Abstract
The maintenance of high-quality standards for prolonging the shelf life of fruit and preserving sensory and nutritional quality is a priority for horticultural products. The aim of this work is to test the effectiveness of a single treatment of edible coating based on Aloe arborescens (EC) and a combined treatment of 1-methylcycyclopropene (1-MCP) and edible coating to prolong the shelf life of “Settembrina” white flesh peach fruit. White flesh peach fruit were harvested at the commercial ripening stage, treated with an edible coating (EC) or 1-MCP + EC or 1-MCP, and stored for 28 days at 1 °C. After 7, 14, 21, and 28 days, fruits were removed from cold storage, transferred at 20 °C and then analyzed immediately (cold out) and after 6 days (shelf life) to evaluate the combined effect of cold storage and room temperature. The fruits were tested for carotenoids content, phenolic content, reducing activity (ABTS). The physicochemical traits were measured in terms of the titratable acidity, total soluble content, weight loss, and vitamin C content. Moreover, their sensory profile was analyzed by a semi-trained panel. Fruit treated with EC and 1-MCP + EC kept their marketing values better than control after 14 days of storage and 6 days of simulated shelf life in terms of flesh firmness, total soluble solids and titratable acidity, as well as sensory parameters. After 21 days of storage, all treatments showed a deterioration of all the quality parameters. The single and combined application of Aloe-based coating (with 1-MCP) slowed down the maturation processes of the fruit, limited the weight loss, and preserved its organoleptic characteristics.
1. Introduction
Peach is a climacteric fruit that dramatically increases ethylene production during ripening [1]. White flesh melting peaches rapidly soften after commercial maturity, and are highly sensitive to chilling injuries and can be easily damaged during shelf life [2]. In recent years, several postharvest treatments have been studied to control chilling injury, including salicylic acid, jasmonic acid, 1-methylcyclopropene, heat-stock treatment, peach-gum coating, controlled atmosphere, ozone and radiation; these treatments have proven to be effective to improve fruit quality during postharvest storage [3,4,5,6,7,8,9,10,11]. Physical techniques appeared more effective of all other chemical treatments to maintain postharvest quality and aroma volatiles, since chemical agents may cause residual odor.
The development of new technologies in controlling the fruit ripening process allows us to prolong its shelf life, reducing distribution loss and supplying high-quality fruit to the market. Among the numerous factors involved, the use of cyclopropenes (1-MCP), a volatile and active compound at very low concentrations [12,13], has shown an inhibitory effect by occupying the ethylene receptors and entering into the physiological processes in which the hormone is involved [14]. Widespread use of 1-MCP has been found in climacteric fruits [15,16] and not-climacteric fruit [13]. Several studies showed that the application of 1-MCP had a high effect on respiratory rate, total soluble solids, firmness, and titratable acid content at harvest and during storage [17,18], depending on the fruit ripening stage at harvest and the time of application [14]. The effect of 1-MCP on the physiological and biochemical processes of peach ripening was studied by some authors with conflicting results: [19] highlighted that 1-MCP blocked ethylene biosynthesis, but in other paper, it appeared ineffective [20,21,22,23]. Liu et al. [24] found that 1-MCP delayed peach fruit ripening. However, Kluge and Jacomino [25], among others, suggested that the utility of 1-MCP is limited because it can delay peach ripening only when it is applied during the pre-climacteric state. Several studies implemented 1-MCP to preserve fruit quality during its storage and shelf life of yellow-flesh peach genotypes; nevertheless, few studies were carried out on white-flesh peach. We know that 1-MCP is able to delay the progression of important maturation and senescence processes, e.g., softening, aroma evolution, and color development [26], but the effect is limited to the first 2–3 days of ripening after harvest [21]. In addition to the use of 1-MCP, there are other techniques used to maintain the quality of the harvested fruit during cold storage and shelf life, e.g., edible coatings (EC). The edible coating is made of a thin layer of edible material that is laid on the surface of the fruit [27,28]. A further advantage of this methodology is the possibility to add substances that act as carriers of antimicrobial agents and slow-release substances [29,30]. Aloe is a genus known for its medicinal properties, comprising over 500 species. The pharmacological properties of Aloe were demonstrated in several studies, both in vitro and in vivo; these properties included antioxidant, antimicrobial, and anti-inflammatory activity. Aloe Arborescens Mill. is one of the most abundant species of the Aloe genus, which is a native of South Africa and has been imported into many countries as an ornamental and medicinal plant due to its proven biological effects, namely, antimicrobial, anti-inflammatory, and scab healing properties. Beyond this, A. arborescens is used for the extraction of active ingredients with cosmetic and nutraceutical interest [31].
Today, new nutraceutical foods have been developed and marketed by communicating to consumers their ability to be blessed for human health. These nutraceuticals are gaining considerable consumer interest because of the increasingly fast and stressful lifestyle. Their main benefits, in addition to providing nutritional value, are that they are functional and offer high amounts of beneficial, mostly natural, low-calorie compounds. In addition, consumers’ current concern about the amount of chemicals generally added to food and/or pharmaceutical and cosmetic products has meant that products with few additives and, when necessary, of natural type, are preferred by consumers as they are perceived as less harmful to human health [31].
Aloe gels are often used for food preservation as edible coatings. Edible coatings generally provide a thin layer on the fruit surface, which acts as a barrier to atmospheric gases. Thanks to aloe gels, respiration and transpiration of fresh produce can be reduced, and postharvest deterioration of foods is often delayed; this promotes food preservation [29]. Edible coatings are generally applied by dipping the foods, spraying, or brushing [32]. The mucilage or gel of the Aloe leaf consists of approximately 99.5% water and 0.5% of solid material that includes compounds like polysaccharides, vitamins, minerals, enzymes, phenolic compounds, and organic acids [33]. The major polysaccharides include not only cellulose and hemicellulose but also storage polysaccharides like glucomannans, mannose derivatives, and acetylated compounds. According to literature, that largely described the composition of polysaccharides in the aloe pulp, acetylated glucomannan molecules are mainly responsible for the thick, mucilage-like properties of the raw Aloe gel [34]. The use of Aloe vera gel has some advantages, such as its antimicrobial activity and ease of preparation, that make it viable for suitable for an edible coating. Moreover, other studies highlighted that Aloe coatings (Aloe arborescens and Aloe vera) reduce weight loss and ethylene production in raw peaches and plums [35]. In a recent study with fresh-cut kiwifruit, Aloe vera was proved to be effective in reducing pectin depolymerization and microbial proliferation. Furthermore, the sensorial quality of fruit was enhanced [36].
Recent studies have been carried out on the post-harvest application of Aloe gel to preserve the quality characteristics of fruits [36,37]. This coating effect has also been characterized in strawberries [38,39], apples [40], sweet cherries [41], pomegranate [42], litchi [43,44], and papaya [45].
However, detailed information on the influence of EC on quality and post-harvest behavior of white flesh melting peaches is not yet available. Indeed, in Italy, the marketability of white flesh melting peaches, with a remarkable organoleptic value [46,47,48,49,50], is hindered by their high susceptibility to post-harvest injuries. Peach genotypes in Southern Italy, which include the white-flesh “Pesca di Bivona” (Murtiddara, Bianca, Agostina, and Settembrina), are characterized by a persistent aroma and an excellent flavor that is highly appreciated by consumers.
This study was carried out in order to widen the marketability of white flesh melting peaches based on the hypothesis that the implementation of 1-MCP with EC might help to better regulate the ripening process. We evaluated the interaction between the antioxidant and antimicrobial properties of the Aloe arborescens gel EC [32,34,36,38] and the 1-MCP [4,13,14,19,37], which delays the ripening of the fruit, testing the storage at low temperatures (1 ± 0.5 °C) and then the shelf life at 20 °C for 6 days.
2. Materials and Methods
2.1. Plant Material and Treatments
The trial was carried out in a commercial orchard located in Bivona (Sicily, Italy) (37°37′ N, 13°26′ E, 503 m a.s.l.) consisting of 15-year-old trees of the local white, melting flesh peach (Prunus persica Batsch) cultivar Settembrina [48,49,50], grafted on GF677 rootstock (P. persica x P. amygdalus) and trained to a vase. Two hundred and twenty fruits were hand-picked from six trees, using the flesh firmness (6.5 ± 0.8 kg cm−2) as a maturity index to determine the ripening stage of the whole sample. The samples immediately after 1 h were sterilized with sodium hypochlorite solution (150 ppm active chlorine) and were irradiated with ultraviolet-C (UV-C) to control microbial spoilage [10,51]. Aloe arborescens gel was prepared from 1 kg of leaves taken from 10-year-old plants. The leaves were cleaned externally with a knife removing the margin and were then cut lengthwise. The parenchyma (from which the gel is obtained) was separated from the epidermis. The gelatinous parenchyma was homogenized with Ultra-Turrax (Ultra-Turax T25, Janke and Kunkle, IKa Labortechnik, Breisgau, Germany) for 5 min at 24.500 rpm, thus obtaining a mucilaginous gel, subsequently filtered to eliminate the fibrous portion. A gelling agent (Gellan 0.56% w/v) and glycerol 0.89% w/v were added to improve the viscosity and plasticity of the film. Subsequently, following further homogenization, a 90 °C/40 min heat treatment was applied to stabilize the solution from a microbiological point of view. Finally, a solution containing ascorbic acid 1% w/v was added to prevent further darkening [52] and citric acid 1% w/v to maintain the pH value below 3 [53].
2.2. Experimental Design
To understand the effect of 1-MCP and the edible coating, the experiment was designed according to a full randomized block design with 4 main treatments: 1-MCP (MCP); 1-MCP + edible coating (1-MCP + EC); edible coating (EC); control (CTR). Five storage times were tested: 0 (T0), 7, 14, 21, 28 days each, followed by 6 days at 20 °C and 70% RH to simulate domestic shelf life. Then, 300 fruits were sampled and used as follows: 15single fruit replicates × 4treatments × 4times of storage + 15 single fruit replicates × 4treatments analyzed before storage at 1 °C. At each storage time and treatment (1-MCP (MCP); 1-MCP + edible coating (1-MCP + EC); edible coating (EC); control (CTR)), 15 single fruit replicates were either analyzed immediately after 7, 14, 21, 28 days of cold storage (cold out—0 days). Subsequently, samples were removed from cold storage after 7, 14, 21, and 28 days and then transferred at 20 °C to simulate a commercialization period for another 6 days (SL6).
Therefore, the four treatments were as follows:
- CTR: only hydrogen peroxide and peroxyacetic acid (0.5% w/v)
- EC: coating Aloe arborescens gel 40% (v/w);
- MCP: 0.14% 1-methylcyclopropene (1-MCP);
- MCP+EC: 0.14% 1-methylcyclopropene (1-MCP) and 40% (v/w) of Aloe arborescens gel (EC);
Fruit were washed with distilled water (5 °C), and a solution of hydrogen peroxide and peroxyacetic acid (OXVIRIN 0.5% w/v for 3 min) was added. Subsequently, the fruit was treated with 1-methylcyclopropene (1-MCP) (SmartFreshSM, AgroFresh Italia srl Milano, Italy), within a bag of high-density polyethylene (HDPE), with a capacity of 5 L, for 21 h, at a temperature of 2 °C. A test tube containing the fruits was placed inside the bag, with 1 µL−1 of 0.14% 1-MCP, and distilled water at 40 °C was added to the test tube (5 mL) just before treatment. The test tube was immediately closed, shaken, and placed in the bag with the fruit; the bag was sealed, and the test tubes opened inside the bags. At the end of the treatment with 1-MCP, 160 fruits were divided: The first group of 80 fruit was treated only with 1-MCP, and the second group with the fruit with fruits previously treated with 1-MCP were submitted to the dipping treatment with an edible coating based on Aloe arborescens (1-MCP+EC). The third group of 80 fruit was treated only with the dipping treatment with an edible coating (EC) based on Aloe arborescens. The fourth group (CTR) of 80 fruit was treated with a dip in distilled water.
All fruit were stored in a 25 m2 cold-storage chamber (21 kPa O2/0.03 kPa CO2) at 1 °C RH 85% for 28 days. A sample of 15 fruits for each treatment was removed from cold storage after 7, 14, 21 and 28 days and transferred at 20 °C to be analyzed immediately (cold out—0 day) or after 6 days (shelf life—+6 days) to evaluate the effect of cold storage and room temperature. Five fruit per treatment at each storage time were subjected to sensory analysis after shelf life.
2.3. Physical-Chemical Analysis
Fruit fresh weight loss, firmness (FF), total soluble solids content (TSSC), titratable acidity (TA), and peel color (PC) were analyzed. The loss of fresh weight was measured at each storage time using a digital scale (model BP4100; Sartorius Inc., Edgewood, NY, USA), reporting the results as a cumulative percentage of weight loss during storage (7, 14, 21, 28 days at 1 °C, 85 ± 5% RH). Fruit firmness (kg cm−2) was measured with a digital penetrometer (53205, TR Turoni, Forlì, Italia) on the two sides of the fruit. Juice was extracted with a centrifuge, and TSSC (°Brix) was measured by digital refractometer Atago Palette PR–32 (Atago Co., Ltd., Tokyo, Japan) and TA (g/L of malic acid) using a Crison compact tritator (Crison Instruments, SA, Barcelona, Spain. Weight loss was expressed as the percentage reduction with respect to the initial time, using the following Equation (1):
Weight loss %: [(Initial fruit weight − Final fruit weight)/Initial fruit weight] × 100
The shelf life values were calculated before storage (T0) and after 6 days at 20 °C. Weight of individual fruit was recorded immediately after the treatment (day 0) and at the different sampling times (3, 5, 7, and 12 days during storage).
Peel color was evaluated on the two opposite sides of each fruit. Two readings per fruit were taken using a Minolta colorimeter model CR-400 (Minolta Co., Ramsey, NJ, USA) to obtain variables of lightness (L*), a* and b*. The instrument was calibrated using the manufacturer’s standard white plate. Within the CIELAB uniform space, a psychometric index of lightness, L* (ranging from 0, black, to 100, white) and two color coordinates—a* (which takes positive values for reddish colors and negative values for greenish ones) and b* (positive for yellowish colors and negative for the bluish ones)—were defined [52]. Color changes were quantified in L*, a* and b* color space, and ΔE was calculated by considering the difference between the color measured just before storage (T0) and the color measured at 7, 14, 21 and 28 d of storage. Total color difference (ΔE*) expressed the magnitude of difference between the initial non-aged color pulp (zero time) and storage-aged samples. Total color difference (ΔE*) was calculated according to the following Formula (2):
where Δa* = a* − a0*, Δb* = b* − b0*, ΔL* = L* − L0*; a0*, b0* and L0* are the corresponding blank values of control sample.
ΔE∗ = (ΔL∗2 + Δa∗2 + Δb∗2)1/2
2.4. Bioactive Properties
2.4.1. Vitamin C Content
The vitamin C content in the ethanol extracts was measured by enzyme colorimetric assay, with a maximum absorbance at 570 nm. The vitamin C concentrations were calculated with reference to a calibration curve constructed using increasing concentrations of ascorbic acid and expressed as milligrams of ascorbic acid in 100 g of FW. All measurements were done in triplicate.
2.4.2. Total Phenol Content
The total phenolic content (TPC) was determined by the reduction of phosphotungstic-phosphomolybdic acid (Folin-Ciocalteu’s reagent) to a blue pigment in alkaline solution, according to Folin and Denis [54]. Accordingly, 10 μL of the diluted sample was mixed with 4 mL of Folin-Ciocalteu reagent (previously diluted eight-fold), and the mixture was allowed to stand at room temperature for 5 min. Then, 2 mL of 10% (w/v) Na2CO3 solution was added, and the volume made to 10 mL with water. After incubation at room temperature in the dark for 90 min, the absorbance was read at 740 nm, using a spectrophotometer (Beckman Coulter DU-800, Fullerton, CA, USA). GA was used to construct the calibration curve, and the results were expressed as milligrams of gallic acid equivalents (GAE) per 100 g FW. All measurements were done in triplicate.
2.4.3. Total Carotenoids
Three aliquots of pulp (ca. 5 g) obtained from 1 slice peach fruit previously ground to a fine powder under liquid nitrogen were mixed for 20 min with 50 mL of extracting solvent (hexane/acetone/ethanol, 50:25:25, v/v). The organic phase containing carotenoids was recovered and then used for analyses after suitable dilution with hexane. Total carotenoid determination was carried out on an aliquot of the hexane extract by measuring absorbance at 450 n min with a Beckman DU 640 spectrophotometer (Midland, ON, Canada). Total carotenoids were calculated according to the method of Ritter and Purcell (1981) [55], using an extinction coefficient of β-carotene of ε% = 2505 [56]. Average values were calculated from the results of 6 measurements in different fruit for each sample.
2.4.4. Total Antioxidant Activity
The total antioxidant activity (TAA) of each cultivar was analyzed using the ABTS radical scavenging capacity assay [57]. These methods are distinguished by their mechanism of action and complement the analysis of the antioxidant potential of the fruits. The ABTS method is based on colorimetric monitoring of the decay of the ABTS•+ radical cation, caused by the oxidation of ABTS•+ radicals when contacting an antioxidant. ABTS•+ was prepared by reacting ABTS with potassium persulphate [58]. The mixture was incubated in the dark at room temperature for at least 16 h and was then diluted with ethanol to an absorbance of 0.70 ± 0.05 at 734 nm. Once prepared, the ABTS•+ solution (990 mL) was mixed with the aqueous sample (10 μL, diluted 10–40 times), and the absorbance was recorded at 0.0 and 2.5 min. Samples were analyzed in triplicate within the linearity range of the assay, as previously described [59]. The TAA evaluated by the ABTS protocol was expressed as mmol Trolox equivalents (TE)/100 g FW.
2.5. Sensory Analysis
Sensory analysis was performed on a sample of 5 fruit (a) before storage and (b) after 7, 14, 21, 28 of cold storage + 6 days of simulated domestic shelf life. The sensory analysis was conducted at the postharvest laboratory of the University of Palermo in September 2019. The sensory evaluation test was performed by an evaluation team consisting of 11 panelists (six men and five women, 25–60 years old) with a good background and knowledge of the details of this evaluation. All panelists were trained and had broad expertise in sensory evaluation of fruits. During preliminary meetings, 15 descriptors were selected for the definition of the sensory profile, generated on the basis of the citation frequency (>60%) and listed below: external color uniformity (ECU); compactness (COM); pulp color intensity (PCI); peach smell (PS); herbaceous smell (HS); floral odor (FO); pasty (PA); sweet (S); acid (A); bitter (B); juicy (JU); peach flavor (FIF); herbaceous flavor (HF); floral flavor (FF) and comprehensive evaluation (CE). The evaluation was carried out from 10.00 to 12.00 a.m. in a special room with individual booths under white lights. Samples were presented in a white plastic plate and tasted 1 h after they were taken out of the cold room [60]. Each panelist received in a random order a sample of 3 anonymous slices per treatment labeled with numbers, and water was provided for rinsing between samples.
The judges evaluated the intensity of each descriptor by assigning categorical scores of 1 (absence of sensation), 2 (just recognizable), 3 (very weak), 4 (weak), 5 (slight), 6 (moderate), 7 (intense), 8 (very intense) and 9 (extremely intense) [61]. During the evaluation, all 11 panelists completed a short questionnaire covering the quality indicators independently.
2.6. Statistical Analysis
The study was planned with a randomized sampling design. Statistical differences with p-values ≤0.05 were considered significant. The Tukey test was used for comparing the averages of measured values. Data for the physical, chemical, and sensory parameters were subjected to analysis of variance. Sources of variation were the time of storage and treatments. Mean comparisons were performed using the Tukey HSD test to examine if differences between treatments and storage time were significant at p ≤ 0.05. All analyses were performed with XLStat® software version 9.0 (Addinsoft, Paris, France).
3. Results and Discussion
3.1. Cold Out (0 Day)
Treatments had a significant effect on flesh firmness, which decreased linearly in the untreated fruit during the cold storage period; this is consistent with what is generally reported for peach fruit after harvest [62]. Flesh softening is related to the action of cell wall degrading enzymes, which hydrolyze starch to soluble sugars and protopectin to water-soluble pectin.
In fruit, EC with mucilage caused the changes of several organic acids in comparison to untreated control (Figure 1), increasing the amount of carbohydrates and other key metabolites, such as beta-sitosterol and uracil [63]. Therefore, 1-MCP combined with Aloe coating could have positively influenced the firmness of peach fruits by reducing cell wall degradation through the inhibition of microbial propagation and delaying fruit senescence (Figure 2). Indeed, no significant reduction (p ≤ 0.05) in flesh firmness occurred in EC- and 1-MCP + EC-treated fruit, with no significant difference between the two treatments (Figure 2). Differences between treated and untreated fruit occurred from the first week after storage and increased during the whole storage period. After 14 days of cold storage, there were significant differences between 1-MCP, EC, and 1-MCP + EC treatment, while there were no differences between EC and 1-MCP treatment (p ≤ 0.05). At day 21, there were no significant differences between EC and 1-MCP+EC treatments, which were the best compared to the other treatments.
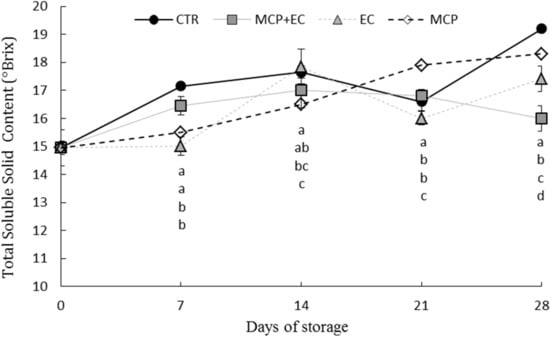
Figure 1.
Evolution of fruit total soluble solids content (°Brix) in white flesh melting peaches (P. persica) cv Settembrina treated with the edible coating (EC), with 1-MCP + EC, 1-MCP (MCP) and untreated (CTR) stored at 1 °C for 7, 14, 21 28 days. Data correspond to the means ± standard error (SE) of 15 replicates. Means with different letters are significantly different at p ≤ 0.05 using Tukey’s test. ns = not significant. Different lowercase letters denote significant differences (p ≤ 0.05) among different treatments for the same sampling time.
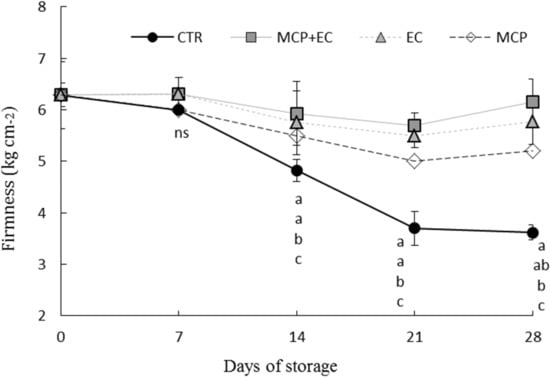
Figure 2.
Evolution of fruit firmness (Kg cm−2) in white flesh melting peaches (P. persica) cv Settembrina treated with the edible coating (EC) and 1-MCP + EC, 1-MCP (MCP) and untreated (CTR) stored at 1 °C for 7, 14, 21 28 days. Data correspond to the means ± standard error (SE) of 15 replicates. Means with different letters are significantly different at p ≤ 0.05 using Tukey’s test. ns = not significant. Different lowercase letters denote significant differences (p ≤ 0.05) among different treatments for the same sampling time.
After 14 and 21 days of cold storage, significant differences occurred between the EC and 1-MCP treatments, while no differences between EC and 1-MCP treatments (p ≤ 0.05) were found.
Total soluble solid content (TSSC) increased significantly during the storage period in all treatments after 21 days, with a different rate pattern (Figure 1). The TSSC results show significant differences between treatments after 7 and 28 days of storage. The treatment with 1-MCP shows a significant increase from (day 0) until day 21 of storage. 1-MCP + EC -treated fruit showed significantly lower TSSC values than CTR fruit from 7 up to 28 days after storage; a significant increase in TSSC values occurred in 1-MCP + EC fruit only during the last two weeks of storage (Figure 1). The ripening process is normally characterized by an increase in sugars and a reduction in organic acids (TTA). Indeed, all treated and untreated fruit showed a sharp reduction of TA during the first two weeks of storage, when 1-MCP + EC fruit showed the highest values and CTR fruit the lowest ones (Figure 3).
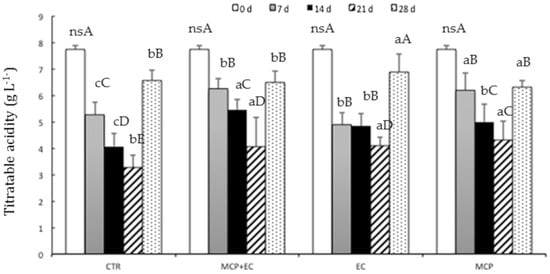
Figure 3.
Evolution of fruit titratable acidity (g L-1) in white flesh melting peaches (P. persica) cv Settembrina treated with the edible coating (EC) with 1-MCP + EC, with 1-MCP (MCP) and untreated (CTR) stored at 1 °C for 7, 14, 21 28 days. Data correspond to the means ± standard error (SE) of 15 replicates. Means with different letters are significantly different at p ≤ 0.05 using Tukey’s test. ns = not significant. Different lowercase letters denote significant differences (p ≤ 0.05) among different treatments for the same sampling time. Different capital letters denote significant differences (p ≤ 0.05) among different sampling times for the same treatment.
The evolution of weight loss (Figure 4) show a significant increase during storage time for all treatments. CTR fruits had the highest weight loss both after storage and after shelf life (SL+6), regardless of the storage period. After 28 days of storage, the treatment 1-MCP + EC shows significant differences between all treatments and registered the lowest weight loss (%) of all treatments.
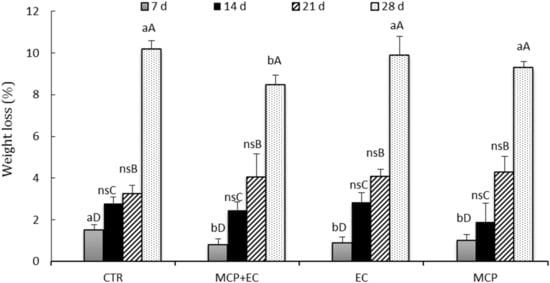
Figure 4.
Evolution of fruit treatments on weight loss (%) in white flesh melting peaches (P. persica) cv Settembrina treated with the edible coating (EC) with 1-MCP + EC, 1-MCP (MCP) and untreated (CTR) stored at 1 °C for 7, 14, 21, and 28 days. Data correspond to the means ± standard error (SE) of 15 replicates. Means with different letters are significantly different at p ≤ 0.05 using Tukey’s test. ns = not significant. Different lowercase letters denote significant differences (p ≤ 0.05) among different treatments for the same sampling time. Different capital letters denote significant differences (p ≤ 0.05) among different sampling times for the same treatment.
3.2. Shelf Life
The effect of simulated shelf life changed with length of storage period and with treatments. Fruit firmness significantly decreased in all treatments during all the storage period. However, in CTR, the fruit showed a continuous decrease from 7 to 28 days, respectively, 5.2 and 2.0 kg cm−2, while the treatments 1-MCP + EC, 1-MCP, and EC showed a significant decrease from the 7th day, with values, respectively, of 5.5 ± 0.4, 4.6 ± 0.3 and 5.3 ± 0.3 kg cm−2, until the 28th day, with values, respectively, of 3.7 ± 0.5, 3.0 ± 0.1, and 3.2 ± 0.2 kg cm−2.
Fruit firmness apparently increased in all fruit stored up to 28 d and after 6 d of simulated shelf life (SL6). This could be attributed to the prolongedstorage, following prolonged periods of refrigeration [2,64], due to a reduction in polygalacturonase (PG), resulting in a high level of pectins that are not hydrolyzed.
At this stage (28 d), CTR, EC, and 1-MCP + EC fruit showed the lowest TA values, while CTR and EC fruit still retained a higher TSSC content than 1-MCP + EC. On the whole, CTR fruit were almost overripe after 14 d of storage followed by 6 d of simulated shelf life, in terms of lack of firmness, low TT, and very high TSSC content (Table 1). Eventually, 1-MCP + EC and EC fruit retained marketable firmness, TSSC, and TA values until 14 d of storage followed by 6 d of simulated shelf life, even though, at this stage, EC fruit showed lower TA and higher TSS values than 1-MCP + EC fruit (Table 1).

Table 1.
Evolution of firmness (kg cm−2), total soluble solid content (TSSC), and total titratable acidity (TA), in white flesh melting peaches, cv Settembrina, during shelf life at 20 °C (SL6) at six days after cold storage (7, 14, 21, 28 days).
After shelf life (SL + 6) period, EC treated fruit showed a significantly higher weight loss than 1-MCP + EC fruit after all cold storage periods, indicating an effect of 1-MCP in reducing weight loss (Table 1).
This result disagrees with previous findings [65]. Finally, skin color change unevenly, as shown by the ΔE values and by the visual analysis (Figure 5 and Figure 6). Figure 6 shows the ΔE value of the fruits during the cold storage at 0 °C and during the shelf life at 20 °C. With regard to fruit stored at 0 ° C, the results show a significant color change after 14 days of storage, while in the first two weeks of storage, no significant differences were observed between all the treatments. After 14 days, the 1-MCP + EC treatment shows significant differences compared to the CTR and 1-MCP treatments, but no significant difference was detected with the EC treatment. The shelf life analysis shows the effectiveness of the 1-MCP + EC treatment at the 7th and 14th days of storage at 1 °C with significant differences from CTR, 1-MCP, and EC treatments (Figure 6). At the 21st day of storage + SL6 of shelf life, the EC treatment showed higher results (p ≤ 0.05) than the other treatments; thus, it was observed an increase in the color change of the peel. (Figure 5 and Figure 6).

Figure 5.
Conversion of the chromatic parameters L* a* b* in RGB using “e-paint.co.uk Convert Lab” software and subsequent comparison with photos of the examined fruits. n = 15 for each stage and treatment.
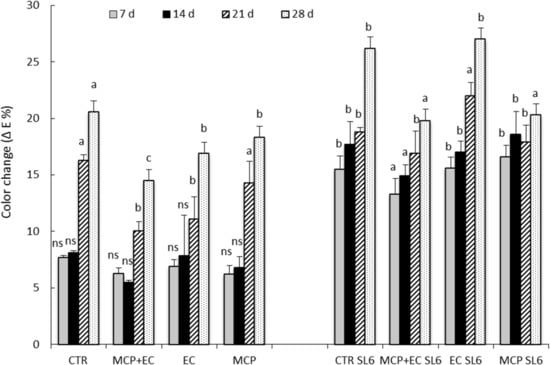
Figure 6.
Color change (ΔE %) of “peach” fruit, cv. “Settembrina” coated with an edible coating made of Aloe arborescens pure mucilage (EC), 1-MCP + coating, treated (CTR), and stored for 7, 14, 21 and 28 days at 1 °C + color change during shelf life after 6 days at 20 °C (SL6). Data correspond to the means ± standard error bars (SE) of 15 replicates. Means with different letters are significantly different at p ≤ 0.05 using Tukey’s test. ns = not significant. Different lowercase letters denote significant differences (p ≤ 0.05) among different treatments for the same sampling time. SL6 = cold storage days (0, 7, 14, 21, 28) at 1 °C, followed by shelf life for 6 days at 20 °C.
3.3. Bioactive Properties
3.3.1. Ascorbic Acid Content
The ascorbic acid content in the 1-MCP + EC treatment did not show a significant decrease from 0 d to the 21st day; contrarily, the control at 3 d shows a significant difference (p > 0.5) with values at 7 d. At 21 d, CTR, 1-MCP, and EC show a decrease of ascorbic acid content values, respectively, of 11%, 2%, 15.2%, and 18% with regard to values of 14 d. At this stage (21 d), significant differences occurred between 1-MCP + EC and all treatments, showing the highest value of ascorbic acid content. After 21 d, ascorbic acid content had a sharp, significant decrease in all treatments. On the contrary, as shown by Ahmed et al., (2019) [66], the values of ascorbic acid in the white-fleshed nectarines cultivar “Arctic Snow” stored at low temperatures, after 3 weeks of cold storage and Aloe vera gel-coated, and control did not exhibit any significant differences. It was shown that uncoated fruit had a mean value of ascorbic acid 16% higher compared to Aloe vera gel-coated fruit during ripening after cold storage.
Moreover, Table 2 shows ascorbic acid content in fresh peaches after 6 d shelf life at 20 °C. Acid ascorbic content, after harvest and after 6 d of shelf life, decreases in CTR and 1-MCP treatments, but increases in other treatments. The same trend was identified by Gallotta et al. [67], probably because after six days of shelf life at 20 °C, all the enzymatic processes involving the maturation of the peach are activated. After 14 d, values of all treatments after SL6 show a minimal decrease (not significant) compared to the treatments without shelf life (CTR, 1-MCP, 1-MCP + EC, and EC). At 7 d, the 1-MCP + SL6 treatment had the highest values compared to 1-MCP and EC, while no differences occurred with CTR. At 28 d + SL6, CTR peach fruits registered the lowest value (4.4) compared to 1-MCP, 1-MCP + EC and EC treatments, respectively, with values of 8.8, 7.7, and 7.2.

Table 2.
Vitamin C, expressed as ascorbic acid equivalents (mg) per 100 g fresh weight (FW) of four different treatments.
3.3.2. Total Phenol Content
In peach fruit during fruit ripening, the total phenolic content (TPC) first decreased sharply and then increased dramatically [68]. The same trend was observed in Ruby Rich® peach harvested and processed at two ripening stages: commercially ripe and ripe on tree [69]. We observed a different trend for each treatment (Table 3). In particular, the phenolic contents of CTR increased significantly from 0 to 28 d. This behavior has been studied by Amodio et al. [70], who stated that in general, the increase in phenolic content is the response of plants to biotic and abiotic stresses, such as pathogen attack, wounding, high levels of visible light, and cold stress. However, 1-MCP + EC and EC treatments showed an increase during 21 d, then decreased at 28 d. The effect of treatment with Aloe edible coating (EC) also showed, in EC and 1-MCP + EC treatments, higher values both during shelf life at 20 °C and until 14 d. 1-MCP + EC and EC treatments caused the highest peak of TPC at two times (7 d + SL6 and 21 d), and 1-MCP-treated fruits showed the highest TPC at day 21. It has been reported that Aloe, as an edible coating, retained the TPC in sapodilla fruits [71], strawberries [72], and apricot [73]. It might be considered that 1-MCP + EC and EC induced accumulation of TPC, which in turn reduced decay incidence and extended storage and shelf life of peach fruits. Phenolic compounds in plants, such as tannin, flavonoids, and phenolic acids, are directly involved in defense mechanisms by preventing pathogen growth and strengthening the host tissues. Therefore maintaining a high level of phenolic compounds in fruits during storage and the shelf life period is important [71].

Table 3.
Total phenol content (TPC, expressed as gallic acid equivalents (GAE, mg) per 100 g fresh weight (FW) of four different treatments, CTR, MCP, MCP + EC, and EC.
3.3.3. Total Carotenoid Content
During storage, progressive degradation of carotenoids in all treatments was not observed until 21 d, although at 28 d, it is possible to observe a decrease of carotenoids content (Table 4).

Table 4.
Total carotenoid content expressed as equivalents (mg) per 100 g fresh weight (FW) of four different treatments.
At 7 and 28 d, carotenoid content did not show significant differences between treatments; nevertheless, at 14 and 21 d, significant differences occurred between 1-MCP + EC and EC with CTR and 1-MCP.
A sharp decrease occurred soon after harvest and at 6 d of shelf life (0 d + SL6) in CTR (52%), 1-MCP (52%), 1-MCP+EC (51%), and EC (53%). No significant differences occurred between treatment at 7 d + SL6, 21 d + SL6, and 28 d + SL6, while at 14 and 21 d, 1-MCP + EC showed the highest carotenoid content with respect to 1-MCP, 1-MCP + EC, and EC treatments (Table 4).
3.3.4. Antioxidant Activity
The antioxidant activity trend of all peach fruit values showed a progressive increase during storage time until 28 d, while the highest values were observed for EC (278.8 mg−1) followed by 1-MCP + EC (258.9 mg−1). 1-MCP + EC treatment showed significant differences with regard to other treatments at 7 and 14 d, while after 21 d, EC treatment showed the highest values. 1-MCP + EC + SL6 showed a significantly higher increase then 1-MCP + EC at 7, 14, and 21 d; in contrast, CTR + SL6 exhibited a decrease of values compared to CTR (Table 5).

Table 5.
Total antioxidant activity (TAA, expressed as Trolox equivalents (Mm Trolox) per 100 g fresh weight (FW) of juices of different treatment (CTR, MCP, MCP + EC, and EC) and stage (days) determined by the ABTS assay.
4. The Sensory Profile
The sensory profile of the Settembrina (Figure 7 and Figure 8) peaches changed with the time of storage and treatment. Indeed, the effect of 1-MCP + EC and EC treatments clearly appeared for most of the descriptors (external color uniformity (ECU), compactness (COM), pulp color intensity (PCI), peach smell (PS), and the comprehensive evaluation (CE), after 14 d of storage. At this stage, the descriptors measured in 1-MCP + EC and EC fruit kept the same values of the fresh fruit. The relatively low values of herbaceous smell (HS) and herbaceous flavor (HF) indicate that the fruit were harvested at a proper stage of ripeness (Figure 8). No significant differences between treatments occurred 7, 14, and 28 d after storage. Indeed, after 7 d of storage, the fruit of all treatments retained the same sensory characteristic (same values) observed at T0, while 28 d after storage, the sensory profile was very much reduced regardless of the treatment since all fruit were almost overripe (Figure 8). Values of all descriptors were significantly reduced 21 and 28 d after storage, with a single score never higher than 5.
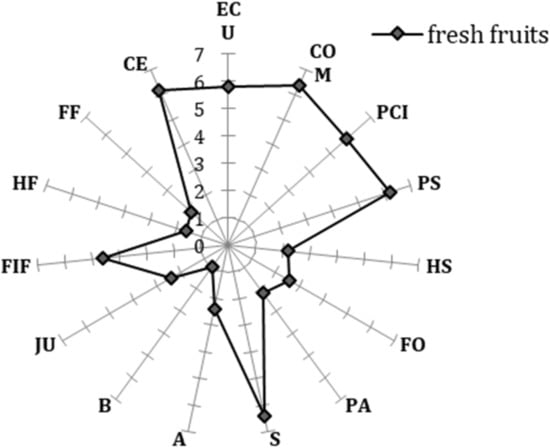
Figure 7.
Sensory profile in white flesh melting peaches (P. persica) cv Settembrina after harvest. n = 15 for each stage and treatment. Descriptors: external color uniformity (ECU); compactness (COM); pulp color intensity (PCI); peach smell (PS); herbaceous smell (HS); floral odor (FO); pasty (PA); sweet (S); acid (A); bitter (B); juicy (JU); peach flavor (FIF); herbaceous flavor (HF); floral flavor (FF), and comprehensive evaluation (CE).
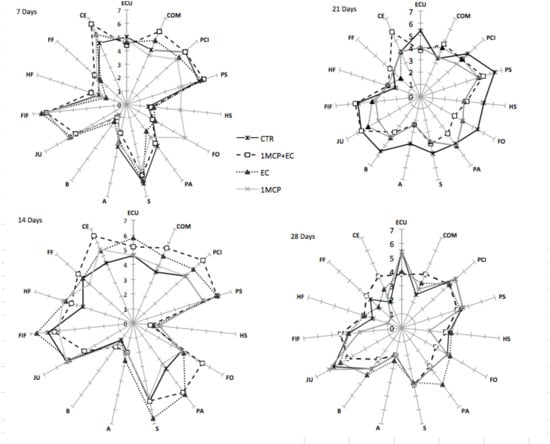
Figure 8.
Evolution of scores for the sensory analysis in white flesh melting peaches (P. persica) cv Settembrina treated with the edible coating (EC) and 1-MCP + EC, 1-MCP, and untreated (CTR) after shelf life at 20 °C. n = 15 for each stage and treatment. Descriptors: External color uniformity (ECU); compactness (COM); pulp color intensity (PCI); peach smell (PS); herbaceous smell (HS); floral odor (FO); pasty (PA); sweet (S); acid (A); bitter (B); juicy (JU); peach flavor (FIF); herbaceous flavor (HF); floral flavor (FF), and comprehensive evaluation (CE).
5. Conclusions
Based on our results, the coating of fruits with Aloe arborescens and 1-MCP lead to maintaining peach quality-related features and, consequently, the storage life of coated fruits.
Both the application of the Aloe-based coating (EC) and the combined Aloe coating with 1-MCP (1-MCP + EC) resulted in a significant slowdown of the ripening processes of Settembrina and could be effective for melting flesh peach groups. Indeed, fruit treated with EC and MCP + EC had values of flesh firmness higher than 6 kg cm−2 after 28 days of cold storage, which is an excellent result for commercial purposes, particularly if it occurs together with optimal values of total soluble solids and titratable acidity content. However, treated fruits kept their quality when stored no longer than 14 d associated with 6 d of simulated shelf life (6 d at 20 °C). At 14 days + SL6, treated and untreated fruit also differed in terms of sensory descriptors.
We demonstrate for the first time that 1-MCP added to Aloe arborescens EC maintained fruit firmness, color, and weight to a greater extent than 1-MCP or aloe coating alone.
Nevertheless, after 21 days of cold storage, the effects of 1-MCP on some evaluated parameters such as total phenolic content, TSSC, and TA were greater than EC alone or 1-MCP plus Aloe arborescens EC.
Taking in to account some sensory attributes such as external visual aspect, peach flavor, pulp color intensity, and comprehensive evaluation, it is advisable to use 1-MCP + EC or EC alone.
Coatings make a layer on the surface of the fruit and operate as a protective barrier that decreases respiration and transpiration through the fruit surface. Finally, this study indicates that combined treatment with Aloe coating and 1-MCP significantly delays the ripening of melting-flesh. This opens perspectives of potential diffusion and future research activity.
Author Contributions
Conceptualization, G.S. and V.F.; methodology, G.S. and V.F.; validation, V.F., F.S., and S.P.; formal analysis, G.S., F.S., A.A., and D.S.; investigation, G.S., V.F., and F.S.; resources, V.F., F.S., P.I., and G.S.; software, S.P., D.S., and F.S.; data curation, G.S., V.F., F.S., and A.A.; writing—Original draft preparation, G.S., V.F., P.I., and A.A.; writing—Review and editing, G.S., V.F., and P.I.; visualization G.S. and V.F. Supervision, G.S. and V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tonutti, P.; Bonghi, C.; Ramina, A. Fruit firmness and ethylene biosynthesis in three cultivars of peach (Prunus persica L. Batsch). J. Hort. Sci. 1996, 71, 141–147. [Google Scholar] [CrossRef]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Palou, L.; Crisosto, C.H.; Smilanick, J.L.; Adaskaveg, J.E.; Zoffoli, J.P. Effects of Continuous 0.3 Ppm Ozone Exposure on Decay Development and Physiological Responses of Peaches and Table Grapes in Cold Storage. Postharvest Biol. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Liguori, G.; Weksler, A.; Zutahi, Y.; Lurie, S.; Kosto, I. Effect of 1-methylcyclopropene on ripening of melting flesh peaches and nectarines. Postharvest Biol. Technol. 2004, 31, 263–268. [Google Scholar] [CrossRef]
- Gonzalez Aguilar, G.; Wang, C.Y.; Buta, G.J. UV-C irradiation reduces breakdown and chilling injury of peaches during cold storage. J. Sci. Food Agric. 2004, 84, 415–422. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; López, M.L.; Echeverría, G.; Lara, I. Volatile ester-synthesising capacity in ‘Tardibelle’ peach fruit in response to controlled atmosphere and 1-MCP treatment. Food Chem. 2010, 123, 698–704. [Google Scholar] [CrossRef]
- Gornik, K.; Badowiec, A.; Weidner, S. The effect of seed conditioning, short-term heat shock and salicylic, jasmonic acid or brasinolide on sunflower (Helianthus annuus L.) chilling resistance and polysome formation. Acta Physiol. Plant. 2014, 36, 2547–2554. [Google Scholar] [CrossRef]
- Chen, H.; Han, S.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Postharvest hot air and hot water treatments affect the antioxidant system in peach fruit during refrigerated storage. Postharvest Biol. Technol. 2017, 126, 1–14. [Google Scholar] [CrossRef]
- Li, P.; Dai, S.J.; Zhao, B.; Zhang, Y.S.; Liao, K.; Xu, F.; Du, C.L.; Leng, P. Effect of low temperatures on pulp browning and endogenous abscisic acid and ethylene concentrations in peach (Prunus persica L.) fruit during post-harvest storage. J. Hortic. Sci. Biotechnol. 2014, 89, 686–692. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, Y.; Li, M.; Zhu, T.; Tu, K. Postharvest Hot Air and UV-C Treatments Enhance Aroma-Related Volatiles by Simulating the Lipoxygenase Pathway in Peaches During Cold Storage. Food Chem. 2019, 292, 294–303. [Google Scholar] [CrossRef]
- Zhang, L.; Kou, X.; Huang, X.; Li, G.; Liu, J.; Ye, J. Peach-gum: A promising alternative for retarding the ripening and senescence in postharvest peach fruit. Postharvest Biol. Technol. 2020, 161, 111088. [Google Scholar] [CrossRef]
- Sisler, E.C.; Serek, M.; Dupille, E.; Goren, R. Inhibition of ethylene response by 1-methylcyclopropene and 3-methylcyclopropene. Plant Growth Regulation. 1999, 27, 105–111. [Google Scholar] [CrossRef]
- Sortino, G.; Inglese, P.; Allegra, A. Effect of 1-MCP on cactus pear fruit at different maturity stages during storage. Acta Hortic. 2019, 1247, 221–228. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Fan, X.; Blankenship, S.; Mattheis, J.P. 1-Methyl-cyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar] [CrossRef]
- Valero, D.; Martínez-Romero, D.; Valverde, J.M.; Guillén, F.; Castillo, S.; Serrano, M. Could the 1-MCP Treatment Effectiveness in Plum Be Affected by Packaging? Postharvest Biol. Technol. 2004, 34, 295–303. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Chen, X.; Xu, W.; Wang, N.; Xu, F.; Shao, X. Postharvest strategy combining maturity and storage temperature for 1MCP treated peach fruit. J. Food Process. Preserv. 2020, e14388. [Google Scholar] [CrossRef]
- Zoffoli, J.; Balbontin, S.; Rogríguez, J.; Retamales, J.; Defilippi, B. Effectiveness of 1-mcp on postharvest deterioration of nectarines and peaches stored at different temperatures. Acta Hortic. 2002, 592, 567–572. [Google Scholar] [CrossRef]
- Mariño-González, L.; Buitrago, C.; Balaguera Lopez, H.; Martínez-Quintero, E. Effect of 1-methylcyclopropene and ethylene on the physiology of peach fruits (Prunus persica L.) cv. Dorado during storage. Rev. Colomb. Cienc. Hortíc. 2019, 13, 46–54. [Google Scholar] [CrossRef]
- Dal Cin, V.; Rizzini, F.M.; Botton, A.; Tonutti, P. The ethylene biosynthetic and transduction pathways are differently affected y 1-MCP in apple and peach fruit. Postharvest Biol. Technol. 2006, 42, 125–133. [Google Scholar] [CrossRef]
- Hayama, H.; Tatsuki, M.; Nakamura, Y. Combined treatment of aminoethoxyvinylglycine (AVG) and 1-methylcyclopropene (1-MCP) reduces peach fruit softening. Postharvest Biol. Technol. 2008, 50, 228–230. [Google Scholar] [CrossRef]
- Tadiello, A.; Ziosi, V.; Negri, A.S. On the role of ethylene, auxin and a golven-like peptide hormone in the regulation of peach ripening. BMC Plant Biol. 2016, 16, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, Y.; Wang, Y.; Pan, L.; Niu, L.; Lu, Z.; Cui, G.; Zeng, W.; Wang, Z. Genes involved in ethylene signal transduction in peach (Prunus persica) and their expression profiles during fruit maturation. Sci. Hortic. 2017, 224, 306–316. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Changes in phenolics and antioxidant property of peach fruit during ripening and responses to 1-methylcyclopropene. Postharvest Biol. Technol. 2015, 108, 111–118. [Google Scholar] [CrossRef]
- Kluge, R.; Jacomino, A. Shelf life of peaches treated whit 1-methylcyclopropene. Sci. Agric. 2002, 59, 69–72. [Google Scholar] [CrossRef]
- Yang, X.; Wei, W.; Lv, P.; Feng, J. Effectiveness of 1-methylcyclopropene treatment on Peach Fruit (Prunus Persica L.) for Extending Storage life. Adv. Mater. Res. 2014, 1089, 159–162. [Google Scholar] [CrossRef]
- McHugh, T.H. Protein-lipid interactions in edible films and coatings. Nahrung 2000, 44, 148–151. [Google Scholar] [CrossRef]
- Miriam del Carmen, M.-G.; Bautista-Baños, S.; Nacary Correa-Pacheco, Z.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Del Río-García, J.C.; de Lorena Ramos-García, M. Effect of Nanostructured Chitosan/Propolis Coatings on the Quality and Antioxidant Capacity of Strawberries During Storage. Coatings 2020, 10, 90. [Google Scholar] [CrossRef]
- Vargas, M.; Pastor, C.; Chiralt, A.; McClements, D.J.; González-Martínez, C. Recent advances in edible coatings for fresh and minimally processed fruits. Crit. Rev. Food Sci. Nutr. 2008, 48, 496–511. [Google Scholar] [CrossRef]
- Bifani, V.; Ramírez, C.; Ihl, M.; Rubilar, M.; García, A.; Zaritzky, M. Effects of murta (Ugni molinae Turcz) extract on gas and water vapor permeability of carboxymetrhycellulose-based edible films. LWT Food Sci. Technol. 2007, 40, 1473–1481. [Google Scholar] [CrossRef]
- Fernandes, F.A.; Carocho, M.; Heleno, S.A.; Rodrigues, P.; Dias, M.I.; Pinela, J.; Prieto, M.A.; Simal-Gandara, J.; Barros, L.; Ferreira, I.C.F.R. Effect of Natural Preservatives on the Nutritional Profile, Chemical Composition, Bioactivity and Stability of a Nutraceutical Preparation of Aloe arborescens. Antioxidants 2020, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Kahramanoğlu, İ.; Chen, C.; Chen, J.; Wan, C. Chemical Constituents, Antimicrobial Activity, and Food Preservative Characteristics of Aloe vera Gel. Agronomy 2019, 9, 831. [Google Scholar] [CrossRef]
- Boudreau, M.D.; Beland, F.A. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J. Environ. Sci. Health Part C 2006, 24, 103–154. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H. Composition and applications of Aloe vera leaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef]
- Guillén, F.; Díaz-Mula, H.M.; Zapata, P.J.; Valero, D.; Serrano, M.; Castillo, S.; Martínez-Romero, D. Aloe arborescens and Aloe vera gels as coatings in delaying postharvest ripening in peach and plum fruit. Postharvest Biol. Technol. 2013, 83, 54–57. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujolà, M. Aloe vera based edible coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar]
- Baez-Sañudo, M.; Siller-Cepeda, J.; Muy-Rangel, D.; Heredia, J.B. Extending the shelf-life of bananas with 1-methylcyclopropene and a chitosan-based edible coating. J. Sci. Food Agric. 2009, 89, 2343–2349. [Google Scholar] [CrossRef]
- Sicari, V.; Loizzo, M.R.; Pellicanò, T.M.; Giuffrè, A.M.; Poiana, M. Evaluation of Aloe Arborescens Gel as New Coating to Maintain the Organoleptic and Functional Properties of Strawberry (Fragaria × Ananassa Cv. Cadonga) Fruits. Int. J. Food Sci. Technol. 2019, 55, 861–870. [Google Scholar] [CrossRef]
- Zapata, P.J.; Navarro, D.; Guillén, F.; Castillo, S.; Martínez-Romero, D.; Valero, D.; Serrano, M. Characterisation of gels from different Aloe spp. as antifungal treatment: Potential crops for industrial applications. Ind. Crop. Prod. 2013, 42, 223–230. [Google Scholar] [CrossRef]
- Farina, V.; Passafiume, R.; Tinebra, I.; Palazzolo, E.; Sortino, G. Use of Aloe Vera Gel-Based Edible Coating with Natural Anti-Browning and Anti-Oxidant Additives to Improve Post-Harvest Quality of Fresh-Cut ‘Fuji’ Apple. Agronomy 2020, 10, 515. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Hossein, M.; Mahmood, G.; Davood, B. Effect of different coatings on post-harvest quality and bioactive compounds of pomegranate (Punica granatum L.) fruits. J. Food Sci. Technol. 2015, 52, 4507–4514. [Google Scholar] [CrossRef]
- Lin, B.; Du, Y.; Liang, X.; Wang, X.; Wang, X.; Yang, J. Effect of chitosan coating on respiratory behavior and quality of stored litchi under ambient temperature. J. Food Eng. 2011, 102, 94–99. [Google Scholar] [CrossRef]
- Farina, V.; Gentile, C.; Sortino, G.; Gianguzzi, G.; D’Asaro, A.; Saletta, F.; Piva, G.; Inglese, P.; Liguori, G. Effects of gellan-based coating application on litchi fruit quality traits. Acta Hortic. 2018, 1194, 335–342. [Google Scholar] [CrossRef]
- Ali, A.; Muhammad, M.T.M.; Sijam, K.; Siddiqui, Y. Effect of chitosan coatings on the physicochemical characteristics of Eksotika II papaya (Carica papaya L.) fruit during cold storage. Food Chem. 2011, 124, 620–626. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Farina, V.; Inglese, P. Effect of passive atmosphere and chemical treatment on fresh cut of white-flesh peach cultivar ‘Settembrina di Bivona. Acta Hortic. 2015, 1084, 765–770. [Google Scholar] [CrossRef]
- Caruso, T.; Di Lorenzo, R.; Barone, E. IL germoplasma del pesco in Sicilia: Aspetti genetici e bioagronomici. In Proceedings of the IV Natl. Congress, Germoplasma Frutticolo Salvaguardia e Valorizzazione Delle Risorse Genetiche, Alghero, Italy, 21–25 September 1992; Volume 1, pp. 285–293. [Google Scholar]
- Sortino, G.; Ingrassia, M.; Allegra, A.; Inglese, P. Sensory evaluation and suitability for fresh-cut produce of white peach [Prunus persica (L.) Batsch] ‘Settembrina di Bivona. Acta Hortic. 2015, 1084, 787–790. [Google Scholar] [CrossRef]
- Sortino, G.; Allegra, A.; Farina, V.; Inglese, P. Postharvest quality and sensory attributes of ‘Pesca di Bivona’ peaches (Prunus persica L.) during storage. Bulg J. Agric. Sci. 2017, 23, 939–946. [Google Scholar]
- Montevecchi, G.; Simone, G.V.; Masino, F.; Bignami, C.; Antonelli, A. Physical and chemical characterization of Pesca bivona, a Sicilian white flesh peach cultivar [Prunus persica (L.) Batsch]. Food Res. Int. 2012, 45, 123–131. [Google Scholar] [CrossRef]
- Liguori, G.; Sortino, G.; De Pasquale, C.; Inglese, P. Effects of modified atmosphere packaging on quality parameters of minimally processed table grapes during cold storage. Adv. Hortic. Sci. 2015, 29, 152–154. [Google Scholar]
- Montero-Calderón, M.; Rojas-Graü, M.A.; Martín-Belloso, O. Effect of packaging conditions on quality and shelf-life of fresh-cut pineapple (Ananas comosus). Postharvest Biol. Technol. 2008, 50, 182–189. [Google Scholar] [CrossRef]
- Passafiume, R.; Perrone, A.; Sortino, G.; Gianguzzi, G.; Saletta, F.; Gentile, C.; Farina, V. Chemical-physical Characteristics, Polyphenolic Content and Total Antioxidant Activity of Three Italian-Grown Pomegranate Cultivars. NFS J. 2019, 16, 9–14. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- De Ritter, E.; Purcell, A.E. Carotenoid analytical methods. In Carotenoids as Colorants and Vitamin A Precursors; Academic Press: Cambridge, MA, USA, 1981; pp. 815–923. [Google Scholar]
- Giusti, M.M.; Jing, P. Analysis of anthocyanins. Food Color. Chem. Funct. Prop. 2008, 6, 479–506. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.A. Spectrophotometric determination of antioxidant activity. Redox Rep. 1996, 2, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gentile, C.; Di Gregorio, E.; Di Stefano, V.; Mannino, G.; Perrone, A.; Avellone, G.; Sortino, G.; Inglese, P.; Farina, V. Food Quality and Nutraceutical Value of Nine Cultivars of Mango (Mangifera Indica L.) Fruits Grown in Mediterranean Subtropical Environment. Food Chem. 2019, 277, 471–479. [Google Scholar] [CrossRef]
- Chironi, S.; Sortino, G.; Allegra, A.; Saletta, F.; Caviglia, V.; Ingrassia, M. Consumer assessment on sensory attributes of fresh table grapes cv ‘Italia’ and ‘Red globe’ after long cold storage treatment. Chem. Eng. Trans. 2017, 58, 421–426. [Google Scholar] [CrossRef]
- Liguori, G.; Sortino, G.; Inglese, P.; Farina, V. Quality changes during postharvest life in white fleshed peach (Prunus Persica L. Batsch) fruits: Preliminary observations. Bulg. J. Agric. Sci. 2016, 22, 497–504. [Google Scholar]
- Bautista-Baños, S.; Hernández-Lauzardo, A.N.; Velázquez-del Valle, M.G.; Hernández-López, M.; Ait Barka, E.; Bosquez-Molina, E. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Allegra, A.; Gallotta, A.; Carimi, F.; Mercati, F.; Inglese, P.; Martinelli, F. Metabolic profiling and post-harvest behavior of “Dottato” fig (Ficus carica L.) fruit covered with an edible coating from O. ficus-indica. Front. Plant Sci. 2018, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Luza, J.G.; Gorsel, R.; Van Polito, V.S.; Kader, A.A. Chilling Injury in Peaches: A Cytochemical and Ultrastructural Cell Wall Study. J. Am. Soc. Hortic. Sci. 1992, 117, 114–118. [Google Scholar] [CrossRef]
- Hazrati, S.; Kashkooli, A.B.; Habibzadeh, F.; Tahmasebi-Sarvestani, Z.; Sadeghi, A.R. Evaluation of Aloe vera Gel as an Alternative Edible Coating for Peach Fruits During Cold Storage Period. Gesunde Pflanzen 2017, 69, 131–137. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Singh, Z.; Khan, A.S. Postharvest Aloe Vera gel-Coating Modulates Fruit Ripening and Quality of ‘Arctic Snow’ Nectarine Kept in Ambient and Cold Storage. Int. J. Food Sci. Technol. 2009, 44, 1024–1033. [Google Scholar] [CrossRef]
- Gallotta, A.; Allegra, A.; Inglese, P.; Sortino, G. Fresh-cut storage of fruit and fresh-cuts affects the behaviour of minimally processed Big Bang nectarines (Prunus persica L. Batsch) during shelf life. Food Pack. Shelf Life 2018, 15, 62–68. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Jiang, L.; Yu, M.; Ma, R.; Yu, Z. 1-MCP treatment affects peach fruit aroma metabolism as revealed by transcriptomics and metabolite analyses. Food Res. Int. 2019, 122, 573–584. [Google Scholar] [CrossRef]
- Sortino, G.; Gallotta, A.; Farina, V.; Allegra, A. Shelf-Life and quality of fresh-cut peach cv ‘Ruby Rich’ at different maturity stage. Chem. Eng. Trans. 2017, 58, 409–414. [Google Scholar]
- Amodio, M.L.; Derossi, A.; Colelli, G. Modeling phenolic content during storage of cut fruit and vegetables: A consecutive reaction mechanism. J. Food Eng. 2014, 140, 1–8. [Google Scholar] [CrossRef]
- Khaliq, G.; Ramzan, M.; Baloch, A.H. Effect of Aloe vera gel coating enriched with Fagonia indica plant extract on physicochemical and antioxidant activity of sapodilla fruit during postharvest storage. Food Chem. 2019, 286, 346–353. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Koushesh, S.M.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Nourozi, F.; Sayyari, M. Enrichment of Aloe vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci. Hortic. 2020, 262, 109041. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).