Evaluation of the Weed Infestation, Grain Health, and Productivity Parameters of Two Spelt Wheat Cultivars Depending on Crop Protection Intensification and Seeding Densities
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of the Experiment—Soil and Climatic Conditions
2.2. Experimental Design and Agronomic Practices
- pro-ecological:
- 2.
- chemical:
- optimum—130 kg ha−1 of cv. ”Rokosz” seeds and 200 kg ha−1 of cv. “Schwabenspelz” spikelets;
- increased—200 kg ha−1 of cv. “Rokosz” seeds and 350 kg ha−1 of cv. “Schwabenspelz” spikelets.
2.3. Evaluation of Yield and Weed Infestation
2.4. Determination of Fungal Contamination of Grain
2.5. Determination of Mycotoxin Contamination of Grain
2.5.1. Sample Preparation and Extraction
2.5.2. HPLC Analysis Conditions
2.5.3. GC/MS Analysis Conditions
2.5.4. Chemical Reagents
2.6. Statistical Analysis
3. Results
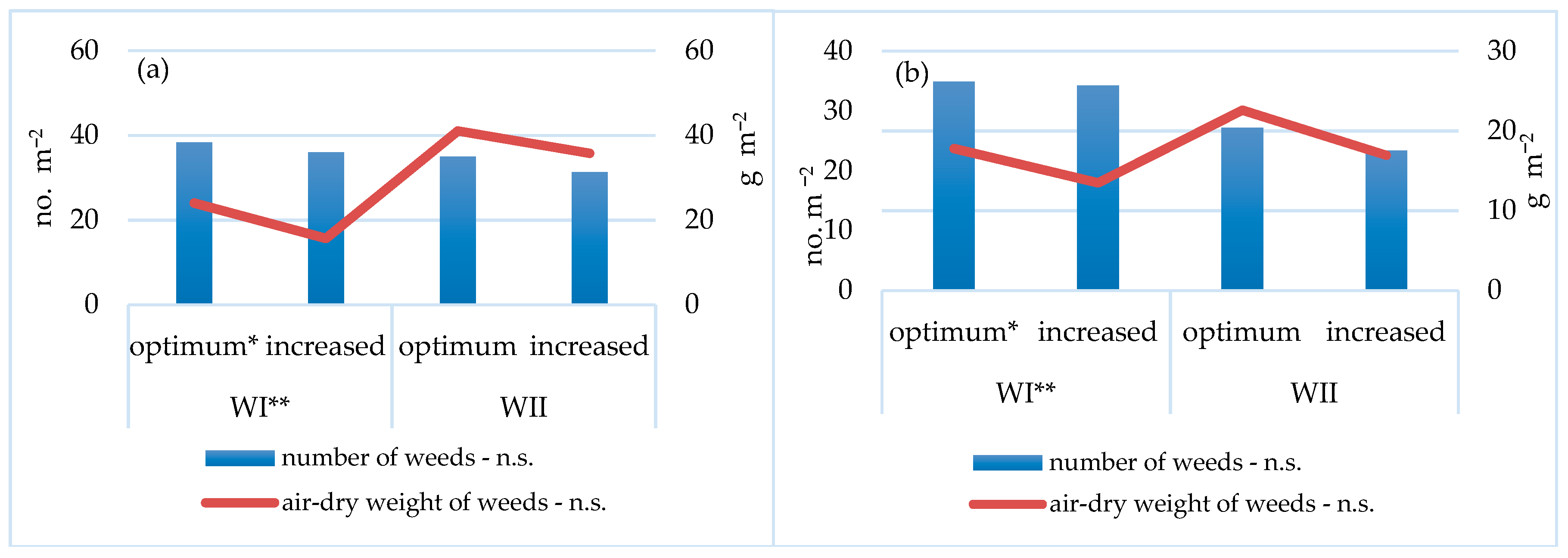
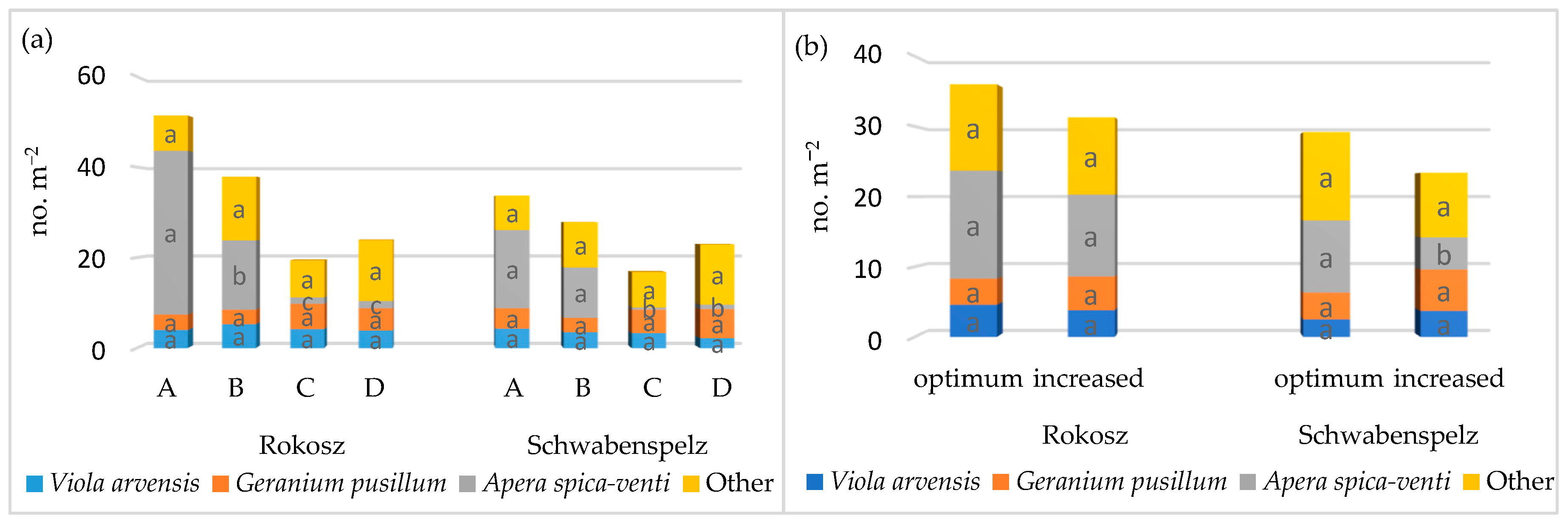
3.1. Weed Infestation of Spelt Wheat
3.2. Yield and Yield Components of Spelt Wheat
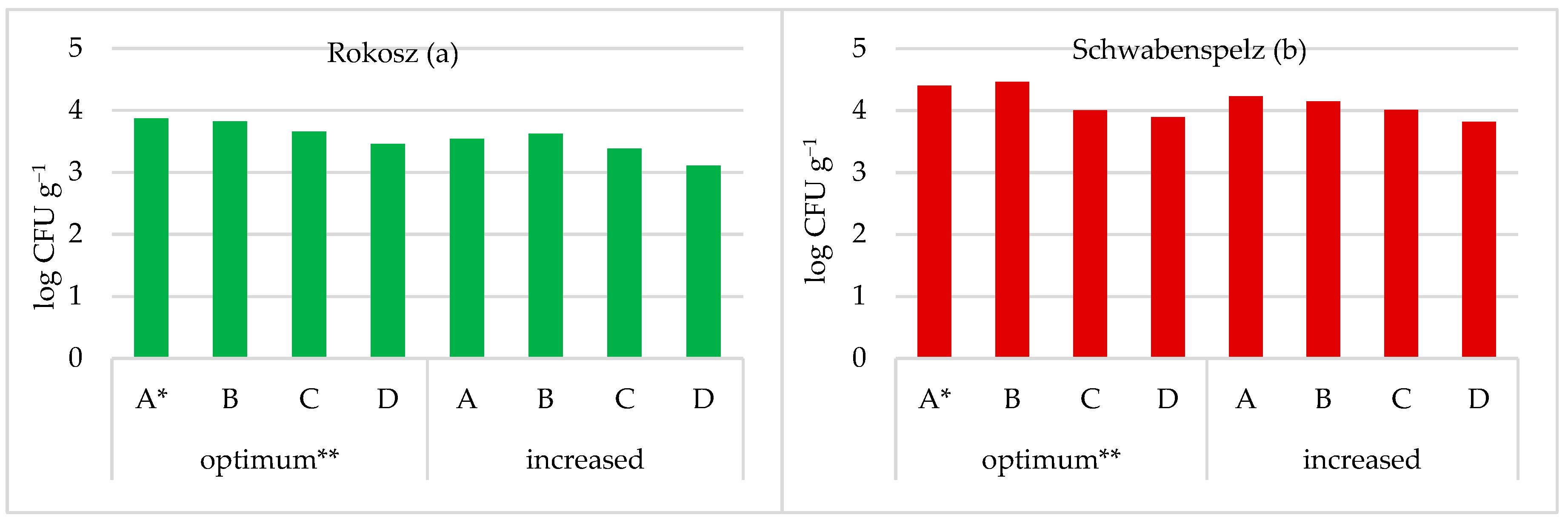
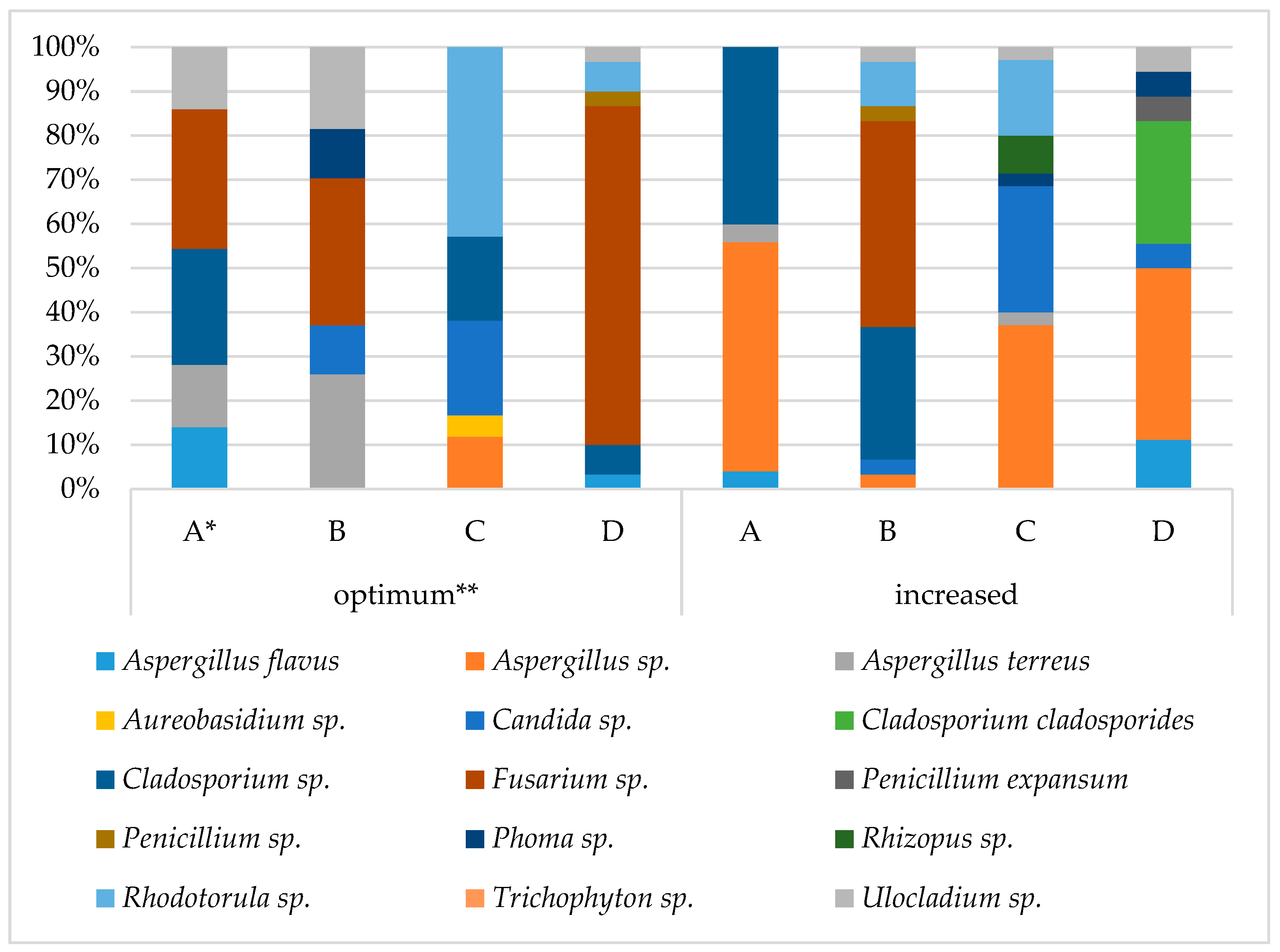
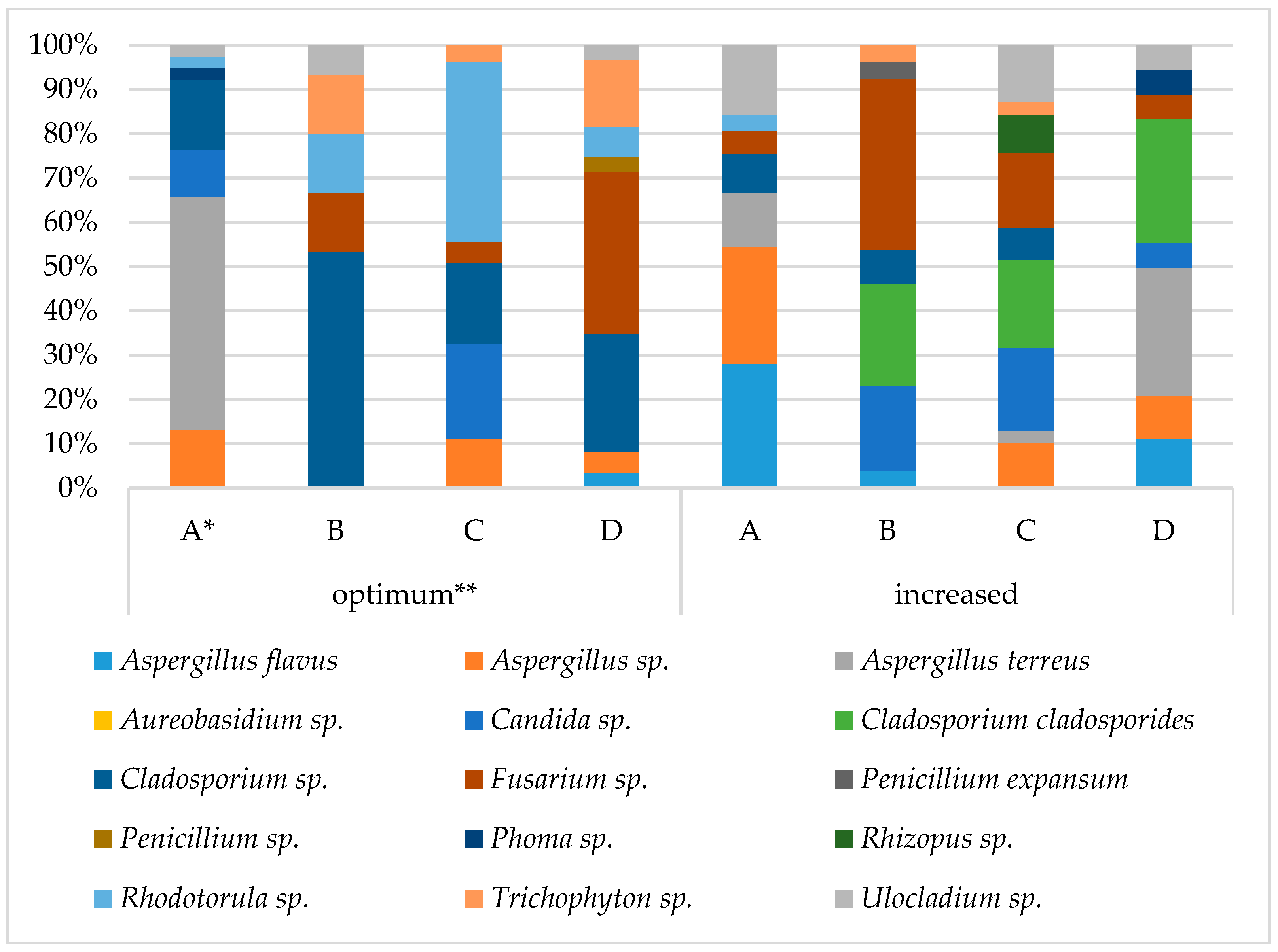
3.3. Grain Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Szumiło, G.; Rachoń, L. Plonowanie i jakość ziarna jarej pszenicy orkisz w zależności od zastosowanego materiału siewnego. Pol. J. Agron. 2015, 20, 9–14. (in Polish). [Google Scholar]
- Stankowski, S.; Pużyński, S.; Sobolewska, M.; Biel, W. Effect of weed control and swing rate on the baking quality of spelt in comparison with common wheat. Bulg. J. Agric. Sci. 2016, 22, 604–610. [Google Scholar]
- Moudrý, J.; Konvalina, P.; Stehno, Z.; Capouchová, I.; Moudrý, J., Jr. Ancient wheat species can extendbiodiversity of cultivated crops. Sci. Res. Essays 2011, 6, 4273–4280. [Google Scholar]
- Andruszczak, S. Spelt wheat grain yield and nutritional value response to sowing rate and nitrogen fertilization. J. Anim. Plant Sci. 2018, 28, 1476–1484. [Google Scholar]
- Kohajdová, Z.; Karovicová, J. Nutritional value and banking applications of pelt wheat. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–14. [Google Scholar]
- Biel, W.; Jaroszewska, A.; Stankowski, S.; Sadkiewicz, J.; Bośko, P. Effects of genotype and weed control on the nutrient composition of Winter spelt (Triticum aestivum ssp. spelta) and common wheat (Triticum aestivum ssp. vulgare). Acta Agric. Scand. B—S. P. 2016, 66, 27–35. [Google Scholar]
- Konvalina, P.; Capouchova, I.; Stehno, Z.; Moudry, J. Agronomic characteristics of the spring forms of the wheat landraces (einkorn, emmer, spelt, intermediate bread wheat) grown in organic farming. J. Agrobiol. 2010, 27, 9–17. [Google Scholar] [CrossRef]
- Vučković, J.; Bodroža-Solarov, M.; Vujić, D.; Bočarov-Stančić, A.; Bagi, F. The protective effect of hulls on the occurrence of Alternaria mycotoxins in spelt wheat. J. Sci. Food Agric. 2013, 93, 1996–2001. [Google Scholar] [CrossRef]
- Kwiatkowski, C.A.; Wesołowski, M.; Pałys, E.; Kraska, P.; Haliniarz, M.; Nowak, A.; Andruszczak, S.; Kwiecińska-Poppe, E. Aspekty proekologicznego gospodarowania w agroekosystemach. Wyd. Perfekta 2014, 169. (in Polish). [Google Scholar]
- Biel, W.; Stankowski, S.; Sobolewska, M.; Radkiewicz, J.; Jaroszewska, A.; Pużyński, S. Effect of selected agronomic factors on the banking quality of winter spelt strains and cultivars (Triticum aestivum ssp. spelta) in comparison with common wheat (Triticum aestivum ssp. vulgare). Rom. Agric. Res. 2016, 33, 1–8. [Google Scholar]
- Petrenko, V.; Spychaj, R.; Prysiazhniuk, O.; Sheiko, T.; Khudolii, L. Evaluation of three wheat species (Triticum aestivum L., T. spelta L., T. dicoccum (Schrank) Schuebl) commonly used in organic cropping systems, considering selected parameters of technological quality. Rom. Agric. Res. 2018, 35, 255–264. [Google Scholar]
- Sulewska, H.; Koziara, W.; Panasiewicz, K.; Ptaszyńska, G. Plonowanie dwóch odmian ozimych orkiszu pszennego w zależności od terminu i ilości wysiewu w warunkach Środkowej Wielkopolski. J. Agric. Eng. Res. 2008, 53, 85–91. [Google Scholar]
- Litwińczuk, M. Przyszłość orkiszu na Zamojszczyźnie. Available online: https://www.agropolska.pl/uprawa/zboza/przyszlosc-orkiszu-na-zamojszczyznie,209.html (accessed on 6 June 2020).
- Kraska, P.; Andruszczak, S.; Kwiecińska-Poppe, E. Reaction of spelt wheat cultivars (Triticum aestivum ssp. spelta) to foliar applications of fertilizers. Agron. Sci. 2019, 74, 37–47. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D. Comparison of phenolic acids profile and antioxidant potential of six varieties of spelt (Triticum spelta L.). J. Agric. Food Chem. 2012, 60, 4603–4612. [Google Scholar] [CrossRef] [PubMed]
- Jablonskytė-Raščė, D.; Maikštėnienė, S.; Mankevičienė, A. Evaluation of productivity and quality of common wheat (Triticum aestivum L.) and spelt (Triticum spelta L.) in relation to nutrition conditions. Zemdirbyste 2013, 100, 45–56. [Google Scholar] [CrossRef]
- Kwiatkowski, C.; Haliniarz, M.; Tomczyńska-Mleko, M.; Mleko, S.; Kawecka-Radomska, M. The content of dietary fiber, amino acids, dihydroxyphenols and some macro- and micronutrients in grain of conventionally and organically grown common wheat, spelt wheat and proso millet. Agr. Food Sci. 2015, 24, 195–205. [Google Scholar] [CrossRef]
- Kraska, P.; Andruszczak, S.; Dziki, D.; Stocki, M.; Stocka, N.; Kwicińska-Poppe, E.; Różyło, K.; Gierasimiuk, P. Green grain of spelt (Triticum aestivum ssp. spelta) harvested at the stage of milk-dough as a rich source of valuable nutrients. Emir. J. Food Agric. 2019, 31, 263–270. [Google Scholar]
- Sliesaravičius, A.; Pekarskas, J.; Rutkovienė, V.; Baranauskis, K. Grain yield and disease resistance of winter cereal varieties and application of biological agent in organic agriculture. Agron. Res. 2006, 4, 371–378. [Google Scholar]
- Schober, T.J.; Beana, S.R.; Kuhn, M. Gluten proteins from spelt (Triticum aestivum ssp. spelta) cultivars: Arheological and size-exclusion high-performance liquid chromatography study. J. Cereal Sci. 2006, 44, 161–173. [Google Scholar] [CrossRef]
- Glamočlija, D.; Žarković, B.; Dražić, S.; Radovanović, V.; Popović, V.; Urgenović, V. Morphological and productive characteristics spelt wheat on the chernozem and degraded soil. In Proceedings of XXVII Conference of Agronomists, Veterinarians, Technologists and Agricultural Economists, Belgrade, Serbia, 20–21 February 2013; Simić, D., Beskorovajni, R., Eds.; Institut PKB Agroekonomik: Belgrade, Serbia, 2013; pp. 23–31. [Google Scholar]
- Ugrenović, V.; Bodroža Solarov, M.; Pezo, L.; Disalov, J.; Popović, V.; Marić, B.; Filipović, V. Analysis of spelt variability (Triticum spelta L.) grown in different conditions of Serbia by organic conditions. Genetika 2018, 50, 635–646. [Google Scholar] [CrossRef]
- Mankevičienė, A.; Jablonskytė-Raščė, D.; Maikštėnienė, S. Occurrence of mycotoxins in spelt and common wheat grain and their products. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Feledyn-Szewczyk, B. Porównanie zdolności konkurencyjnych w stosunku do chwastów oraz plonów ziarna pszenicy orkisz (Triticum aestivum ssp. spelta) z odmianami pszenicy zwyczajnej (Triticum aestivum ssp. vulgare) w ekologicznym systemie produkcji. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2012, 293, 13–26. (in Polish). [Google Scholar]
- Babenko, L.M.; Hospodarenko, H.M.; Rozhkov, R.V.; Pariy, Y.F.; Pariy, M.F.; Babenko, A.V.; Kosakivska, I.V. Triticum spelta: Origin, biological characteristics and perspectives for use in breeding and agriculture. Regul. Mech. Biosyst. 2018, 9, 250–257. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Würschum, T. Genetic variability, heritability and correlation among agronomic and disease resistance traits in a diversity panel and elite breeding material of spelt wheat. Plant. Breed. 2014, 133, 459–464. [Google Scholar] [CrossRef]
- Korkhova, M.; Panfilova, A.; Chernova, A.; Rozhok, O. The effect of pre-sowing seed treatment with biopreparations on productivity of cultivars of Triticum spelta L. Agrolife Sci. J. 2019, 8, 120–127. [Google Scholar]
- Wojtkowiak, K.; Stępień, A. Nutritive value of spelt (Triticum aestivum spp. spelta L.) as influenced by the foliar application of copper, zinc and manganese. Zemdirbyste 2015, 102, 389–396. [Google Scholar] [CrossRef][Green Version]
- Wiwart, M.; Szafranska, A.; Wachowska, U.; Suchowilska, E. Quality parameters and rheological dough properties of 15 Spelt (Triticum spelta L.) varieties cultivated today. Cereal Chem. 2017, 94, 1037–1044. [Google Scholar]
- Zorovski, P.; Popov, V.; Georgieva, T. Growth and development of Triticum monococcum L., Triticum dicoccum Sch. and Triticum spelta L. in organic farming conditions. Contemp. Agric. Serb. J. Agric. Sci. 2018, 67, 45–50. [Google Scholar] [CrossRef]
- Rachoń, L.; Szumiło, G.; Nita, Z. Plonowanie ozimych rodów Triticum durum i Triticum aestivum ssp. spelta w warunkach okolic Lublina. Agron. Sci. 2020, 64, 101–109. (in Polish). [Google Scholar]
- Andruszczak, S.; Kwiecińska-Poppe, E.; Kraska, P.; Pałys, E. Yield of winter cultivars of spelt wheat (Triticum aestivum ssp. spelta L.) cultivated under diversified conditions of mineral fertilization and chemical protection. Acta Sci. Pol. Agric. 2011, 10, 5–14. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering plants and pteridophytes of Poland a checklist. Krytyczna lista roślin naczyniowych Polski; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; Volume 1, p. 442. [Google Scholar]
- PN-ISO 21527–2: “ Mikrobiologia żywności i Pasz. Horyzontalna Metoda Oznaczania Liczby Drożdży i pleśni”; Polish Committee for Standardization: Warsaw, Poland, 2009.
- Woźniak, A.; Nowakowicz-Dębek, B.; Stępniowska, A.; Wlazło, Ł. Effect of ozonation on microbiological and chemical traits of wheat grain Plant. Soil Environ. 2016, 62, 552–557. [Google Scholar] [CrossRef]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi; CRS Press LLC.: Boca Raton, FL, USA, 2002; p. 486. [Google Scholar]
- Valle-Algarra, F.M.; Medina, A.; Gimeno-Adelantado, J.V.; Llorens, A.; Jimenez, M.; Mateo, R. Comparative assessment of solid-phase extraction clean-up procedures, GC columns and perfluoroacylation reagents for determination of type B trichothecenes in wheat by GCECD. Talanta 2005, 66, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Beres, B.L.; Harker, K.N.; Clayton, G.W.; Bremer, E.; Blackshaw, R.E.; Graf, R.J. Weed-competitive ability of spring and winter cereals in the Northern Great Plains. Weed Tech. 2010, 24, 108–116. [Google Scholar] [CrossRef]
- Andruszczak, S.; Kraska, P.; Kwiecińska-Poppe, E.; Pałys, E. Weed infestation of crops of winter spelt wheat (Triticum aestivum ssp. spelta) cultivars grown under different conditions of mineral fertilization and chemical plant protection. Acta Agrobot. 2012, 65, 109–118. [Google Scholar] [CrossRef][Green Version]
- Andruszczak, S.; Kraska, P.; Kwiecińska-Poppe, E.; Pałys, E. The effect of tillage system and herbicide application on weed infestation of crops of winter spelt wheat (Triticum aestivum ssp. spelta L.) cultivars. Acta Agrobot. 2013, 66, 173–184. [Google Scholar] [CrossRef]
- Korres, N.E.; Froud-Williams, R.J. Effect of winter wheat cultivars and seed rate on the biological characteristics of naturally occurring weed flora. Weed Res. 2002, 42, 417–428. [Google Scholar] [CrossRef]
- Mason, H.E.; Spaner, D. Competitive ability of wheat in conventional and organic management systems: A review of the literature. Can. J. Plant. Sci. 2006, 86, 333–343. [Google Scholar] [CrossRef]
- Andruszczak, S. Reaction of winter spelt cultivars to reduced tillage system and chemical plant protection. Zemdirbyste 2017, 104, 15–22. [Google Scholar] [CrossRef]
- Kurstjens, D.A.G.; Kropff, A.M. The impact of uprooting and soil-covering on the effectiveness of weed harrowing. Weed Res. 2001, 41, 211–228. [Google Scholar] [CrossRef]
- Kapeluszny, J.; Dyńska, M.; Haliniarz, M. Wpływ sposobów pielęgnacji na zachwaszczenie dwóch linii pszenicy twardej (Triticum durum Desf.). Prog. Plant Prot. 2012, 52, 287–293. [Google Scholar]
- Hansen, P.K.; Rasmussen, I.A.; Holst, N.; Candreasen, C. Tolerance of four spring barley (Hordeum vulgare) varieties to weed harrowing. Weed Res. 2007, 47, 241–251. [Google Scholar] [CrossRef]
- Lundkvist, A. Effects of pre- and post-emergence weed harrowing on annual weeds in peas and spring cereals. Weed Res. 2009, 49, 409–416. [Google Scholar] [CrossRef]
- Pospišil, A.; Pospišil, M.; Svečnjak, Z.; Matotan, S. Influence of crop management upon the agronomic traits of spelt (Triticum spelta L.). Plant Soil Environ. 2011, 57, 435–440. [Google Scholar] [CrossRef]
- Pospišil, A.; Pospišil, M.; Brčić, M. Influence of seeding rate and nitrogen topdressing upon the agronomic traits of spelt (Triticum spelta L.). Rom. Agric. Res. 2016, 33, 235–240. [Google Scholar]
- Bankina, B.; Bimšteine, G.; Neusa-Luca, I.; Roga, A.; Fridmanis, D. What influences the composition of fungi in wheat grains? Acta Agrobot. 2017, 70, 17–26. [Google Scholar] [CrossRef][Green Version]
- Karron, E.; Runno-Paurson, E.; Lõiveke, H.; Islamov, B.; Kütt, M.L.; Talve, T.; Lauringson, E.; Hõrak, H.; Edesi, L.; Niinemets, Ü. Application of widely used fungicides does not necessarily affect grain yield, and incidence of Fusarium spp. and mycotoxins DON, HT-2 and T-2 in spring barley in northern climates. Kvas. prumys 2020, 66, 215–223. [Google Scholar] [CrossRef]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurence of mycotoxins in commodieties, feeds and feeds ingredients. Anim. Feed Sci. Tech. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Sultan, Y.; Magan, N. Mycotoxigenic fungi in peanuts from different geographic regions of Egypt. Mycotox. Res. 2010, 26, 133–140. [Google Scholar] [CrossRef]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur J. Plant. Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Orlando, B.; Grignon, G.; Vitry, C.; Kashefifard, K.; Valade, R. Fusarium species and enniatin mycotoxins in wheat, durum wheat, triticale and barley harvested in France. Mycotoxin Res. 2019, 35, 369–380. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Rämo, S.; Hussien, T.; Carlobos-Lopez, A.L.; Cumagun, C.J.R. Molecular quantification and genetic diversity of toxigenic Fusarium species in northern Europe as compared with those in southern Europe. Microorganisms 2013, 1, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.B.; Font, G.; Ruiz, M.J.; Ferrer, E. Co-occurrence and risk assessment of mycotoxins in food and diet from Mediterranean area. Food Chem. 2012, 135, 423–429. [Google Scholar] [CrossRef]
- Wiwart, M.; Perkowski, J.; Jackowiak, H.; Packa, D.; Borusiewicz, A.; Buoeko, M. Response of some cultivars of spring spelt (Triticum spelta) to Fusarium culmorum infection. Die Bodenkultur 2004, 55, 29–36. [Google Scholar]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef]
- Beres, B.L.; Brûlé-Babel, A.L.; Ye, Z.; Graf, R.J.; Turkington, T.K.; Harding, M.W.; Kutcher, H.R.; Hooker, D.C. Exploring Genotype × Environment × Management synergies to manage Fusarium head blight in wheat. Can. J. Plant. Pathol. 2018, 40, 179–188. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.E.; Løes, A.K.; Kristoffersen, A.B. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. 2012, 29, 1129–1140. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Vanara, F. Effect of plant density on toxigenic fungal infection and mycotoxin contamination of maize kernels. Field Crop. Res. 2008, 106, 234–241. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Colombari, G.; Pietri, A. Comparison of integrated field programmes for the reduction of fumonisin contamination in maize kernels. Field Crop. Res. 2009, 111, 284–289. [Google Scholar] [CrossRef]
- Edwards, S.G. Influence of agricultural practices on Fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Romani, M.; Rastelli, S.; Giorni, P. Mycotoxins and Related Fungi in Italian Paddy Rice During the Growing Season and Storage. Toxins 2019, 11, 151. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium head blight incidence and mycotoxin accumulation in three durum wheat cultivars in relation to sowing date and density. Sci. Nat. 2018, 105, 2. [Google Scholar] [CrossRef] [PubMed]
- van der Burgt, G.J.H.M.; Timmermans, B.G.H.; Scholberg, J.M.S.; Osman, A.M. Fusarium head blight and deoxynivalenol contamination in wheat as affected by nitrogen fertilization. NJAS—Wagen. J. Life Sci. 2011, 58, 123–129. [Google Scholar] [CrossRef]
- Bérubé, M.E.; Vanasse, A.; Rioux, S.; Bourget, N.; Dion, Y.; Tremblay, G. Effect of glyphosate on Fusarium head blight in wheat and barley under different soil tillages. Plant. Dis. 2012, 96, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EC) No 1126/2007. Polish Nutritional Standards 2014. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/PDF/?uri=CELEX:32007R1126&from=EN (accessed on 17 April 2020).

| Years | Months | September – August | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| September | October | November | December | January | February | March | April | May | June | July | August | ||
| Rainfalls (mm) | Sum | ||||||||||||
| 2012/ 2013 | 40.4 | 110.7 | 29 | 17.4 | 60.3 | 30 | 37.6 | 53.2 | 103.3 | 108.3 | 44.3 | 26.6 | 661.1 |
| 2013/ 2014 | 49.5 | 7.3 | 60.6 | 13.7 | 54.5 | 5.8 | 49.1 | 63.9 | 230.2 | 110.2 | 61.4 | 102 | 808.2 |
| 2014/ 2015 | 21.8 | 27.5 | 24.1 | 57.8 | 50.9 | 15.8 | 48.6 | 39.1 | 169.6 | 13.5 | 52.6 | 5.9 | 527.2 |
| LTA 1963–2010 | 59.5 | 45.6 | 41 | 36.9 | 30.3 | 29.2 | 31.3 | 42.4 | 63.5 | 72.7 | 80 | 69.5 | 601.9 |
| Temperature (°C) | Mean | ||||||||||||
| 2012/ 2013 | 14.6 | 7.7 | 5 | −3.4 | −4.4 | −1.3 | −2.6 | 7.4 | 14.9 | 18.1 | 18.7 | 18.7 | 7.8 |
| 2013/ 2014 | 11.3 | 9.5 | 4.9 | 1.7 | −2.9 | 0.3 | 4.9 | 8.9 | 13 | 15.2 | 19.6 | 18.3 | 8.7 |
| 2014/ 2015 | 14.0 | 9.7 | 4.6 | −0.1 | 1.0 | −1.1 | 2.8 | 6.5 | 11.5 | 16.1 | 19 | 21.9 | 8.8 |
| LTA 1963–2010 | 13.1 | 7.9 | 2.9 | −1.3 | −3.0 | −1.7 | 1.8 | 7.7 | 13.6 | 16.5 | 18.3 | 17.7 | 7.8 |
| Years | Crop Protection | NW I (no. m−2) | WW I (g m−2) | NW II (no. m−2) | WW II (g m−2) |
|---|---|---|---|---|---|
| 2013 | A | 90.8 a | 68.66 a | 86.3 a | 158.06 a |
| B | 78.7 ab | 62.12 a | 58.3 a | 119.1 ab | |
| C | 27.2 c | 5.68 b | 18 a | 21.7 c | |
| D | 28.5 c | 8.18 b | 21.2 a | 25.33 c | |
| 2014 | A | 53 abc | 25.4 b | 56 a | 39.39 bc |
| B | 37.7 bc | 13.71 b | 42 a | 19.37 c | |
| C | 28 c | 7.79 b | 32.3 a | 13.17 c | |
| D | 31.3 bc | 6.51 b | 42 a | 19.87 c | |
| 2015 | A | 21.3c | 10.99b | 12 a | 13.13 c |
| B | 22.7c | 13.6b | 13.3 a | 17.25 c | |
| C | 13.3c | 7.02b | 7.8 a | 5.09 c | |
| D | 13.3c | 8.31b | 8.7 a | 9.11 c | |
| Comparison of the pro-ecological and chemical crop protection (mean for 2013–2015) | |||||
| pro-ecological (A, B) | 50,7 a | 32.41 a | 44.7 a | 61.05 a | |
| chemical (C, D) | 23,6 b | 7.25 b | 21.7 b | 15.71 b | |
| Crop Protection | NW I (no. m−2) | WW I (g m−2) | NW II (no. m−2) | WW II (g m−2) |
|---|---|---|---|---|
| A | 44.3 a | 26.35 a | 33.7 a | 32.58 a |
| B | 43.8 ab | 21.95 a | 27.8 a | 25.22 a |
| C | 26.2 bc | 6.21 b | 16.8 a | 8.42 a |
| D | 24.2 c | 8.05 b | 22.9 a | 12.89 a |
| Comparison of the pro-ecological and chemical crop protection | ||||
| pro-ecological (A, B) | 44 a | 24.15 a | 30.7 a | 28.9 a |
| chemical (C, D) | 25.2 b | 7.13 b | 19.9 a | 10.66 a |
| Crop Protection | HP (cm) | LE (cm) | NE (no. m−2) | WG (g) |
|---|---|---|---|---|
| A | 106a | 8.3a | 417a | 1.1a |
| B | 104.2a | 8.4a | 421a | 1.15a |
| C | 105.5a | 8.6a | 413a | 1.15a |
| D | 99.9a | 8.5a | 459a | 1.33a |
| Comparison of the pro-ecological and chemical crop protection | ||||
| pro-ecological (A, B) | 105.1a | 8.4a | 419a | 1.13a |
| chemical (C, D) | 102.7a | 8.5a | 436a | 1.24a |
| Crop Protection | 2013 | 2014 | 2015 |
|---|---|---|---|
| t ha−1 | |||
| A | 2.43 d | 3.23 cd | 5.62 ab |
| B | 2.73 d | 3.31 cd | 5.66 ab |
| C | 3.57 cd | 3.19 cd | 5.73 a |
| D | 4.7 abc | 4.05 bcd | 6.1 a |
| Comparison of the pro-ecological and chemical crop protection (mean for 2013–2015) | |||
| pro-ecological (A, B) | 3.83 a | ||
| chemical (C, D) | 4.56 b | ||
| Crop Protection | HP (cm) | LE (cm) | NE (no. m−2) | WG (g) | Y (t ha−1) |
|---|---|---|---|---|---|
| A | 127.9 ab | 11.5 a | 357 a | 1.03 a | 3.12 a |
| B | 131.4 a | 11.5 a | 353 a | 1.14 a | 3.02 a |
| C | 128.9 ab | 11.2 a | 372 a | 1.09 a | 3.16 a |
| D | 119.3 b | 11.4 a | 389 a | 1.13 a | 3.56 a |
| Comparison of the pro-ecological and chemical crop protection | |||||
| pro-ecological (A, B) | 129.6 a | 11.5 a | 355 a | 1.09 a | 3.07 a |
| chemical (C, D) | 124.1 a | 11.3 a | 381 a | 1.11 a | 3.36 a |
| Seeding Density | HP (cm) | LE (cm) | NE (no. m−2) | WG (g) | Y (t ha−1) |
|---|---|---|---|---|---|
| cv. “Rokosz” | |||||
| optimum | 105.7 a | 8.7 a | 412 a | 1.22 a | 4.25 a |
| increased | 102.2 a | 8.3 b | 443 a | 1.15 a | 4.14 a |
| cv. “Schwabenspelz” | |||||
| optimum | 125.9 a | 11.3 a | 350 a | 1.11 a | 3.24 a |
| increased | 127.9 a | 11.5 a | 386 a | 1.09 a | 3.25 a |
| Experimental Factors | NIV | DAS | 15-AcDON | T-2 | DON | ZEA | HT-2 Toxin | 3-AcDON | Fuzarenon X | Sum |
|---|---|---|---|---|---|---|---|---|---|---|
| Crop protection | ||||||||||
| A | 243b | 167b | 78b | 13a | 203a | 6b | 5c | 100b | 51c | 866b |
| B | 277b | 216a | 145a | 11a | 89b | 51a | 49b | 47c | 24c | 909b |
| C | 317a | 69c | 126a | 20a | 75b | 7b | 154a | 299a | 205a | 1272a |
| D | 16c | 0.5d | 75b | - | 11c | - | - | 295a | 150b | 548c |
| Seeding density | ||||||||||
| optimum | 345a | 87b | 132a | 6a | 141a | 9a | 100a | 193a | 99a | 1111a |
| increased | 82b | 139a | 79b | 17a | 48b | 23a | 4b | 177a | 117a | 685b |
| Experimental Factors | NIV | DAS | 15-AcDON | T-2 | DON | ZEA | HI-2 Toxin | 3-AcDON | Fuzarenon X | Sum |
|---|---|---|---|---|---|---|---|---|---|---|
| Crop protection | ||||||||||
| A | 227a | 47a | 135a | 31 | 6b | - | - | 259a | 132a | 836a |
| B | 245a | 61a | 61b | - | 54a | - | 27a | 176b | 90c | 712a |
| C | 221a | 60a | 110a | - | 14a | - | 11a | 206ab | 107bc | 727a |
| D | 39b | 5b | 110a | - | 11a | - | - | 165b | 128ab | 457b |
| Seeding density | ||||||||||
| optimum | 258a | 73a | 110a | 16 | 16a | - | 5a | 264a | 163a | 904a |
| increased | 108b | 13b | 98a | - | 26a | - | 14b | 139b | 65b | 462b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haliniarz, M.; Gawęda, D.; Nowakowicz-Dębek, B.; Najda, A.; Chojnacka, S.; Łukasz, J.; Wlazło, Ł.; Różańska-Boczula, M. Evaluation of the Weed Infestation, Grain Health, and Productivity Parameters of Two Spelt Wheat Cultivars Depending on Crop Protection Intensification and Seeding Densities. Agriculture 2020, 10, 229. https://doi.org/10.3390/agriculture10060229
Haliniarz M, Gawęda D, Nowakowicz-Dębek B, Najda A, Chojnacka S, Łukasz J, Wlazło Ł, Różańska-Boczula M. Evaluation of the Weed Infestation, Grain Health, and Productivity Parameters of Two Spelt Wheat Cultivars Depending on Crop Protection Intensification and Seeding Densities. Agriculture. 2020; 10(6):229. https://doi.org/10.3390/agriculture10060229
Chicago/Turabian StyleHaliniarz, Małgorzata, Dorota Gawęda, Bożena Nowakowicz-Dębek, Agnieszka Najda, Sylwia Chojnacka, Justyna Łukasz, Łukasz Wlazło, and Monika Różańska-Boczula. 2020. "Evaluation of the Weed Infestation, Grain Health, and Productivity Parameters of Two Spelt Wheat Cultivars Depending on Crop Protection Intensification and Seeding Densities" Agriculture 10, no. 6: 229. https://doi.org/10.3390/agriculture10060229
APA StyleHaliniarz, M., Gawęda, D., Nowakowicz-Dębek, B., Najda, A., Chojnacka, S., Łukasz, J., Wlazło, Ł., & Różańska-Boczula, M. (2020). Evaluation of the Weed Infestation, Grain Health, and Productivity Parameters of Two Spelt Wheat Cultivars Depending on Crop Protection Intensification and Seeding Densities. Agriculture, 10(6), 229. https://doi.org/10.3390/agriculture10060229







