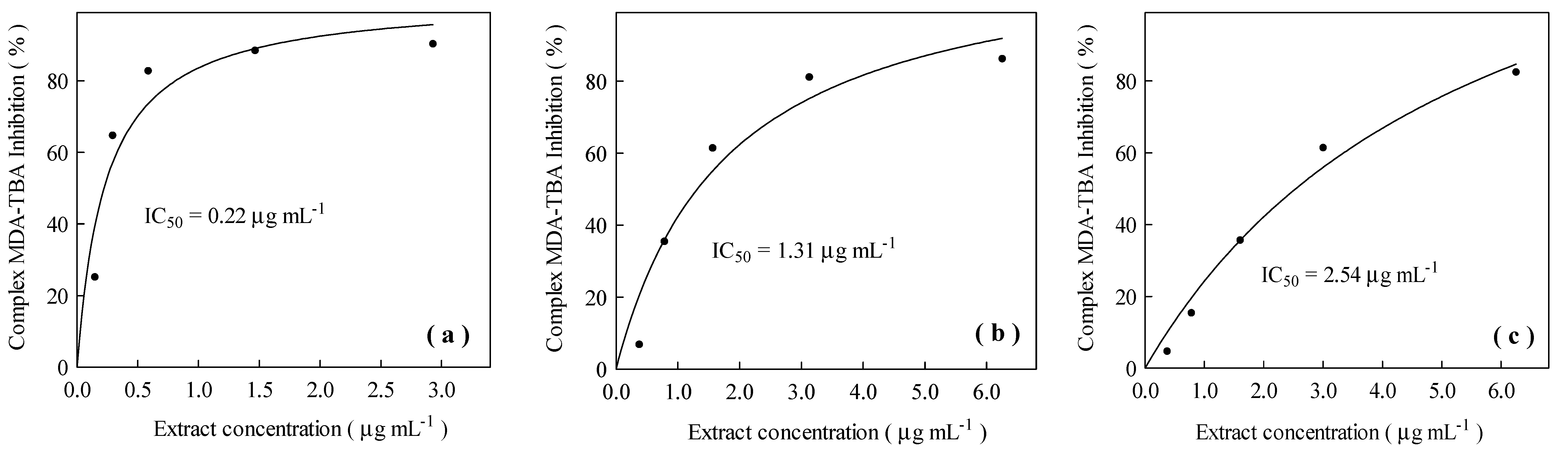
3.3. Antioxidant Activity of the Concentrated and Dried Rosemary Extracts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zielińska, A.; Ferreira, N.R.; Feliczak-Guzik, A.; Nowak, I.; Souto, E.B. Loading, release profile and accelerated stability assessment of monoterpenes-loaded Solid Lipid Nanoparticles (SLN). Pharm. Dev. Technol. 2020, 1–13. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals-shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharm. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Santini, A. Nutraceuticals in Human Health. Foods 2020, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharm. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Campos, J.R.; Severino, P.; Ferreira, C.S.; Zielinska, A.; Santini, A.; Souto, S.B.; Souto, E.B. Linseed Essential Oil—Source of Lipids as Active Ingredients for Pharmaceuticals and Nutraceuticals. Curr. Med. Chem. 2019, 26, 4537–4558. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Novellino, E.; Souto, E.B.; Daliu, P.; Santini, A. Abelmoschus esculentus (L.): Bioactive Components’ Beneficial Properties-Focused on Antidiabetic Role-For Sustainable Health Applications. Molecules 2018, 24, 38. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kregiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E.; Armini, V.; Ritieni, A. State of the art of Ready-to-Use Therapeutic Food: A tool for nutraceuticals addition to foodstuff. Food Chem. 2013, 140, 843–849. [Google Scholar] [CrossRef]
- Amarowicz, R.; Carle, R.; Dongowski, G.; Durazzo, A.; Galensa, R.; Kammerer, D.; Maiani, G.; Piskula, M.K. Influence of postharvest processing and storage on the content of phenolic acids and flavonoids in foods. Mol. Nutr. Food Res. 2009, 53, S151–S183. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arraez-Roman, D.; Martinez-Ferez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef]
- Santos, I.S.; Ponte, B.M.; Boonme, P.; Silva, A.M.; Souto, E.B. Nanoencapsulation of polyphenols for protective effect against colon-rectal cancer. Biotechno. Adv. 2013, 31, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Severino, P.; Basso, R.; Santana, M.H. Encapsulation of antioxidants in gastrointestinal-resistant nanoparticulate carriers. Methods Mol. Bio. 2013, 1028, 37–46. [Google Scholar] [CrossRef]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P.; et al. From Plant Compounds to Botanicals and Back: A Current Snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Camilli, E.; D’Addezio, L.; Piccinelli, R.; Mantur-Vierendeel, A.; Marletta, L.; Finglas, P.; Turrini, A.; Sette, S. Development of Dietary Supplement Label Database in Italy: Focus of FoodEx2 Coding. Nutrients 2019, 12, 89. [Google Scholar] [CrossRef]
- Souza, C.R.F.; Oliveira, W.P. Spouted bed drying of Bauhinia forficata link extract: The effects of feed atomizer position and operating conditions on equipment performance and product properties. Braz. J. Chem. Eng. 2005, 22, 239–247. [Google Scholar] [CrossRef]
- Runha, F.P.; Cordeiro, D.S.; Pereira, C.A.M.; Vilegas, J.; Oliveira, W.P. Production of Dry Extracts of Medicinal Brazilian Plants by Spouted Bed Process: Development of the Process and Evaluation of Thermal Degradation During the Drying Operation. Food Bioprod. Process. 2001, 79, 160–168. [Google Scholar] [CrossRef]
- Lemos Senna, E.; Petrovick, P.R.; Gonzalez Ortega, G.; Bassani, V.L. Preparation and Characterization of Spray-dried Powders from Achyrocline satureioides (Lam.) DC Extracts. Phytother. Res. 1997, 11, 123–127. [Google Scholar] [CrossRef]
- Tran, T.T.A.; Nguyen, H.V.H. Effects of Spray-Drying Temperatures and Carriers on Physical and Antioxidant Properties of Lemongrass Leaf Extract Powder. Beverages 2018, 4, 84. [Google Scholar] [CrossRef]
- Oliveira, W.P.; Bott, R.F.; Souza, C.R.F. Manufacture of Standardized Dried Extracts from Medicinal Brazilian Plants. Dry. Technol. 2006, 24, 523–533. [Google Scholar] [CrossRef]
- Cordeiro, D.S.; Oliveira, W.P. Technical aspects of the production of dried extract of Maytenus ilicifolia leaves by jet spouted bed drying. Int. J. Pharm. 2005, 299, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Passos, M.L.; Massarani, G.; Freire, J.T.; Mujumdar, A.S. Drying Of Pastes In Spouted Beds Of Inert Particles: Design Criteria And Modeling. Dry. Technol. 1997, 15, 605–624. [Google Scholar] [CrossRef]
- Mathur, K.B.; Epstein, N. Dynamics of Spouted Beds. In Advances in Chemical Engineering; Drew, T.B., Cokelet, G.R., Hoopes, J.W., Vermeulen, T., Eds.; Academic Press: Cambridge, MA, USA, 1974; Volume 9, pp. 111–191. [Google Scholar]
- Durazzo, A.; Lucarini, M. Extractable and Non-Extractable Antioxidants. Molecules 2019, 24, 1933. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M. A Current shot and re-thinking of antioxidant research strategy. Braz. J. Anal. Chem. 2018, 5, 9–11. [Google Scholar] [CrossRef]
- Durazzo, A. Extractable and Non-extractable polyphenols: An overview. In Non-Extractable Polyphenols and Carotenoids: Importance in Human Nutrition and Health; Saura-Calixto, F., Pérez-Jiménez, J., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 1–37. [Google Scholar]
- Cermak, R.; Durazzo, A.; Maiani, G.; Böhm, V.; Kammerer, D.R.; Carle, R.; Wiczkowski, W.; Piskula, M.K.; Galensa, R. The influence of postharvest processing and storage of foodstuffs on the bioavailability of flavonoids and phenolic acids. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S184–S193. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Souza, C.R.F.; Schiavetto, I.A.; Thomazini, F.C.F.; Oliveira, W.P. Processing of Rosmarinus officinalis linne extract on spray and spouted bed dryers. Braz. J. Chem. Eng. 2008, 25, 59–69. [Google Scholar] [CrossRef][Green Version]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Souza, C.R.F.; Bott, R.F.; Oliveira, W.P. Optimization of the extraction of flavonoids compounds from herbal material using Experimental Design and Multi-response Analysis. Lat. Am. J. Pharm. 2007, 65, 682–690. [Google Scholar]
- Bajaj, K.L.; Devsharma, A.K. A colorimetric method for the determination of tannins in tea. Microchim. Acta 1977, 68, 249–253. [Google Scholar] [CrossRef]
- Tzeng, G.S.; Chen, H.J.; Wang, Y.Y.; Wan, C.C. The effects of roughening on teflon surfaces. Surf. Coat. Technol. 1997, 89, 108–113. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Oliveira, W.P. Physicochemical Properties of Phytopharmaceutical Preparations as Affected by Drying Methods and Carriers. Dry. Technol. 2012, 30, 921–934. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-epoxide-loaded SLN: Evaluation of drug release, antioxidant activity and cytotoxicity in HaCaT cell line. Int. J. Mol. Sci. 2020, 21, 1449. [Google Scholar] [CrossRef]
- Souto, E.B.; Souto, S.B.; Zielinska, A.; Durazzo, A.; Lucarini, M.; Santini, A.; Horbańczuk, O.K.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. Perillaldehyde 1,2-epoxide loaded SLN-tailored mAb: Production, physicochemical characterization and in vitro cytotoxicity profile in MCF-7 cell lines. Pharmaceutics 2020, 12, 161. [Google Scholar] [CrossRef]
- Fernandes, M.R.; Azzolini, A.E.; Martinez, M.L.; Souza, C.R.; Lucisano-Valim, Y.M.; Oliveira, W.P. Assessment of antioxidant activity of spray dried extracts of Psidium guajava leaves by DPPH and chemiluminescence inhibition in human neutrophils. BioMed Res. Int. 2014, 2014, 382891. [Google Scholar] [CrossRef]
- Rodrigues, T.; Santos, A.C.; Pigoso, A.A.; Mingatto, F.E.; Uyemura, S.A.; Curti, C. Thioridazine interacts with the membrane of mitochondria acquiring antioxidant activity toward apoptosis-potentially implicated mechanisms. Br. J. Pharm. 2002, 136, 136–142. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar]
- Zainol, M.K.M.; Hamid, A.A.; Bakar, F.A.; Dek, M.S.P. Effect of different drying methods on the degradation of selected flavonoids in Centella asiatica. Int. Food Res. J. 2009, 16, 531–537. [Google Scholar]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic Acid and its pharmacological potential. Isrn Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.B.E.; Van Den Berg, D.-J.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Sandulachi, E. Water activity concept and its role in food preservation. Meridian Ing. 2012, 4, 40–48. [Google Scholar]
- Labuza, T.P.; Altunakar, L. Water Activity Prediction and Moisture Sorption Isotherms. In Water Activity in Foods; Barbosa-Cánovas, G.V., Fontana, A.J., Schmidt, S.J., Labuza, T.P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; Volume 5, pp. 109–154. [Google Scholar] [CrossRef]
- Anandharamakrishnan, C.; Padma Ishwarya, S. Spray Drying Techniques for Food Ingredient Encapsulation; Wiley-Blackwell, John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 22–23. [Google Scholar]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kuhne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Lopez-Machado, A.L.; Bonilla, V.L.; Pizarro, P.G.; Silva, A.M.; Souto, E.B. Lipid nanoparticles as carriers for the treatment of neurodegeneration associated with Alzheimer’s disease and glaucoma: Present and future challenges. Curr. Pharm. Des. 2020, 26, 1235–1250. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Egea, M.A.; Davis, B.M.; Guo, L.; Espina, M.; Silva, A.M.; Calpena, A.C.; Souto, E.M.B.; Ravindran, N.; Ettcheto, M.; et al. Memantine-Loaded PEGylated Biodegradable Nanoparticles for the Treatment of Glaucoma. Small 2018, 14. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Cano, A.; Calpena, A.C.; Camins, A.; Carmona, N.; Silva, A.M.; Souto, E.B.; et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: In vitro and in vivo characterization. J. Nanobiotechnol. 2018, 16, 32. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Fangueiro, J.F.; Andreani, T.; Souto, E.B. Comparison of antiproliferative effect of epigallocatechin gallate when loaded into cationic solid lipid nanoparticles against different cell lines. Pharm. Dev. Technol. 2019, 24, 1243–1249. [Google Scholar] [CrossRef]
- Doktorovova, S.; Santos, D.L.; Costa, I.; Andreani, T.; Souto, E.B.; Silva, A.M. Cationic solid lipid nanoparticles interfere with the activity of antioxidant enzymes in hepatocellular carcinoma cells. Int. J. Pharm. 2014, 471, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A., Jr.; Borin, M.F.; Lopez, R.F.; Fonseca, M.J. In vitro evaluation of quercetin cutaneous absorption from topical formulations and its functional stability by antioxidant activity. Int. J. Pharm. 2007, 328, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.T.M.C.; Casagrande, R.; Georgetti, S.R.; Bentley, M.V.L.B.; Fonseca, M.J.V. Influence of Vehicle on Antioxidant Activity of Quercetin: A Liquid Crystalline Formulation. Lat. Am. J. Pharm. 2007, 26, 805. [Google Scholar]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Souza, C.R.F.; Georgetti, S.R.; Salvador, M.J.; Fonseca, M.J.V.; Oliveira, W.P. Antioxidant activity and physical-chemical properties of spray and spouted bed dried extracts of Bauhinia forficata. Braz. J. Pharm. Sci. 2009, 45, 209–218. [Google Scholar] [CrossRef]
- Bankole, V.O.; Osungunna, M.O.; Souza, C.R.F.; Salvador, S.L.; Oliveira, W.P. Spray-Dried Proliposomes: An Innovative Method for Encapsulation of Rosmarinus officinalis L. Polyphenols. AAPS PharmSciTech 2020, 21, 143. [Google Scholar] [CrossRef]

| Tgi (°C) | Ws/Wmax (%) | Ws (g min−1) |
|---|---|---|
| 80 | 15 | 6.0 |
| 80 | 45 | 18.0 |
| 80 | 75 | 30.0 |
| 150 | 15 | 10.0 |
| 150 | 45 | 33.0 |
| 150 | 75 | 49.0 |
| Tgi (°C) | Ws/Wmax (%) | TF (mg.QE g−1) * | Tp (mg.GAE g−1) * | Xp (% w/w) |
|---|---|---|---|---|
| 80 | 15 | 6.4 ± 0.05 | 13.1 ± 0.98 | 13.7 ± 1.12 |
| 80 | 45 | 5.6 ± 0.03 | 25.0 ± 1.87 | 8.7 ± 0.87 |
| 80 | 75 | 4.7 ± 0.04 | 36.6 ± 2.11 | 13.9 ± 0.95 |
| 150 | 15 | 5.0 ± 0.04 | 32.4 ± 1.13 | 2.0 ± 0.05 |
| 150 | 45 | 3.8 ± 0.05 | 48.6 ± 2.05 | 7.9 ± 0.55 |
| 150 | 75 | 3.7 ± 0.01 | 49.9 ± 1.29 | 11.1 ± 1.03 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, C.R.F.; Baldim, I.; Bankole, V.O.; da Ana, R.; Durazzo, A.; Lucarini, M.; Cicero, N.; Santini, A.; Souto, E.B.; Oliveira, W.P. Spouted Bed Dried Rosmarinus officinalis Extract: A Novel Approach for Physicochemical Properties and Antioxidant Activity. Agriculture 2020, 10, 349. https://doi.org/10.3390/agriculture10080349
Souza CRF, Baldim I, Bankole VO, da Ana R, Durazzo A, Lucarini M, Cicero N, Santini A, Souto EB, Oliveira WP. Spouted Bed Dried Rosmarinus officinalis Extract: A Novel Approach for Physicochemical Properties and Antioxidant Activity. Agriculture. 2020; 10(8):349. https://doi.org/10.3390/agriculture10080349
Chicago/Turabian StyleSouza, Claudia R. F., Iara Baldim, Victor O. Bankole, Raquel da Ana, Alessandra Durazzo, Massimo Lucarini, Nicola Cicero, Antonello Santini, Eliana B. Souto, and Wanderley P. Oliveira. 2020. "Spouted Bed Dried Rosmarinus officinalis Extract: A Novel Approach for Physicochemical Properties and Antioxidant Activity" Agriculture 10, no. 8: 349. https://doi.org/10.3390/agriculture10080349
APA StyleSouza, C. R. F., Baldim, I., Bankole, V. O., da Ana, R., Durazzo, A., Lucarini, M., Cicero, N., Santini, A., Souto, E. B., & Oliveira, W. P. (2020). Spouted Bed Dried Rosmarinus officinalis Extract: A Novel Approach for Physicochemical Properties and Antioxidant Activity. Agriculture, 10(8), 349. https://doi.org/10.3390/agriculture10080349










