Microflora in the Reproductive Tract of Cattle: A Review
Abstract
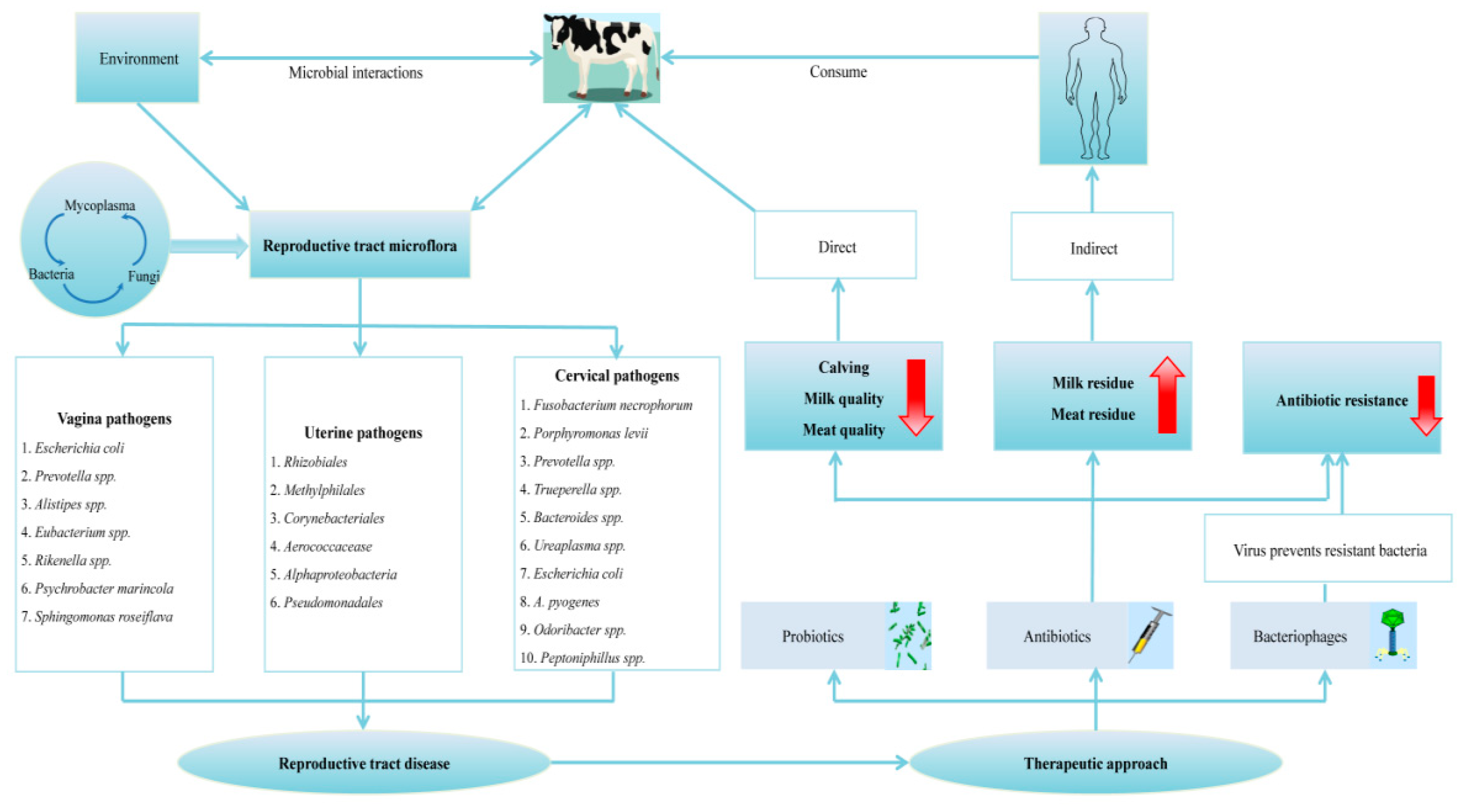
:1. Introduction
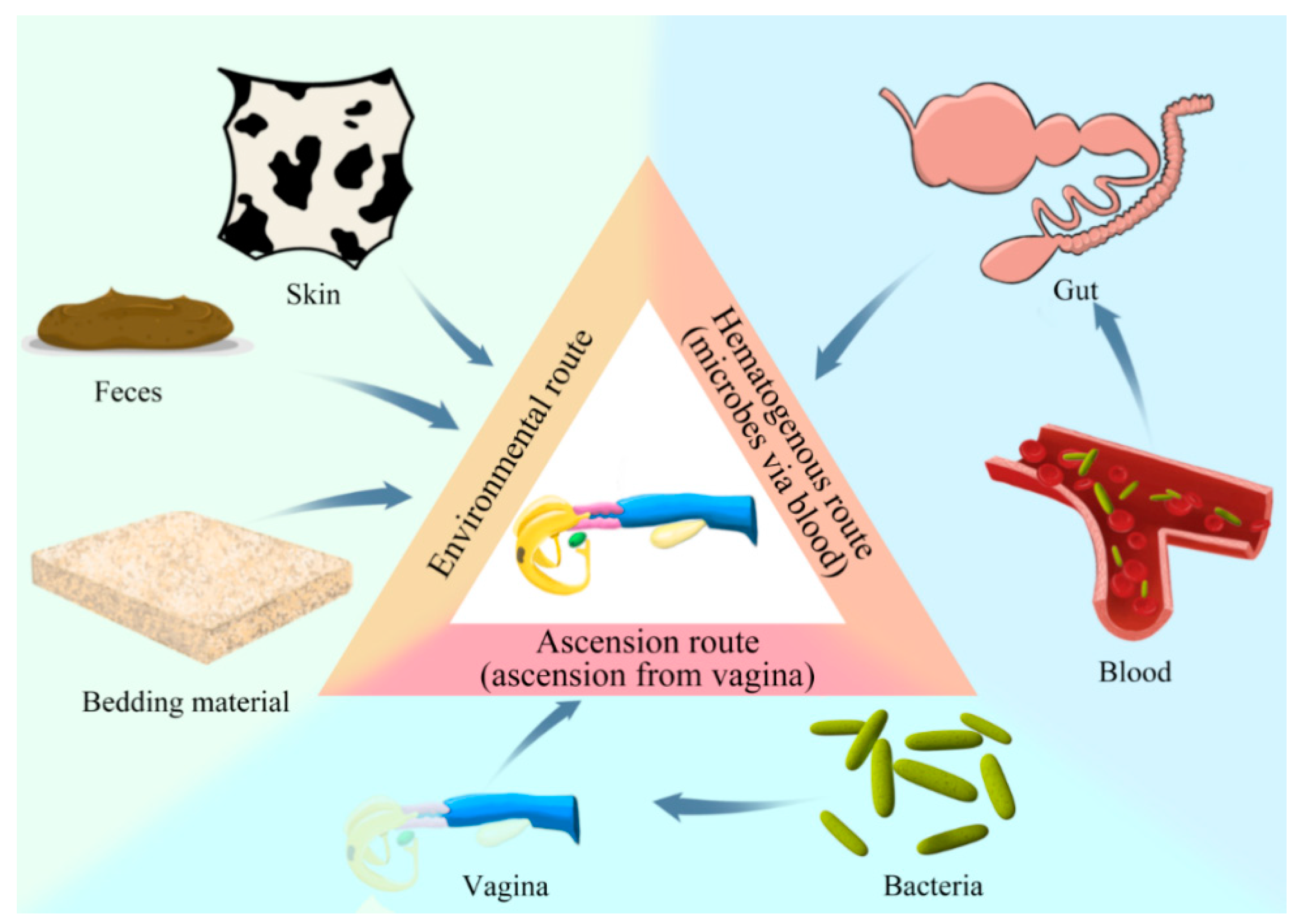
2. Prospective Origin(s) of the Reproductive Tract Microflora
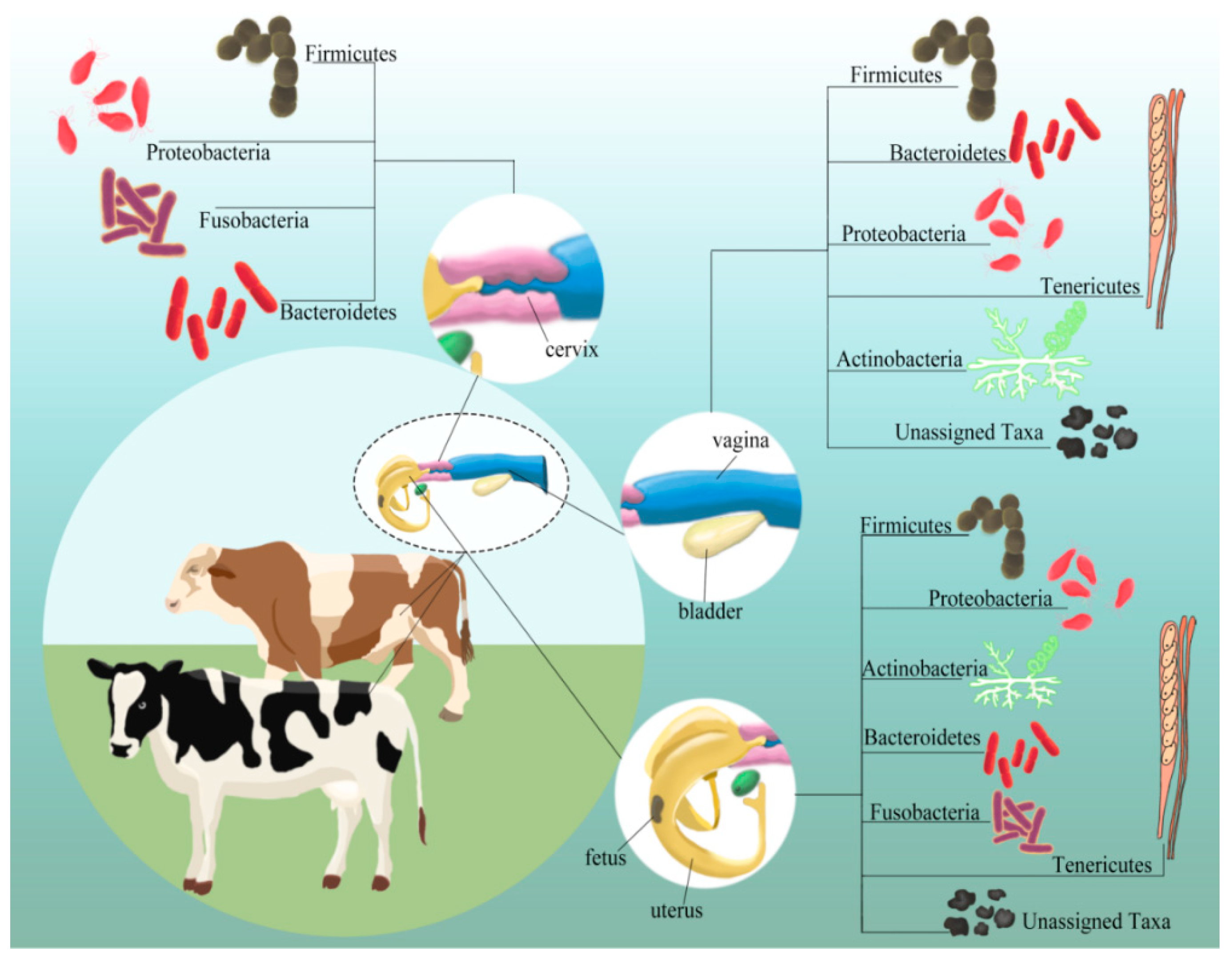
3. Microflora Composition(s) in Various Niches of the Reproductive Tract
3.1. Vaginal Microflora
3.2. Cervical Microflora
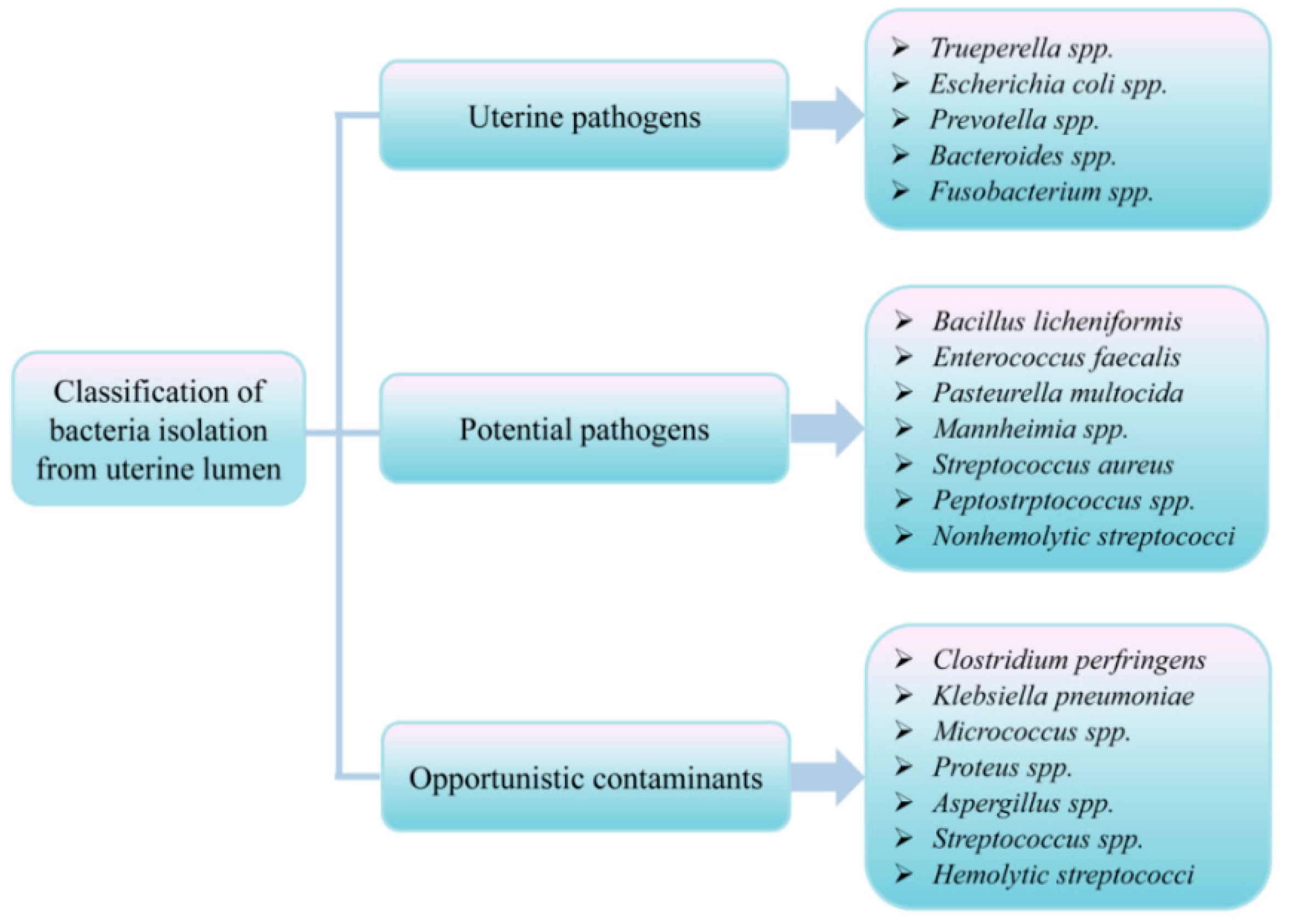
3.3. Uterine Microflora
4. Functional Role of Microflora in the Reproductive Tract
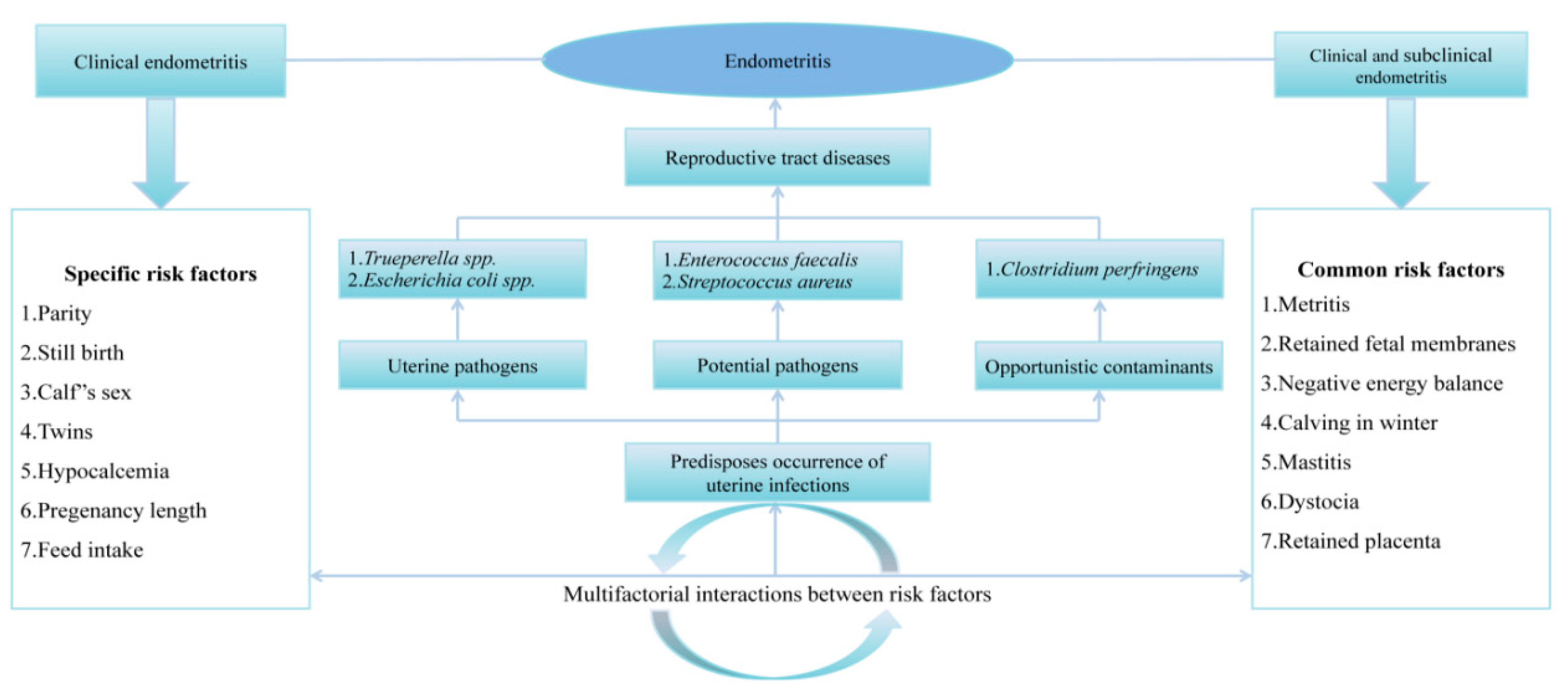
4.1. Bacterial Microflora Association with Reproductive Disorders and Failures
4.1.1. Metritis
4.1.2. Clinical and Sub-Clinical Endometritis
5. Prophylaxis and Therapeutic Approaches Modulating Reproductive Tract Microflora
5.1. Antibiotics
5.2. Probiotics: A New Approach to Modulate the Reproductive Tract Microflora
5.2.1. Lactic Acid Bacteria
5.2.2. Bacteriophages: Virus-Preventing Pathogens
6. Conclusions and Future Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HMP | Human Microbiome Project; |
| OTU | Operational Taxonomic Units; |
| SE | Subclinical endometritis; |
| CE | Clinical endometritis; |
| SCE | Subclinical endometritis; |
| AI | Artificial Insemination; |
| OR | Odds Ratio; |
| OTC | Operational Taxonomic Classification; |
| CF | Clinical Formative; |
| CP | Clinical Postpartum; |
| CG | Clinical Gestation; |
| DIM | Days in Milk; |
| DPP | Days Postpartum; |
| PVD | Purulent Vaginal Discharge; |
| ABR | Antibiotic Resistance; |
| MIC | minimum inhibitory concentration; |
| CCFA | ceftiofur crystalline free acid; |
| CM | chitosan microparticles; |
| APM | Acute Puerperal Metritis; |
| LAB | Lactic Acid Bacteria; |
| CB | Cephapirin Benzathine; |
| DEX | Dextrose solution; |
| OTC | Oxytetracycline; |
| LP | liquid paraffin; |
| CON | control; |
| PG | prostaglandin; |
| PP | Postpartum; |
| MOI | Multiplicity of Infection; |
| S. aureus | Staphylococcus aureus; |
| F. necrophorum | Fusobacterium necrophorum; |
| T. pyogenes | Trueperella pyogenes; |
| CIN | cervical intraepithelial neoplasia; |
| HPV | human papillomavirus; |
| CVM | Cervico-vaginal mucus; |
| COD | cystic ovarian disease; |
| SAT | Samia-treat; |
| CoNS | coagulase-negative staphylococci; |
| RTD | reproductive tract disease; |
| AMU | antimicrobial use; |
| AMR | antimicrobial resistance; |
| ARMs | antimicrobial resistant microorganisms; |
| EB | estradiol benzoate; |
| CL | corpus luteum; |
| αPGF2 | prostaglandin αF2; |
| nAbts | nanoantibiotics; |
| VMT | vaginal microbiota transplant; |
| FDA | Foods and Drug Authority; |
| MSc | mesenchymal stem cells; |
| IVF | in vitro fertilization |
| IVF-ICFSI | in vitro fertilization-intracytoplasmic sperm injection |
| MSS | Metagenomic Shotgun Sequencing; |
| RP | retained placenta; |
| MDR | multi-drug resistant; |
| ROS | reactive oxygen species. |
| GnRH | Gonadotropin-releasing hormone |
References
- Parfrey, L.W.; Moreau, C.S.; Russell, J.A. Introduction: The host-associated microbiome: Pattern, process and function. Mol. Ecol. 2018, 27, 1749–1765. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Zilber-Rosenberg, I. Microbes drive evolution of animals and plants: The hologenome concept. MBio 2016, 7, e01395-15. [Google Scholar] [CrossRef] [Green Version]
- Koedooder, R.; Mackens, S.; Budding, A.; Fares, D.; Blockeel, C.; Laven, J.; Schoenmakers, S. Identification and evaluation of the microbiome in the female and male reproductive tracts. Hum. Reprod. Update 2018, 25, 298–325. [Google Scholar] [CrossRef]
- Schoenmakers, S.; Steegers-Theunissen, R.; Faas, M. The matter of the reproductive microbiome. Obst. Med. 2019, 12, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Song, X.; Wei, W.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Scolari, F.; Attardo, G.M.; Aksoy, E.; Weiss, B.; Savini, G.; Takac, P.; Abd-Alla, A.; Parker, A.G.; Aksoy, S.; Malacrida, A.R. Symbiotic microbes affect the expression of male reproductive genes in Glossina m. morsitans. BMC Microbiol. 2018, 18, 169. [Google Scholar] [CrossRef]
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine microbiota: Residents, tourists, or invaders? Front. Immunol. 2018, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Moreno, I.; Simon, C. Deciphering the effect of reproductive tract microbiota on human reproduction. Reprod. Med. Biol. 2019, 18, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.J.; Vieira-Neto, A.; Gobikrushanth, M.; Daetz, R.; Mingoti, R.D.; Parize, A.C.B.; De Freitas, S.L.; Da Costa, A.N.L.; Bicalho, R.C.; Lima, S.; et al. Uterine microbiota progression from calving until establishment of metritis in dairy cows. Appl. Environ. Microbiol. 2015, 81, 6324–6332. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, L.R.V.; Karstrup, C.C.; Pedersen, H.G.; Agerholm, J.S.; Jensen, T.K.; Klitgaard, K. Revisiting bovine pyometra—New insights into the disease using a culture-independent deep sequencing approach. Vet. Microbiol. 2015, 175, 319–324. [Google Scholar] [CrossRef]
- Neckovic, A.; van Oorschot, R.A.; Szkuta, B.; Durdle, A. Investigation of direct and indirect transfer of microbiomes between individuals. Forensic Sci. Int. Genet. 2020, 45, 102212. [Google Scholar] [CrossRef] [PubMed]
- Piersanti, R.L.; Bromfield, J.J. [AN354] The Consequence of Postpartum Uterine Disease on Dairy Cow Fertility. EDIS 2019, 2019, 1–4. [Google Scholar]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82–83, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Cunha, F.; Ma, X.; Martinez, N.; Vieira-Neto, A.; Daetz, R.; Bicalho, R.C.; Lima, S.; Santos, J.E.; Jeong, K.C. Uterine microbiota and immune parameters associated with fever in dairy cows with metritis. PLoS ONE 2016, 11, e0165740. [Google Scholar] [CrossRef] [Green Version]
- Lima, F.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.; Ganda, E.K.; de Oliveira Filho, J.C.; Lorenzo, G.; Trojacanec, P.; Bicalho, R.C. Prepartum and Postpartum Rumen Fluid Microbiomes: Characterization and Correlation with Production Traits in Dairy Cows. Appl. Environ. Microbiol. 2015, 81, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Bicalho, M.; Santin, T.; Rodrigues, M.; Marques, C.; Lima, S.; Bicalho, R. Dynamics of the microbiota found in the vaginas of dairy cows during the transition period: Associations with uterine diseases and reproductive outcome. J. Dairy Sci. 2017, 100, 3043–3058. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Bromfield, J.J. Tolerance and innate immunity shape the development of postpartum uterine disease and the impact of endometritis in dairy cattle. Ann. Rev. Anim. Biosci. 2019, 7, 361–384. [Google Scholar] [CrossRef] [Green Version]
- Galvão, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. J. Dairy Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef]
- Nagaraja, T.; Lechtenberg, K.F. Liver abscesses in feedlot cattle. Vet. Clin. N. Am. Food Anim. Pract. 2007, 23, 351–369. [Google Scholar] [CrossRef]
- Kutzer, P.; Schulze, C.; Engelhardt, A.; Wieler, L.H.; Nordhoff, M. Helcococcus ovis, an Emerging Pathogen in Bovine Valvular Endocarditis. J. Clin. Microbiol. 2008, 46, 3291–3295. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.J.; Cunha, F.; Vieira-Neto, A.; Bicalho, R.C.; Lima, S.; Bicalho, M.L.; Galvão, K.N. Blood as a route of transmission of uterine pathogens from the gut to the uterus in cows. Microbiome 2017, 5, 109. [Google Scholar] [CrossRef]
- Laguardia-Nascimento, M.; Branco, K.M.; Gasparini, M.R.; Giannattasio-Ferraz, S.; Leite, L.R.; Araujo, F.M.; Salim, A.C.; Nicoli, J.R.; Oliveira, G.C.; Barbosa-Stancioli, E.F. Vaginal microbiome characterization of Nellore cattle using metagenomic analysis. PLoS ONE 2015, 10, e0143294. [Google Scholar] [CrossRef] [PubMed]
- Amos, M.R.; Healey, G.D.; Goldstone, R.J.; Mahan, S.M.; Düvel, A.; Schuberth, H.-J.; Sandra, O.; Zieger, P.; Dieuzy-Labaye, I.; Smith, D.G. Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle. Biol. Reprod. 2014, 54, 1–13. [Google Scholar]
- Hansen, P.J. Physiology and Endocrinology Symposium: Maternal immunological adjustments to pregnancy and parturition in ruminants and possible implications for postpartum uterine health: Is there a prepartum-postpartum nexus? J. Anim. Sci. 2013, 91, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Young, W.; Hine, B.C.; Wallace, O.A.; Callaghan, M.; Bibiloni, R. Transfer of intestinal bacterial components to mammary secretions in the cow. PeerJ 2015, 3, e888. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, C.J.; Ishaq, S.L.; Bichi, E.; Olivo, S.K.; Lowe, J.; Aldridge, B.M. Biogeographical differences in the influence of maternal microbial sources on the early successional development of the bovine neonatal gastrointestinal tract. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Knight, R.; Callewaert, C.; Marotz, C.; Hyde, E.R.; Debelius, J.W.; McDonald, D.; Sogin, M.L. The microbiome and human biology. Ann. Rev. Genom. Hum. Genet. 2017, 18, 65–86. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Liu, C.; Yang, Y.; Lu, W. Comparison of vaginal microbial community structure in healthy and endometritis dairy cows by PCR-DGGE and real-time PCR. Anaerobe 2016, 38, 1–6. [Google Scholar] [CrossRef]
- Nesengani, L.T.; Wang, J.; Yang, Y.; Yang, L.; Lu, W. Unravelling vaginal microbial genetic diversity and abundance between Holstein and Fleckvieh cattle. RSC Adv. 2017, 7, 56137–56143. [Google Scholar] [CrossRef] [Green Version]
- El-Hayek, S.; Clarke, H.J. Control of oocyte growth and development by intercellular communication within the follicular niche. In Molecular Mechanisms of Cell Differentiation in Gonad Development; Springer: Berlin/Heidelberg, Germany, 2016; pp. 191–224. [Google Scholar]
- Giannattasio- Ferraz, S.; Laguardia-Nascimento, M.; Gasparini, M.R.; Leite, L.R.; Araujo, F.M.G.; de Matos Salim, A.C.; de Oliveira, A.P.; Nicoli, J.R.; de Oliveira, G.C.; da Fonseca, F.G. A common vaginal microbiota composition among breeds of Bos taurus indicus (Gyr and Nellore). Braz. J. Microbiol. 2019, 50, 1115–1124. [Google Scholar] [CrossRef]
- Saini, P.; Singh, M.; Kumar, P. Fungal endometritis in bovines. Open Vet. J. 2019, 9, 94–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, F.; McClure, M.; Rorie, R.; Wang, X.; Chai, J.; Wei, X.; Lai, S.; Zhao, J. The vaginal and fecal microbiomes are related to pregnancy status in beef heifers. J. Anim. Sci. Biotechnol. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Ault, T.B.; Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.; Edwards, J.L.; Myer, P.R.; Pohler, K.G. Uterine and vaginal bacterial community diversity prior to artificial insemination between pregnant and nonpregnant postpartum cows. J. Anim. Sci. 2019, 97, 4298–4304. [Google Scholar] [CrossRef]
- Mahalingam, S.; Dharumadurai, D.; Archunan, G. Vaginal microbiome analysis of buffalo (Bubalus bubalis) during estrous cycle using high-throughput amplicon sequence of 16S rRNA gene. Symbiosis 2019, 78, 97–106. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Nesengani, L.T.; Gong, Y.; Yang, Y.; Yang, L.; Lu, W. Comparison of vaginal microbial community structure of beef cattle between luteal phase and follicular phase. Indian J. Anim. Res. 2019, 53, 1298–1303. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Goltsman, D.S.A.; Sun, C.L.; Proctor, D.M.; DiGiulio, D.B.; Robaczewska, A.; Thomas, B.C.; Shaw, G.M.; Stevenson, D.K.; Holmes, S.P.; Banfield, J.F. Metagenomic analysis with strain-level resolution reveals fine-scale variation in the human pregnancy microbiome. Genome. Res. 2018, 28, 1467–1480. [Google Scholar] [CrossRef] [Green Version]
- Azawi, O. Postpartum uterine infection in cattle. Anim Reprod. Sci. 2008, 105, 187–208. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, M.; Kumar, P.; Sharma, A.; Neelam, A.M.J.; Sharma, P. Postpartum Uterine Infections in Cows and Factors Affecting it–A Review. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 1020–1028. [Google Scholar]
- Deguillaume, L.; Geffré, A.; Desquilbet, L.; Dizien, A.; Thoumire, S.; Vornière, C.; Constant, F.; Fournier, R.; Chastant-Maillard, S. Effect of endocervical inflammation on days to conception in dairy cows. J. Dairy Sci. 2012, 95, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Singer, E.; Bushnell, B.; Coleman-Derr, D.; Bowman, B.; Bowers, R.M.; Levy, A.; Gies, E.A.; Cheng, J.-F.; Copeland, A.; Klenk, H.-P. High-resolution phylogenetic microbial community profiling. The ISME J. 2016, 10, 2020–2032. [Google Scholar] [CrossRef]
- Galvão, K. Postpartum uterine diseases in dairy cows. Anim Reprod. 2018, 9, 290–296. [Google Scholar]
- Wang, Y.; Wang, J.; Li, H.; Fu, K.; Pang, B.; Yang, Y.; Liu, Y.; Tian, W.; Cao, R. Characterization of the cervical bacterial community in dairy cows with metritis and during different physiological phases. Theriogenology 2018, 108, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Curty, G.; de Carvalho, P.S.; Soares, M.A. The Role of the Cervicovaginal Microbiome on the Genesis and as a Biomarker of Premalignant Cervical Intraepithelial Neoplasia and Invasive Cervical Cancer. Int. J. Mol. Sci. 2020, 21, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnane, M.; Meade, K.G.; O’Farrelly, C. Cervico-vaginal mucus (CVM)–an accessible source of immunologically informative biomolecules. Vet. Res. Commun. 2018, 42, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, I.; Franasiak, J.M. Endometrial microbiota—new player in town. Fertil. Steril. 2017, 108, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Pelzer, E.; Gomez-Arango, L.F.; Barrett, H.L.; Nitert, M.D. Maternal health and the placental microbiome. Placenta 2017, 54, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, C.; Mangogna, A.; Bossi, F.; Ricci, G.; Kishore, U.; Bulla, R. Uterine immunity and microbiota: A shifting paradigm. Front. Immunol. 2019, 10, 2387. [Google Scholar] [CrossRef] [Green Version]
- Moore, S.; Ericsson, A.; Poock, S.; Melendez, P.; Lucy, M. Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci. 2017, 100, 4953–4960. [Google Scholar] [CrossRef] [Green Version]
- Karstrup, C.C.; Klitgaard, K.; Jensen, T.K.; Agerholm, J.S.; Pedersen, H.G. Presence of bacteria in the endometrium and placentomes of pregnant cows. Theriogenology 2017, 99, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.A.; Gilbert, R.O.; Bicalho, R.C. Metagenomic analysis of the uterine bacterial microbiota in healthy and metritic postpartum dairy cows. J. Dairy Sci. 2011, 94, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.M.; Bicalho, R.C. Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows. PLoS ONE 2012, 7, e53048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-CasoLuengo, R.; Lu, J.; Williams, E.J.; Miranda-CasoLuengo, A.A.; Carrington, S.D.; Evans, A.C.; Meijer, W.G. Delayed differentiation of vaginal and uterine microbiomes in dairy cows developing postpartum endometritis. PLoS ONE 2019, 14, e0200974. [Google Scholar] [CrossRef] [Green Version]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.J.; Meade, K.G.; Williams, E.J.; O’Farrelly, C.; Grant, J.; Evans, A.C.; Beltman, M.E. Purulent vaginal discharge diagnosed in pasture-based Holstein-Friesian cows at 21 days postpartum is influenced by previous lactation milk yield and results in diminished fertility. J. Dairy Sci. 2020, 103, 666–675. [Google Scholar] [CrossRef]
- Canadas, E.R.; Herlihy, M.; Kenneally, J.; Grant, J.; Kearney, F.; Lonergan, P.; Butler, S. Associations between postpartum phenotypes, cow factors, genetic traits, and reproductive performance in seasonal-calving, pasture-based lactating dairy cows. J. Dairy Sci. 2020, 103, 1016–1030. [Google Scholar] [CrossRef] [Green Version]
- Rojas Canadas, E.R.; Herlihy, M.M.; Kenneally, J.; Grant, J.; Kearney, F.; Lonergan, P.; Butler, S.T. Associations between ovarian cyclicity, uterine health, indicators of bioenergetic status, and genetic traits during early lactation in seasonal-calving, pasturebased lactating dairy cows. J. Dairy Sci. 2020, 103, 1002–1015. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Santos, N.R. Dynamics of postpartum endometrial cytology and bacteriology and their relationship to fertility in dairy cows. Theriogenology 2016, 85, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, S.; Duffield, T.; Leslie, K.; Bateman, K.; Keefe, G.P.; Walton, J.; Johnson, W. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef]
- De Boer, M.; Buddle, B.M.; Heuer, C.; Hussein, H.; Zheng, T.; LeBlanc, S.J.; McDougall, S. Associations between intrauterine bacterial infection, reproductive tract inflammation, and reproductive performance in pasture-based dairy cows. Theriogenology 2015, 83, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.; Molinari, P.C.; Ormsby, T.J.; Bromfield, J.J. Preventing postpartum uterine disease in dairy cattle depends on avoiding, tolerating and resisting pathogenic bacteria. Theriogenology 2020, 150, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Silva, T. Adaptive immunity in the postpartum uterus: Potential use of vaccines to control metritis. Theriogenology 2020, 150, 201–209. [Google Scholar] [CrossRef]
- Pascottini, O.B.; LeBlanc, S.J. Modulation of immune function in the bovine uterus peripartum. Theriogenology 2020, 150, 193–200. [Google Scholar] [CrossRef] [PubMed]
- König, S.; May, K. Invited review: Phenotyping strategies and quantitative-genetic background of resistance, tolerance and resilience associated traits in dairy cattle. Animal 2019, 13, 897–908. [Google Scholar]
- Gilbert, R.O. Symposium review: Mechanisms of disruption of fertility by infectious diseases of the reproductive tract. J. Dairy Sci. 2019, 102, 3754–3765. [Google Scholar] [CrossRef] [Green Version]
- Chase, C.; Kaushik, R.S. Mucosal Immune System of Cattle: All Immune Responses Begin Here. Vet. Clin. Food Anim. Pract 2019, 35, 431–451. [Google Scholar] [CrossRef]
- Helfrich, A.L.; Reichenbach, H.-D.; Meyerholz, M.M.; Schoon, H.-A.; Arnold, G.J.; Fröhlich, T.; Weber, F.; Zerbe, H. Novel sampling procedure to characterize bovine subclinical endometritis by uterine secretions and tissue. Theriogenology 2020, 141, 186–196. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Owens, S.E. Postpartum uterine infection and endometritis in dairy cattle. Anim. Reprod. (AR) 2018, 14, 622–629. [Google Scholar] [CrossRef]
- Bruun, J.; Ersbøll, A.; Alban, L. Risk factors for metritis in Danish dairy cows. Prev. Vet. Med. 2002, 54, 179–190. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Giuliodori, M.J.; Magnasco, R.; Becu-Villalobos, D.; Lacau-Mengido, I.; Risco, C.; de la Sota, R.L. Metritis in dairy cows: Risk factors and reproductive performance. J. Dairy Sci. 2013, 96, 3621–3631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossein-Zadeh, N.G.; Ardalan, M. Cow-specific risk factors for retained placenta, metritis and clinical mastitis in Holstein cows. Vet. Res. Commun. 2011, 35, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Kaidi, R.; Hanzen, C.; England, G.C. Risk factors of clinical and subclinical endometritis in cattle: A review. Turk. J. Vet. Anim. Sci. 2017, 41, 1–11. [Google Scholar] [CrossRef]
- Imhof, S.; Luternauer, M.; Hüsler, J.; Steiner, A.; Hirsbrunner, G. Therapy of retained fetal membranes in cattle: Comparison of two treatment protocols. Anim. Reprod. Sci. 2019, 206, 11–16. [Google Scholar] [CrossRef]
- Cunha, F.; Jeon, S.J.; Kutzer, P.; Jeong, K.C.; Galvão, K.N. Draft Genome Sequences of Helcococcus ovis Strains Isolated at Time of Metritis Diagnosis from the Uterus of Holstein Dairy Cows. Microbiol. Resour. Announc. 2019, 8, e00402-19. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Wang, Y.; Hang, S.; Zhu, W. Microbial diversity in uterus of healthy and metritic postpartum Holstein dairy cows. Folia Microbiol. 2013, 58, 593–600. [Google Scholar] [CrossRef]
- Sicsic, R.; Goshen, T.; Dutta, R.; Kedem-Vaanunu, N.; Kaplan-Shabtai, V.; Pasternak, Z.; Gottlieb, Y.; Shpigel, N.Y.; Raz, T. Microbial communities and inflammatory response in the endometrium differ between normal and metritic dairy cows at 5–10 days post-partum. Vet Res. 2018, 49, 77. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Saini, S.; Ansari, S.; Jamwal, S.; Malakar, D. 220 Exploring the use of mesenchymal stem cells for treatment of mastitis and metritis in cattle. Reprod. Fertil. Dev. 2020, 32, 238. [Google Scholar] [CrossRef]
- Kelly, E.; McAloon, C.; O’Grady, L.; Duane, M.; Somers, J.; Beltman, M. Cow-level risk factors for reproductive tract disease diagnosed by 2 methods in pasture-grazed dairy cattle in Ireland. J. Dairy Sci. 2020, 103, 737–749. [Google Scholar] [CrossRef]
- Piersanti, R.L.; Zimpel, R.; Molinari, P.C.; Dickson, M.J.; Ma, Z.; Jeong, K.C.; Santos, J.E.; Sheldon, I.M.; Bromfield, J.J. A model of clinical endometritis in Holstein heifers using pathogenic Escherichia coli and Trueperella pyogenes. J Dairy Sci. 2019, 102, 2686–2697. [Google Scholar] [CrossRef]
- Salah, N.; Yimer, N. Cytological endometritis and its agreement with ultrasound examination in postpartum beef cows. Vet. World. 2017, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Ricci, A.; Gallo, S.; Molinaro, F.; Dondo, A.; Zoppi, S.; Vincenti, L. Evaluation of subclinical endometritis and consequences on fertility in Piedmontese beef cows. Reprod. Domest. Anim. 2015, 50, 142–148. [Google Scholar] [CrossRef] [PubMed]
- De Cássia Bicudo, L.; Oba, E.; Bicudo, S.D.; da Silva Leite, D.; Siqueira, A.K.; de Souza Monobe, M.M.; Nogueira, M.; de Figueiredo Pantoja, J.C.; Listoni, F.J.P.; Ribeiro, M.G. Virulence factors and phylogenetic group profile of uterine Escherichia coli in early postpartum of high-producing dairy cows. Anim. Prod. Sci. 2019, 59, 1898–1905. [Google Scholar]
- Madoz, L.V.; Giuliodori, M.J.; Jaureguiberry, M.; Plöntzke, J.; Drillich, M.; de la Sota, R.L. The relationship between endometrial cytology during estrous cycle and cutoff points for the diagnosis of subclinical endometritis in grazing dairy cows. J. Dairy Sci. 2013, 96, 4333–4339. [Google Scholar] [CrossRef] [Green Version]
- Werner, A.; Suthar, V.; Plöntzke, J.; Heuwieser, W. Relationship between bacteriological findings in the second and fourth weeks postpartum and uterine infection in dairy cows considering bacteriological results. J. Dairy Sci. 2012, 95, 7105–7114. [Google Scholar] [CrossRef] [PubMed]
- Mogheiseh, A.; Ahmadi, M.R.; Nazifi, S.; Mirzaei, A.; Fallah, E. Destination of corpus luteum in postpartum clinical endometritis cows and factors affecting self-recovery. Vet. Anim Sci. 2020, 9, 100067. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Xu, J.; An, L.; Wang, J.; Zhu, Y. Uterine Microbiota of Dairy Cows With Clinical and Subclinical Endometritis. Front. Microbiol. 2018, 9, 2691. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Nishimura, R.; Gunji, Y.; Hishinuma, M. Research of postpartum endometritis in Japanese Black cattle with cystic ovarian disease by vaginal mucus test and endometrial cytology. Arch. Anim. Breed. 2020, 63, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, Z.; Mann, G.; Robinson, R. Impact of endometritis on post-partum ovarian cyclicity in dairy cows. Vet. J. 2019, 248, 8–13. [Google Scholar] [CrossRef]
- Salzano, A.; Pesce, A.; D’Andrea, L.; Paciello, O.; della Ragione, F.; Ciaramella, P.; Salzano, C.; Costagliola, A.; Licitra, F.; Neglia, G. Inflammatory response in repeat breeder buffaloes. Theriogenology. 2020, 145, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Basu, S.; Mishra, P.; Ray, K. Effect of immunomodulators on certain haematological parameters in ameliorating bovine Endometritis. IJCS 2019, 7, 1736–1739. [Google Scholar]
- Djuricic, D.; Vince, S.; Ablondi, M.; Dobranic, T.; Samardzija, M. Effect of preventive intrauterine ozone application on reproductive efficiency in Holstein cows. Reprod. Domest. Anim. 2012, 47, 87–91. [Google Scholar] [CrossRef]
- Đuričić, D.; Lipar, M.; Samardžija, M. Ozone treatment of metritis and endometritis in Holstein cows. Vet. Arhiv. 2014, 84, 103–110. [Google Scholar]
- Đuričić, D.; Valpotić, H.; Samardžija, M. Prophylaxis and therapeutic potential of ozone in buiatrics: Current knowledge. Anim. Reprod. Sci. 2015, 159, 1–7. [Google Scholar] [CrossRef]
- Roope, L.S.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; San Tan, P.; Walker, A.S.; Robotham, J.V. The challenge of antimicrobial resistance: What economics can contribute. Science. 2019, 364, eaau4679. [Google Scholar] [CrossRef]
- Kleczkowski, M.; Kluciński, W.; Czerski, M.; Kudyba, E. Association between acute phase response, oxidative status and mastitis in cows. Vet. Stn. 2017, 48, 177–186. [Google Scholar]
- Zobel, R.; Martinec, R.; Ivanović, D.; Rošić, N.; Stančić, Z.; Žerjavić, I.; Flajsig, B.; Plavec, H.; Smolec, O. Intrauterine ozone administration for improving fertility rate in Simmental cattle. Vet. Arhiv. 2014, 84, 1–8. [Google Scholar]
- Proudfoot, K.L. Maternal Behavior and Design of the Maternity Pen. Vet. Clin. Food Anim. Pract. 2019, 35, 111–124. [Google Scholar] [CrossRef]
- Szenci, O.; Szelényi, Z.; Lénárt, L.; Buják, D.; Kovács, L.; Fruzsina Kézér, L.; Han, B.; Horváth, A. Importance of monitoring the peripartal period to increase reproductive performance in dairy cattle. Vet. Stn. 2018, 49, 297–307. [Google Scholar]
- Gomez, D.E.; Galvão, K.N.; Rodriguez-Lecompte, J.C.; Costa, M.C. The Cattle Microbiota and the Immune System: An Evolving Field. Vet. Clin. Food Anim. Pract. 2019, 35, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, A.H.; Goss, E.M.; Havelaar, A.; van Diepeningen, A.D.; Finckh, M.R.; Morris, J.G. One Health-Cycling of diverse microbial communities as a connecting force for soil, plant, animal, human and ecosystem health. Sci. Total Environ. 2019, 664, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Grout, A.H.; Goss, E.M.; Havelaar, A.; van Diepeningen, A.D.; Finckh, M.R.; Morris, J.G. A Review of Potential Public Health Impacts Associated with the Global Dairy Sector. GeoHealth 2020, 4, e2019GH000213. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Hughes, J.M. Critical importance of a one health approach to antimicrobial resistance. EcoHealth 2019, 16, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Jee, Y.; Carlson, J.; Rafai, E.; Musonda, K.; Huong, T.T.G.; Daza, P.; Sattayawuthipong, W.; Yoon, T. Antimicrobial resistance: A threat to global health. The Lancet. Infect. Dis. 2018, 18, 939–940. [Google Scholar] [CrossRef]
- Rochford, C.; Sridhar, D.; Woods, N.; Saleh, Z.; Hartenstein, L.; Ahlawat, H.; Whiting, E.; Dybul, M.; Cars, O. Global governance of antimicrobial resistance. Lancet 2018, 391, 1976–1978. [Google Scholar] [CrossRef]
- Ma, Z.; Lee, S.; Jeong, K.C. Mitigating antibiotic resistance at the livestock-environment interface: A review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef]
- Hommerich, K.; Ruddat, I.; Hartmann, M.; Werner, N.; Käsbohrer, A.; Kreienbrock, L. Monitoring Antibiotic Usage in German Dairy and Beef Cattle Farms—A Longitudinal Analysis. Front. Vet. Sci 2019, 6, 244. [Google Scholar] [CrossRef] [Green Version]
- Nak, Y.; Dagalp, S.; Cetin, C.; Nak, D.; Alkan, F.; Borum, E.; Tuna, B. Course and severity of postpartum metritis cases following antibiotic and PGF2α administration in postpartum metritis cows infected with BoHV-4. Transboud. Emerg. Dis. 2011, 58, 31–36. [Google Scholar] [CrossRef]
- El-Khadrawy, H.; Ahmed, W.; Zaabal, M.; Hanafi, E. Strategies for diagnosis and treatment of uterine infection in bovines. Global Vet. 2015, 15, 98–105. [Google Scholar]
- Lima, F.; Vieira-Neto, A.; Vasconcellos, G.; Mingoti, R.; Karakaya, E.; Solé, E.; Bisinotto, R.; Martinez, N.; Risco, C.; Galvao, K. Efficacy of ampicillin trihydrate or ceftiofur hydrochloride for treatment of metritis and subsequent fertility in dairy cows. J. Dairy Sci. 2014, 97, 5401–5414. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, T.; Kiku, Y.; Mizuno, M.; Inumaru, S.; Kushibiki, S.; Shingu, H.; Matsubara, T.; Takahashi, H.; Hayashi, T. Effect of intramammary infusion of rbGM-CSF on SCC and expression of polymorphonuclear neutrophil adhesion molecules in subclinical mastitis cows. Vet. Res. Commun. 2012, 36, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.; Giguère, S.; Guardabassi, L.; Morley, P.; Papich, M.; Ricciuto, D.; Sykes, J.E. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J. Vet. Intern. Med. 2015, 29, 487–498. [Google Scholar] [CrossRef]
- Haimerl, P.; Arlt, S.; Borchardt, S.; Heuwieser, W.P. Antibiotic treatment of metritis in dairy cows—A meta-analysis. J. Dairy Sci. 2017, 100, 3783–3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, E.B.; Cunha, F.; Daetz, R.; Figueiredo, C.C.; Chebel, R.C.; Santos, J.E.; Galvão, K.N. Using chitosan microparticles to treat metritis in lactating dairy cows. J. Dairy Sci. 2019, 102 (Suppl. 1), 286. [Google Scholar]
- Jeon, S.J.; Lima, F.S.; Vieira-Neto, A.; Machado, V.S.; Lima, S.F.; Bicalho, R.C.; Santos, J.E.P.; Galvão, K.N. Shift of uterine microbiota associated with antibiotic treatment and cure of metritis in dairy cows. Vet. Microbiol. 2018, 214, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Von Krueger, X.; Scherpenisse, P.; Roiger, S.; Heuwieser, W. Determination of ceftiofur derivatives in serum, endometrial tissue, and lochia in puerperal dairy cows with fever or acute puerperal metritis after subcutaneous administration of ceftiofur crystalline free acid. J. Dairy Sci. 2013, 96, 1054–1062. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.R.; Mogheiseh, A.; Mirzaei, A.; Nazifi, S.; Fallah, E. Treatment of cows with clinical endometritis III as cows affected by pyometra-Non antibiotic treatment of severe clinical endometritis. Asian Pac. J. Reprod. 2018, 7, 185. [Google Scholar]
- Bandai, K.; Kusaka, H.; Miura, H.; Kikuchi, M.; Sakaguchi, M. A simple and practical short-term timed artificial insemination protocol using estradiol benzoate with prostaglandin F2α in lactating dairy cows. Theriogenology 2020, 141, 197–201. [Google Scholar] [CrossRef]
- El-Rheem, S.M.A.; Ghallab, R.S.; El-Sharkawy, S. SAT, a New Approach in Understanding and Treatment of Subclinical Endometritis in Dairy Cows. Open J. Vet. Med. 2019, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Maan, M.K.; Sattar, A.; Mi, K.; Shabbir, M.A.B.; Xie, S.; Xin, L.; Ahmed, S.; Algharib, S.A.; Huang, L.; Yuan, Z. Integration of PK/PD for dose optimization of aditoprim against Trueperella pyogenes causing endometritis in bovines. Microb. Pathog. 2020, 142, 104097. [Google Scholar] [CrossRef] [PubMed]
- Aghamiri, S.M.; Ahmadi, M.R.; Haghkhah, M.; Derakhshandeh, A. Identification of pathogenic microorganisms of repeat breeder dairy cows and a hyperimmune treatment approach. Asian Pac. J. Reprod. 2020, 9, 44. [Google Scholar]
- Colombo, M.; Castilho, N.P.; Todorov, S.D.; Nero, L.A. Beneficial properties of lactic acid bacteria naturally present in dairy production. BMC Microbiol. 2018, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Bertulat, S.; Borchardt, S.; Burfeind, O.; Heuwieser, W. Randomized, controlled clinical trial on the efficacy of nonsteroidal antiinflammatory drugs for the treatment of acute puerperal metritis in dairy cows. J. Dairy Sci. 2016, 99, 8241–8249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinedo, P.; Velez, J.; Bothe, H.; Merchan, D.; Piñeiro, J.; Risco, C. Effect of intrauterine infusion of an organic-certified product on uterine health, survival, and fertility of dairy cows with toxic puerperal metritis. J. Dairy Sci. 2015, 98, 3120–3132. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Wang, S.; Wang, L.; Wang, H.; Li, X.; Liu, Y. Prophylactic strategy with herbal remedy to reduce puerperal metritis risk in dairy cows: A randomized clinical trial. Livest. Sci. 2015, 181, 231–235. [Google Scholar] [CrossRef]
- Daetz, R.; Cunha, F.; Bittar, J.; Risco, C.; Magalhaes, F.; Maeda, Y.; Santos, J.; Jeong, K.; Cooke, R.; Galvão, K. Clinical response after chitosan microparticle administration and preliminary assessment of efficacy in preventing metritis in lactating dairy cows. J. Dairy Sci. 2016, 99, 8946–8955. [Google Scholar] [CrossRef]
- Jeon, S.J.; Ma, Z.; Kang, M.; Galvao, K.N.; Jeong, K.C. Application of chitosan microparticles for treatment of metritis and in vivo evaluation of broad spectrum antimicrobial activity in cow uteri. Biomaterials 2016, 110, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/chitosan derivatives and their interactions with microorganisms: A comprehensive review and future perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A. Nanoantibiotics: A Novel Rational Approach to Antibiotic Resistant Infections. Curr. Drug Metab. 2019, 20, 720–741. [Google Scholar] [CrossRef]
- Makki, M.; Ahmadi, M.R.; Gheisari, H.R.; Nazifi, S. Cure rate of postpartum endometritis after different treatments in high produce dairy cows. Comp. Clin. Pathol. 2017, 26, 921–928. [Google Scholar] [CrossRef]
- Brick, T.A.; Schuenemann, G.M.; Bas, S.; Daniels, J.B.; Pinto, C.; Rings, D.M.; Rajala-Schultz, P.J. Effect of intrauterine dextrose or antibiotic therapy on reproductive performance of lactating dairy cows diagnosed with clinical endometritis. J. Dairy Sci. 2012, 95, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.R.; Makki, M.; Mirzaei, A.; Gheisari, H.R. Effects of hypertonic dextrose and paraffin solution as non-antibiotic treatments of clinical endometritis on reproductive performance of high producing dairy cows. Reprod. Domest. Anim. 2019, 54, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, L.J. Bombay Veterinary College Parel, Mumbai-400 012. Ph.D. Thesis, Maharashtra Animal and Fishery Sciences University, Nagpur, India, 2016. [Google Scholar]
- Machado, V.; Oikonomou, G.; Ganda, E.; Stephens, L.; Milhomem, M.; Freitas, G.; Zinicola, M.; Pearson, J.; Wieland, M.; Guard, C. The effect of intrauterine infusion of dextrose on clinical endometritis cure rate and reproductive performance of dairy cows. J. Dairy Sci. 2015, 98, 3849–3858. [Google Scholar] [CrossRef] [PubMed]
- Maquivar, M.; Barragan, A.; Velez, J.; Bothe, H.; Schuenemann, G.M. Effect of intrauterine dextrose on reproductive performance of lactating dairy cows diagnosed with purulent vaginal discharge under certified organic management. J. Dairy Sci. 2015, 98, 3876–3886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doehring, C.; Sundrum, A. Efficacy of homeopathy in livestock according to peer-reviewed publications from 1981 to 2014. Vet. Rec. 2016, 179, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghayedi, M.; Ahmadzadeh, H.; Ghazvini, K.; Goharshadi, E. Neglected antibacterial activity of ethylene glycol as a common solvent. Microb. Pathog. 2017, 107, 457–461. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar]
- Novik, G.; Savich, V. Beneficial microbiota. Probiotics and pharmaceutical products in functional nutrition and medicine. Microbes Infect. 2020, 22, 8–18. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5. [Google Scholar] [CrossRef]
- Cameron, A.; McAllister, T. Could probiotics be the panacea alternative to the use of antimicrobials in livestock diets? Benef. Microbes. 2019, 10, 773–799. [Google Scholar] [CrossRef] [PubMed]
- VT Nair, D.; Venkitanarayanan, K.; Kollanoor Johny, A. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.I.; Sadekuzzaman, M.; Ha, S.-D. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- Abdul-Abbas, S.J.; Al-Badran, A.E.; Al-bayyar, A.H.A.; Al-Sherifi, H.R. Isolation and identification of alocalstrain of probiotic bacterial Lactobacillus plantarum and studied the tolerance ability for different levels of ph. Basrah J. Vet. Res. 2016, 15, 329–345. [Google Scholar] [CrossRef]
- Gorzelak, M.A.; Gill, S.K.; Tasnim, N.; Ahmadi-Vand, Z.; Jay, M.; Gibson, D.L. Methods for improving human gut microbiome data by reducing variability through sample processing and storage of stool. PLoS ONE 2015, 10, e0134802. [Google Scholar] [CrossRef]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal lactic acid bacteria modulated local and systemic immune responses and lowered the incidence of uterine infections in periparturient dairy cows. PLoS ONE 2015, 10, e0124167. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Berardo, N.; Giraudo, J.; Nader-Macías, M.; Bogni, C. Bovine mastitis prevention: Humoral and cellular response of dairy cows inoculated with lactic acid bacteria at the dry-off period. Benef. Microbes. 2017, 8, 589–596. [Google Scholar] [CrossRef]
- Niu, C.; Cheng, C.; Liu, Y.; Huang, S.; Fu, Y.; Li, P. Transcriptome Profiling Analysis of Bovine Vaginal Epithelial Cell Response to an Isolated Lactobacillus Strain. MSystems 2019, 4, e00268-19. [Google Scholar] [CrossRef] [Green Version]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Genís, S.; Bach, À.; Fàbregas, F.; Arís, A. Potential of lactic acid bacteria at regulating Escherichia coli infection and inflammation of bovine endometrium. Theriogenology 2016, 85, 625–637. [Google Scholar] [CrossRef]
- Genís, S.; Sánchez-Chardi, A.; Bach, À.; Fàbregas, F.; Arís, A. A combination of lactic acid bacteria regulates Escherichia coli infection and inflammation of the bovine endometrium. J. Dairy Sci. 2017, 100, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, J.; Yang, Y.; Li, X.; Sun, C. In vitro assessment of probiotic properties of lactic acid bacteria isolated from vaginas of healthy cows. Indian J. Anim. Res. 2015, 49, 355–359. [Google Scholar] [CrossRef]
- Norenhag, J.; Du, J.; Olovsson, M.; Verstraelen, H.; Engstrand, L.; Brusselaers, N. The vaginal microbiota, human papillomavirus and cervical dysplasia: A systematic review and network meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 171–180. [Google Scholar]
- Superti, F.; De Seta, F. Warding Off Recurrent Yeast and Bacterial Vaginal Infections: Lactoferrin and Lactobacilli. Microorganisms 2020, 8, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramanick, R.; Mayadeo, N.; Warke, H.; Begum, S.; Aich, P.; Aranha, C. Vaginal microbiota of asymptomatic bacterial vaginosis and vulvovaginal candidiasis: Are they different from normal microbiota? Microb. Pathog. 2019, 134, 103599. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Z.; Zhang, X.; Chen, X.; Wang, S. Local probiotic Lactobacillus crispatus and Lactobacillus delbrueckii exhibit strong antifungal effects against vulvovaginal candidiasis in a rat model. Front. Microbiol. 2019, 10, 1033. [Google Scholar] [CrossRef]
- Jang, S.J.; Lee, K.; Kwon, B.; You, H.J.; Ko, G. Vaginal lactobacilli inhibit growth and hyphae formation of Candida albicans. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Koedooder, R.; Singer, M.; Schoenmakers, S.; Savelkoul, P.H.; Morré, S.A.; de Jonge, J.D.; Poort, L.; Cuypers, W.-J.S.; Beckers, N.; Broekmans, F. The vaginal microbiome as a predictor for outcome of in vitro fertilization with or without intracytoplasmic sperm injection: A prospective study. Hum. Reprod. 2019, 34, 1042–1054. [Google Scholar] [CrossRef]
- Vergaro, P.; Tiscornia, G.; Barragán, M.; García, D.; Rodriguez, A.; Santaló, J.; Vassena, R. Vaginal microbiota profile at the time of embryo transfer does not affect live birth rate in IVF cycles with donated oocytes. Reprod. Biomed. Online 2019, 38, 883–891. [Google Scholar] [CrossRef]
- Hoang, T.; Toler, E.; DeLong, K.; Mafunda, N.A.; Bloom, S.M.; Zierden, H.C.; Moench, T.R.; Coleman, J.S.; Hanes, J.; Kwon, D.S. The cervicovaginal mucus barrier to HIV-1 is diminished in bacterial vaginosis. PLoS Pathog. 2020, 16, e1008236. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.S.M.; Lourenço, M.L.M.C.; Vasconcelos, B.M.; Carneiro, V.A. Probiotics Lactobacillus strains: A promising alternative therapy against to biofilm-forming enteropathogenic bacteria? Biology 2019, 13, 544–551. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Oliveira, W.P.; Burgos-Díaz, C.; Rubilar, M.; Shene, C. Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiol. 2019, 65, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, M.; Bukhari, S.M.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.; Shah, S.S. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A. Microbes as a tool to defend against antibiotic resistance in food animal production. Indian J. Anim. Health. 2019, 58, 01–18. [Google Scholar] [CrossRef]
- Cai, R.; Wang, Z.; Wang, G.; Zhang, H.; Cheng, M.; Guo, Z.; Ji, Y.; Xi, H.; Wang, X.; Xue, Y. Biological properties and genomics analysis of vB_KpnS_GH-K3, a Klebsiella phage with a putative depolymerase-like protein. Virus Genes. 2019, 55, 696–706. [Google Scholar] [CrossRef]
- Singh, S.B.; Young, K.; Silver, L.L. Silver, What is an “ideal” antibiotic? Discovery challenges and path forward. Biochem. Pharmacol. 2017, 133, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe. 2019, 25, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Amarillas, L.; Rubí-Rangel, L.; Chaidez, C.; González-Robles, A.; Lightbourn-Rojas, L.; León-Félix, J. Isolation and characterization of phiLLS, a novel phage with potential biocontrol agent against multidrug-resistant Escherichia coli. Front. Microbiol. 2017, 8, 1355. [Google Scholar] [CrossRef]
- Wang, L.; Qu, K.; Li, X.; Cao, Z.; Wang, X.; Li, Z.; Song, Y.; Xu, Y. Use of bacteriophages to control Escherichia coli O157: H7 in domestic ruminants, meat products, and fruits and vegetables. Foodborne Pathog. Dis. 2017, 14, 483–493. [Google Scholar] [CrossRef]
- Cao, F.; Wang, X.; Wang, L.; Li, Z.; Che, J.; Wang, L.; Li, X.; Cao, Z.; Zhang, J.; Jin, L. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Aldoori, A.A.; Mahdii, E.F.; Abbas, A.K.; Jassim, S.A. Bacteriophage biocontrol rescues mice bacteremic of clinically isolated mastitis from dairy cows associated with methicillin-resistant Staphyloccocus aureus. Adv. Microbiol. 2015, 5, 383. [Google Scholar] [CrossRef] [Green Version]
- Biswas, B.; Adhya, S.; Washart, P.; Paul, B.; Trostel, A.N.; Powell, B.; Carlton, R.; Merril, C.R. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 2002, 70, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents. 2019, 53, 16–21. [Google Scholar] [CrossRef] [PubMed]
- El Haddad, L.; Harb, C.P.; Gebara, M.A.; Stibich, M.A.; Chemaly, R.F. Systematic and critical review of bacteriophage therapy against multidrug-Resistant eskape organisms in humans. Clin. Infect. Dis. 2019, 69, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bryan, D.; El-Shibiny, A.; Hobbs, Z.; Porter, J.; Kutter, E.M. Bacteriophage T4 infection of stationary phase E. coli: Life after log from a phage perspective. Front. Microbiol. 2016, 7, 1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, G.-Y.; Park, D.W.; Lee, Y.-D.; Park, J.-H. Isolation and characterization of bacteriophages for the control of Shiga Toxin-producing E. coli. Korean J. Food Sci.Technol. 2018, 50, 594–600. [Google Scholar]
- Domingo-Calap, P.; Georgel, P.; Bahram, S. Back to the future: Bacteriophages as promising therapeutic tools. Hla 2016, 87, 133–140. [Google Scholar] [CrossRef]
- Song, J.; Xia, F.; Jiang, H.; Li, X.; Hu, L.; Gong, P.; Lei, L.; Feng, X.; Sun, C.; Gu, J. Identification and characterization of HolGH15: The holin of Staphylococcus aureus bacteriophage GH15. J. Gen. Virol. 2016, 97, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 31. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appiah, M.O.; Wang, J.; Lu, W. Microflora in the Reproductive Tract of Cattle: A Review. Agriculture 2020, 10, 232. https://doi.org/10.3390/agriculture10060232
Appiah MO, Wang J, Lu W. Microflora in the Reproductive Tract of Cattle: A Review. Agriculture. 2020; 10(6):232. https://doi.org/10.3390/agriculture10060232
Chicago/Turabian StyleAppiah, Michael Osei, Jun Wang, and Wenfa Lu. 2020. "Microflora in the Reproductive Tract of Cattle: A Review" Agriculture 10, no. 6: 232. https://doi.org/10.3390/agriculture10060232
APA StyleAppiah, M. O., Wang, J., & Lu, W. (2020). Microflora in the Reproductive Tract of Cattle: A Review. Agriculture, 10(6), 232. https://doi.org/10.3390/agriculture10060232




