1. Introduction
In the world, few crops have experienced such a sustained economic growth rate as grapevines in the last 30 years. This has been particularly true of new production areas, notably North America, South America, Australia, New Zealand, South Africa, and recently China [
1,
2]. However, such growth has been and is currently challenged by a number of factors that must be addressed in a sustainable manner [
1]. Chief amongst these factors are climate change scenarios that directly impact the agronomic environment of vines [
3,
4]. Invasive species are a factor related to increased international trade levels and climate changes that indirectly impact the development of arthropods.
Although Canada’s viticultural industry experienced tremendous growth, it is basically practiced in cool-climate conditions, and it does not rank amongst major grape producers in the world as of 2019, the five biggest producers of grapes being China (11.7 t), Italy (8.6 t), USA (6.9 t), Spain (6.9 t), and France (6.2 t) [
2]. In 2018, global wine production was 26.7 billion litres, of which Canada represented 0.3% [
5]. Amongst the four major producing areas of wines, the province of Quebec ranks third.
Quebec viticulture went from almost no production in 1980 to 2.3 million bottles produced in 2019 [
6]. As of December 2019, amongst the 135 artisanal permits winery issued by the Régie des alcools, des jeux et des courses du Québec [
7], 83 were members of the Conseil des vins du Québec. The eight biggest wineries produced > 50% of Quebec wine production. In 2019, the demand by Quebecers for Quebec wines largely exceeded the production.
2. Challenges of Quebec Vineyards
Isaacs et al. [
8] and Daane et al. [
1] discussed the challenges of major grape-producing areas. In contrast to major grape-producing areas of the world where water usage is an important concern [
9], the major impediments to producing grapes in Quebec are the winter survival of grapevines [
10], minimum winter temperatures (occasionally reaching −35 °C in southern Quebec) and the duration of the frost-free growing season which, in the warmer viticultural region of Quebec, is currently 148–160 days on average (1979–2008) [
11]. To extend the frost-free season and to prevent cold injury in the spring and in the fall, the biggest vineyards installed wind machines, actioned when near zeros temperatures are predicted. Winter-hardy cultivars such as Chardonnay, Pinot noir and Cabernet franc resist lower temperatures of −17 to −19 °C, while semi-rustic cultivars such as Seyval, Vidal and Marechal Foch resist −20 to −24 °C.
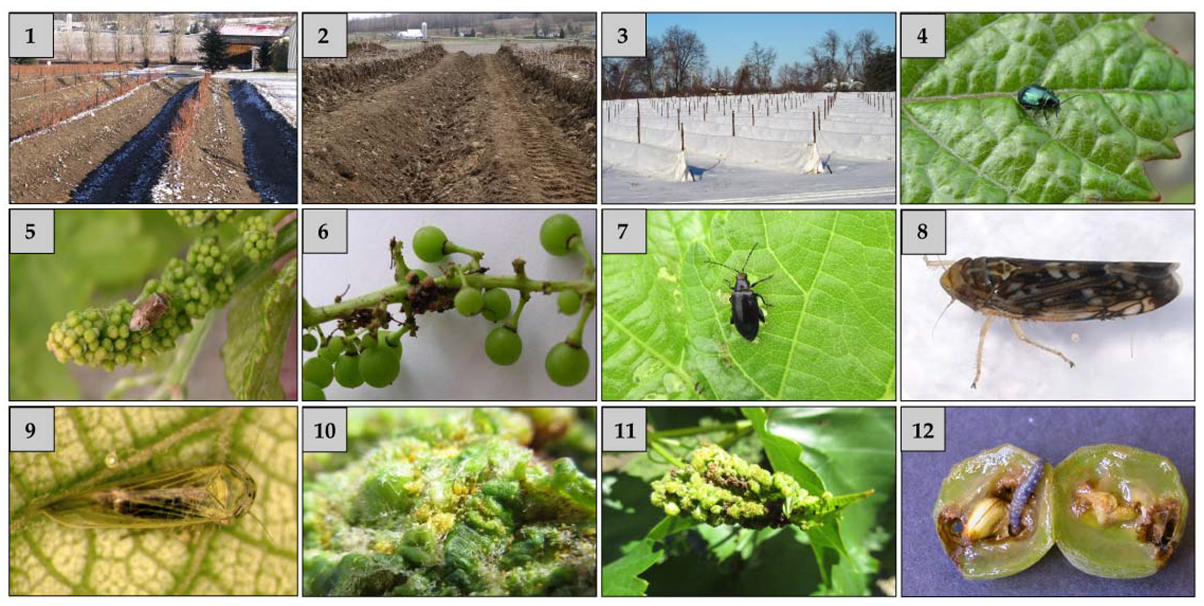
Some Quebec viticulturists mitigate winter losses of vines by hilling soil around the stems in the fall (
Figure 1(1)), which maintain the vines at temperatures > −8 °C, and unearthing them in the spring (
Figure 1(2)). Others cover the vines with geotextiles (
Figure 1(3); [
12]). This method is riskier for
Vinifera and some semi-rustic cultivars because vines experience temperatures of ca. −25 °C under geotextiles when outside temperature is −35 °C (J. Lasnier and C.-H. de Coussergue, pers. comm.).
From a Quebec viticultural point of view, climate change will be an opportunity, as it will allow to produce grapes with cultivars having a relatively higher oenological potential. Although the principles of pest management discussed in [
13] will remain, entomological challenges will be exacerbated in scenarios with increased average temperatures, and trade will likely generate new entomological problems. For example, some arthropods such as the brown marmorated sting bug (
Halyomorpha halys-Pentatomidae) and the spotted lanternfly (
Lycorma delicatula-Fulgoridae) are currently under biovigilance in Quebec. Thus, the sustainability of arthropod management programs will be challenged. Amongst biotic factors, fungal diseases, (i.e., mainly downy mildew (
Plasmopara viticola), powdery mildew (
Erysiphe necator) and bunch rot (
Botrytis cinerea) and, to a lesser extent, bacterial diseases are major drivers of crop protection programs [
14].
In this paper, we review entomological issues that were addressed since 1980’s and will be addressed in the coming years by Quebec viticulturists. We will draw on the relevant literature, notably [
13,
15,
16,
17,
18,
19,
20,
21,
22]. We will refer to a number of arthropod species discussed and illustrated in [
22]. The topics will be discussed in the following sequence: challenges of Quebec vineyards, entomological research in Quebec vineyards, primary pests, secondary pests, pests under biovigilance, natural enemies and conclusive remarks. We used the phenological systems of Baggiolini (letters) and Eichhorn-Lorenz (numbers) as reference points [
23].
3. Entomological Research in Quebec Vineyards
Historically, entomological research in vineyards focused on arthropod pests and consequently, relatively little is known about the biodiversity of arthropods in unmanaged or lightly managed vineyards. A notable exception are the biodiversity studies conducted systematically in the vineyards of Quebec, in which a high level of arthropod biodiversity was found, demonstrating the potentially rich array of natural enemies that may occur in this agroecosystem for biological control of pests [
24].
In Quebec, the first formal research project was initiated in 1997 as a partnership between two commercial vineyards (L’Orpailleur, Dunham, Qc (45° 07′ N, 72° 51′ W) and Dietrich Jooss, Iberville, Qc (45° 16′ N, 73° 11′ W), Ag-cord inc. and Agriculture and Agri-Food Canada [
18]. From the start, the project was designed to establish a base-line of arthropod biodiversity in insecticide-free vineyards. The approach was to work from the ground up, i.e., from general information concerning arthropod fauna towards more specific subjects as the projects and the situation evolved. This was feasible because, in the late 90’s, the agricultural lands devoted to viticulture were relatively small in Quebec and, consequently, the build-up of entomological problems did not occur at the same pace and intensity compared to well established wine-growing areas.
The populations of arthropods were estimated with a variety of methods, notably by visual estimation, by the tapping of clusters over a plastic pot, and with pitfall and flight interception traps [
15]. A major challenge was to sort out the specimens by given taxa such that they were amenable to identification at the species level. Identification of specimens of selected taxa were done by professional systematists that co-authored scientific papers.
4. Primary Pests
In Quebec vineyards, primary pests must be monitored systematically as they entail a high risk of damage if their populations are abundant (
Table 1).
4.1. Coleoptera
The grape flea beetle (
Altica chalybea-Chrysomelidae) and the lesser grape flea beetle (
Figure 1(4)
(Altica woodsi-Chrysomelidae) are two specialist species of flea beetles found in Quebec vineyards [
25,
26]. These foliar pests occasionally require insecticide treatments in spring if massive migrations borned by warm and dominant south-west winds occur. These rare events can cause severe damage at stage C (05-bud swell).
4.2. Hemiptera
The tarnished plant bug (
Lygus lineolaris-Miridae) (
Figure 1(5) is a highly polyphagous (>300 plant species) insect that can complete three generations on vines [
22,
27,
28]. Nymphs and adults puncture and feed on leaves, flower buds, shoots, meristem tissues and grapes. Histological studies conducted in the laboratory have demonstrated that, at stage H (55—flowers separating) and I (65—bloom), nymphs and adults feed on nectaries without damaging the ovaries [
29,
30]. However, field studies where 1–4 nymphs and adults were confined in sleeve cages at stage G (53—single flowers in compact groups) to stage K (75—berries pea-sized) have demonstrated a considerable (ca. 80%) reduction in cluster weight (
Figure 1(6)). The first generation of nymphs (nymphal stages 1–5) may pierce the pedicels of berries after stage fruit set, which can cause berries to drop, causing economic damage if no insecticide has been applied before this stage. The foliar damage caused by adults and nymphs after stage K (75—berries pea-sized) has no economic impact. The toxicity of some insecticides against adults and nymphs have been established by [
31]. Climate change is not expected to change the status or economic impact of tarnished plant bug.
5. Secondary Pests
These species are present in the vineyards every year at population levels that normally do not warrant insecticidal treatment (
Table 1). However, they occasionally require localized insecticidal or biopesticidal treatments.
5.1. Acari
Three phytophagous species are commonly found in Quebec vineyards, notably the grape erineum mite (Colomerus vitis-Eriophyidae), the european red mite (Panonychus ulmi-Tetranychidae) and the two-spotted spider mite (Tetranychus urticae-Tetranychidae). They do not require acaricidal treatments as their populations are under check by conserving natural enemies.
5.2. Coleoptera
The red-headed flea beetle (
Figure 1(7) (
Systena frontalis-Chrysomelidae) can cause severe damage in young vine plantings from mid-July to early September. A number of coleopteran pests are seldom found in low numbers, notably the grape cane girdler (
Ampeloglypter ampelopsis-Curculionidae), the grape cane gallmaker (
Ampeloglypter sesostris-Curculionidae), the black vine weevil (
Otiorhynchus sulcatus-Curculionidae), and the rose chafer (
Macrodactylus subspinosus-Scarabeidae) [
33].
Native from Asia, the japanese beetle (
Popillia japonica-Scarabeidae) was accidentally introduced in the United States in 1916 [
34]. In 1939, it was found for the first time in Lacolle, Qc, a municipality located a few km from the New York State border. In the mid-80’s, it was a pest of rosaceous ornamentals in Bedford, Qc, a municipality near the Quebec-Vermont border (C. Vincent, pers. comm.). Since the early 2000’s, the japanese beetle has considerably extended its geographical distribution in Quebec. When present in vineyards, adults feed on leaves and new shoots of vines. Since 2000, their levels of damage have steadily increased in Quebec vineyards, and they increased significantly since 2015.
5.3. Diptera
Native from Asia, the spotted wing drosophila (
Drosophila suzukii-Drosophilidae) was found for the first time in Quebec vineyards in 2012 [
35]. As grape is not its preferred host, it currently does not warrant insecticidal treatments.
D. suzukii is found on decomposing grapes fallen after harvest. In vineyards, adults are trapped in mid-September. Under a warming scenario,
D. suzukii should rise in importance.
The grape tumid gallmaker (Janetiella brevicauda-Cecidomyiidae) is rarely found in Quebec vineyards. Its populations develop in low and poorly drained vineyards.
5.4. Hemiptera
In Quebec vineyards, [
15] found 59 Cicadellids species assigned to four categories: 1) species strictly associated with grapevine; 2) species using grapevines as secondary hosts; 3) species associated with weeds inside the vineyard; and 4) species associated with neighboring vegetation of the vineyard. Among the species strickly associated with grapevine, five species, the eastern grape leafhopper (
Erythroneura comes-Cicadellidae); the grapevine leafhopper (
Erythroneura vitis-Cicadellidae); the three-banded leafhopper (
Erythroneura tricincta-Cicadellidae); the virginia creeper leafhopper (
Erythroneura ziczac-Cicadellidae); and the vine leafhopper (
Erythroneura vitifex-Cicadellidae), were captured in very low numbers. The most abundant species were
Latalus (
Adarrus)
ocellaris and
Macrosteles quadrilineatus, two species associated with weeds inside vineyard; and
Empoasca spp., i.e., species associated with neighboring vegetation of the vineyards.
Following the first mention of bois noir associated with vines in Canada by [
36], research projects were undertaken to determine the biodiversity of cicadellids and quarantine phytoplasmas, notably flavescence dorée and bois noir. Phytoplasmas, mostly aster yellows, were found in several vineyards of Canada [
37,
38,
39]. No quarantine or regulated phytoplasmas such as flavescence dorée and bois noir were found, although the vector,
Scaphoideus titanus (Cicadellidae) (
Figure 1(8)), a species native to North America, was found in Quebec and Ontario [
32,
38].
A rearing method for
Erythroneura elegantula,
Erythroneura vitis, and
Erythroneura ziczac was developed by [
40], allowing electropenetrography studies to understand the feeding behavior of these three species [
41].
In a later study, ca. 110 cicadellid species have been found in vineyards of Canada [
42]. The most damaging species are Empoasca fabae (mentioned previously) and Erythroneura spp. [
32]. The potato leafhopper (
Empoasca fabae-Cicadellidae) is a highly polyphagous pest that feeds on more than 200 plant species and do not overwinter in Quebec. Generally, this species arrives in mid-July by prevailing winds from the south-west and it does not cause significant damage to vineyards. The vector of aster yellows, Macrosteles quadrilineatus (
Figure 1(9)), is a likely vector of aster yellows in Quebec vineyards.
Leafhopper development takes a variable amount of time depending largely on environmental conditions. Leafhoppers can have two or three generations per year, depending on the species, and can overwinter in various forms. Some species deposits their eggs in the bark of shoots and canes while other species overwinter as adults under leaf debris. A model to predict the abundance of cicadellids in Quebec vineyards was developed [
43].
Native to North America, the grape phylloxera (
Daktulosphaira vitifoliae-Phylloxeridae) was associated with the foliage of wild vines before the plantation of grape cultivars. In Quebec, there are two indigenous wild vines:
Parthenocissus vitacea and
V. riparia, and one introduced species,
P. quinquefolia [
44]. The forms gallicoles and radicicoles do not affect
V. vinifera on North-American rootstocks and on most European and American hybrids. Several vines planted in Quebec in the last two decades were issued from new breeding programs. Generally resulting from crosses between
V. labrusca or
V. riparia, those cultivars are not generally attacked by the radicicoles, but some can be very susceptible to the gallicoles (1(10) and (11)). Planting some of these cultivars in Quebec favored the development of the gallicoles [
45].
5.5. Lepidoptera
The grape leaffolder (Desmia funeralis-Crambidae), the eight-spotted forester (Alypia octomaculata-Noctuidae), and the climbing cutworm (Euxoa messoria-Noctuidae) rarely cause damage to Quebec vineyards.
Native from North America, the grape berry moth (
Paralobesia viteana-Tortricidae) has two generations in Quebec [
15,
22]. The damage caused by the first generation larvae on the developing clusters is less severe than that caused by the second generation (
Figure 1(12)). The injuries they cause promote the spread of the causal fungus of grey mould (
Botrytis cinerea), which may damage part or all of the affected clusters [
46]. In Quebec vineyards, as chemical treatment of first generation larvae has little impact on damage levels at harvest, control efforts are typically carried out when necessary against second generation larvae. Trap catches are used to time insecticide sprays, which are seldom needed in Quebec. As the pressure of grape berry moth is relatively low, mating disruption programs are not currently economically justified. Under a climate change scenario, grape berry moth could have a third, and possibly fourth generation such that it will become a major concern for viticulturists as is currently the case in Ontario and New York State, where mating disruption is not a registered product.
6. Pests under Biovigilance
Biovigilance allows for the detection of significant temporal and spatial trends of several agronomic factors, notably new pests [
47]. The arthropod species in this section are indigenous or invasives. However, biovigilance is in order because they may pose important threats to the viticultural industry in Quebec. For example, grapevine leafroll-associated viruses are vectored by scale insects. Under a climate change scenario, the risks entailed by these species that may extend their current distribution to the north is likely to increase.
6.1. Coleoptera
Native from North America, the grape rootworm (
Fidia viticida-Chrysomelidae) was so far restricted to Eastern USA [
33] and southern Ontario. In 2016,
F. viticida adults were sampled in Laval, Qc, by beating virginia creeper (
Parthenocissus quinquefolia) and riverbank grape (
Vitis riparia) [
48].
F. viticida is currently under biovigilance in Quebec because it was reported as a serious pest in Erie County in Pennsylvania [
49].
6.2. Hemiptera
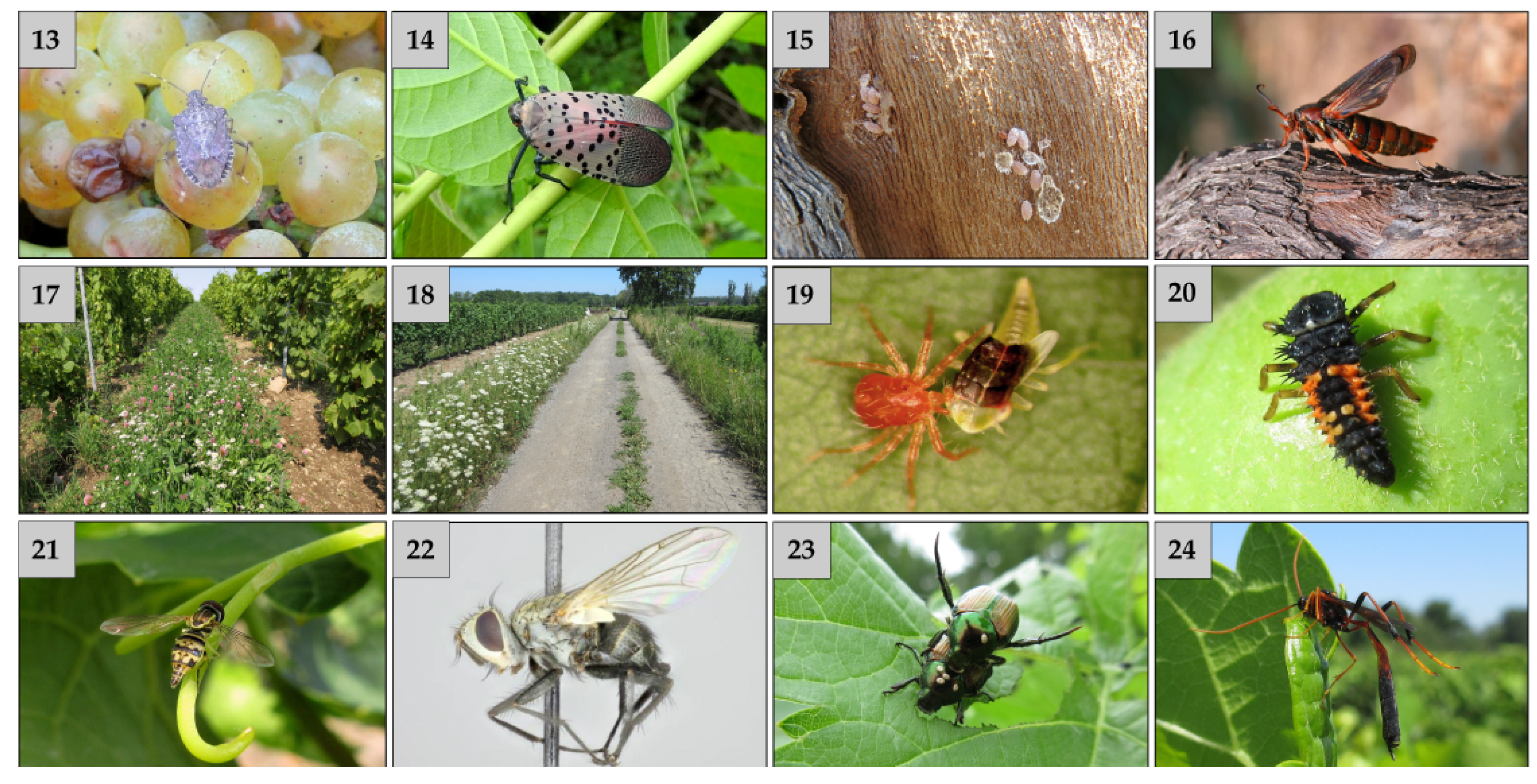
Native to Asia and highly polyphagous, the brown marmorated stink bug (
Hyalyomorpha halys-Pentatomidae) (
Figure 2(13) has caused considerable concerns and damage in several crops where it established in North America [
50]. In Quebec it has been found for the first time in Montreal [
51] and has since slightly expanded its geographical distribution [
52]. Reported to have caused severe damage in vineyards of mid-Atlantic States of USA [
53], it has not so far been found in Quebec vineyards. When adults are pressed with grapes at harvest, they release a defensive chemical that can taint the wine [
54]. It is under biovigilance because of the uncertainty of its geographical expansion under a climate change scenario.
Native from Asia, the spotted lanternfly (
Lycorma delicatula-Fulgoridae) (
Figure 2(14)) has been found for the first time in 2014 in Pennsylvania and, as of 2019, was found in several New England States, and have been intercepted in New York State [
55]. It feeds on > 65 host plants, including
Vitis vinifera, which is a preferred host. Although currently absent from Canada, it has been added to the list of regulated species by the Canadian Food Inspection Agency [
56]. A MAXENT model predicts that Southern Quebec has low potential for the distribution of
L. delicatula [
57]. If present, this species is likely to cause some concerns and impact protection programs.
Absent from Quebec, the grape mealybug (
Pseudococcus maritimus-Pseudococcidae) (
Figure 2(15)) is currently found in Ontario and New York State, where it is a vector of leaf roll virus [
58]. It is currently under biovigilance in Quebec. European fruit lecanium scale (
Parthenolecanium corni-Coccidae) and cottony maple scale (
Pulvinaria innumerabilis-Coccidae), are present in Quebec vineyards, but their populations are low.
6.3. Lepidoptera
The grape root borer (
Vitacea polistiformis-Sesiidae) (
Figure 2(16)) is potentially the most destructive pest of grapevines. The larvae tunnel into the largest roots and up to the crown of the vines. Since larval activity is limited to the vine structures below ground, the symptoms become apparent only when the vines are severely damaged. That is why this pest is often ignored until it becomes a serious problem in a vineyard.
The blotch leafminer (Antispila viticordifoliella-Heliozelidae) and the grape plume moth (Geina periscelidactylu-Pterophoridae) are found in vineyards of Ontario, but not in Quebec.
7. Natural Enemies
The conservation of predators, notably Anystis baccarum (Anystidae), is the cornerstone of Quebec protection programs. Over the years, it allowed phytophagous mite and cicadellid management without pesticidal treatments. As a rule, the least toxic pesticides are timely used to conserve predators. In Quebec vineyards, A. baccarum developed, over the years, some tolerance to pyrethroids sprayed annually at stage H (17—flower separating stage) against tarnished plant bug nymphs and to sulphur sprayed annually against powdery mildew. It also developed resistance to some fungicides used against downy mildew, powdery mildew and botrytis bunch rot, notably Metiram, Captan, Metalaxyl-M, fenhexamid, Boscalide, and sulphur. It is an excellent candidate for conservation-augmentation biological control program.
To foster natural enemies two augmentation techniques were used [
22]. First, if absent or present in insufficient numbers, the predatory mites can be collected on dormant wood in a donor vineyard. The collected wood is transferred to a recipient vineyard. Second, flowering strips can be established in three manners: (1) flower strips in middle rows of vineyards (
Figure 2 (17)); (2) flower strips at the perimeter of vineyards (
Figure 2(18)); and (3) small islands of flowering plants at the end of rows.
Predators and parasitoids that are most attracted by flowering strips are hymenopterans and dipterans [
59]. Flowering strips may also serve as a refugia and hibernation sites for numerous beneficial arthropods such as spiders, carabs, coccinellids, hemipterans, etc.
7.1. Predators
7.1.1. Arachnida
Araneae. In the late 1990s, the spider fauna of vineyards in northern parts of North America were completely unknown and their role was poorly known, even though spiders represent important natural enemies to phytophagous insects occurring in vineyards. Weekly pitfall trapping in 1998 and 1999 in Dunham and Iberville yielded over 4610 spiders belonging to 97 species and 16 families [
60]. Spider assemblages (diversity and community composition) were similar between the two vineyards. The most abundant species were the linyphiid
Tennesseellum formicum, the lycosid
Trochosa ruricola, the linyphiid
Erigone atra, and the linyphiid
Halorates plumosus.
Species turnover was high between sample dates, and activity and species richness of the guilds of web-building and hunting spiders indicated that many species that differ in foraging mode are active during all months of the growing season. The diverse ground-dwelling spider fauna in vineyards was therefore well positioned to prey on phytophagous pests, and their populations should be conserved in these agroecosystems.
Amongst mite predators found in Quebec vineyards, the anystid
Anystis baccarum is the most prevalent species. This generalist and voracious species predates on a wide range of prey, notably two-spotted spider mites (
Tetranychus urticae-Tetranychidae) and european red mite (
Panonychus ulmi-Tetranychidae), leafhoppers (
Figure 2(19)), tarnished plant bug nymphs and lepidopteran eggs.
Flowering strips in row middles of the vineyard favor the presence and establishment of natural enemies. Populations of
Anystis baccarum and parasitoids, mostly hymenopteran, provides natural suppression of leafhopper populations [
22].
Balaustium sp. (Erythraeidae) and Allothrombium sp. (Trombidiidae) are occasionally present in old vineyards. Neoseiulus fallacis and Thyphlodromus caudiglans are dominant phytoseiid predators. On grapevine canopy, they are rarely found because their mite preys are decimated and they are victims of intraguild predation exerted by anystids (J. Lasnier, comm. pers.). On ground-cover, predatory mites of the family Phytoseiidae are very effective natural enemies for the control of two-spotted spider mites. They attack the eggs and mobile stages of the two-spotted spider mite. In addition, they lay their eggs in two-spotted spider mite colonies, allowing future generations to prey directly on the two-spotted spider mites.
To keep phytophagous mites at acceptable levels in a sustainable manner, it is crucial to use pesticides that are least toxic to predatory mites. Such information has been established by [
61].
7.1.2. Coleoptera
Carabidae. Ground beetles are opportunists that eat many types of food. They are primarily predators of insects (including many pest species), arthropods and other organisms such as slugs. A study on the biodiversity of carabids was conducted by [
62]. Most species engage in random search for prey. Several species of ground beetles are phytophagous and have a significant beneficial impact by eating and destroying weed seeds. Typically, ground beetles do not cause any damage to vines.
Coccinellidae. Amongst the 22 species of coccinellids found in Quebec vineyards [
15,
63], the multicoloured asian lady beetle (
Harmonia axyridis-Coccinellidae) (
Figure 2(20)) has a special status because it is a voracious predator of arthropods and, potentially a pest if found in great abundance at harvest [
64,
65]. Found for the first time in Canada in Frelighsburg, Qc (a municipality near the Quebec-Vermont border) in 1994 [
66], it has displaced native coccinellids including
Coccinella spp.,
Coleomegilla sp.,
Hyperaspis spp.,
Hyppodamia spp. [
63]. Larvae and adults of these species are predatory. When pressed with grapes at harvest, adult
H. axyridis can taint wine [
65].
7.1.3. Diptera
Asilidae. Asilidae larvae feed in the soil during their development. The adults are formidable predators, capturing prey that are often larger than themselves in mid-flight, earning them the common name robber fly, also hunt their prey by lying in wait and ambushing it. The larvae and adults are predators.
Syrphidae. They are a large and very diverse group commonly named flower flies or hoverflies (
Figure 2 (21)). The eggs are usually attached singly to leaves or bark close to their prey. The larvae (maggots) of several species of the subfamily Syrphinae are important predators of leafhoppers, aphids, thrips, and phylloxera. Syrphid flies are widespread and frequently found in flower strips, where adults feed on pollen and nectar.
7.1.4. Hemiptera
The main families of hemipterans found in Quebec vineyards are Miridae (
Hyaliodes vitripennis,
Hyaliodes harti,
Campylomma verbasci and
Blepharidopterus sp.), Nabidae (
Hoplistoscelis spp. and
Nabis spp.), Pentatomidae (
Perillus spp.) and Reduviidae (
Zelus spp.) [
22]. These species exert important functions as predators of arthropods.
7.1.5. Neuroptera
The main species, the green lacewing (Chrysoperla carnea-Chrysopidae) and the brown lacewing (Hemerobius humilinus-Hemerobiidae), are generalist predators. The presence of strips of flowering plants in the vineyard promotes the development of colonies of Chrysopidae and Hemerobiidae as they feed on nectar, pollen, and honeydew before and during the egg-laying period.
7.2. Parasitoid Insects
Flower strips attract and harbor an array of species of parasitoids that generally feed on nectar and pollen. For example, a study conducted at the experimental farm of Frelighsburg, Qc, showed an increase of 960% of Hymenoptera (Ichneumonoidae, Chalcidoidea, and Proctotrupoidea) and 983% of Diptera (Cynipoidea, Syrphidae, and Tachinidae) in traps positioned at ca. 15m from flower strips [
59].
7.2.1. Diptera
Tachinidae. Adult Tachinidae feed on nectar and pollen and are very effective natural enemies, particularly against lepidopteran larvae. O’Hara [
67] mentioned
Istocheta aldrichi (Tachinidae) (
Figure 2(22)) in Nepean, On. Its eggs were observed on the scutellum of japanese beetle (
Popillia japonica-Scarabaeidae) adults (
Figure 2(23)) in 2015 in vineyards of Dunham, Qc (J. Lasnier, pers. comm.). Prevalence of parasitism by
I. aldrichi is increasing since as it was 29% in 2018, and 38% in 2019, such that japanese beetle population is conversely decreasing (J. Lasnier, pers. comm.).
7.2.2. Hymenoptera
The main species of parasitoids belong to the families Braconidae, Ichneumonidae (
Figure 2(24)), and the super-family (Chalcidoidea). Most Braconids are endoparasitoids of a number of coleopteran, dipteran, heteropteran, and lepidopteran species. Adult Ichneumonidae feed on nectar, sap, and sometimes small arthropods, most commonly lepidopteran and coleopteran larvae or pupae.
Three important families of Chalcidoidea for natural control of vineyard pests are Aphelinidae, Encyrtidae, and Trichogrammatidae. The great majority of Aphelinidae are ecto- or endoparasitoids of mealybugs, aphids, whiteflies, eggs of Lepidoptera and all stages of Diptera.
8. Technological Transfer
From 1997 up to now, the research results were transposed in an Integrated Pest Management program favoring biocontrol by conservation and augmentation. In parallel, efforts have focused on the transfer of information to viticulturists, as outlined in [
18,
22]
Table 1. Hence, a number of documents were published [
16,
17,
18,
68]. A Guide to the key arthropods of vineyards of Eastern Canada [
22] help viticulturists to quickly identify arthropods in their vineyards. A biovigilance approach has been envisaged to address protection problems before they arise in vineyards [
47]. Information about the management of insects of grapes and viticultural issues is available on the website of MAPAQ [
69].
9. The Future: Conclusions
As reviewed by Gerling [
70], there are several parameters and systems to measure viticultural sustainability. Basically, economic, social, and environmental components must be considered. Because this review is focused on the sustainability of arthropod management programs, we will briefly address the economic and social components, and will conclude with the environmental component.
From an economic point of view, the cost of practicing viticulture is helped by the relatively low cost of land, including in areas where climate is more suitable, i.e., southern Quebec. As with any agri-business, profit margins are slim, even on a medium term (10 years) perspective. As for any perennial crop, the cultivation of grapevines is a long-term commitment. From the start, the choice of cultivar is a major driver of type of wine to be produced and the agronomic practices that will be made to have sustained yields, including protection practices. The economic outlook is positive for Quebec wines of quality [
71].
From a social point of view, viticulture benefits from a positive image in Quebec. Viticulture lends itself to agritourism, bringing more value and customers to the wineries. Some consumers from Quebec have started to make comments about the importance of how viticultural and oenological steps are influencing their choice to buy a local wine (C.-H. de Coussergues, pers. comm.). The societal pressure is likely to be a formidable driver to make protection programs as sustainable as possible.
From an environmental point of view, rainfall is abundant in Quebec, and consequently, water usage is not a factor to take into account as a sustainability benchmark as it is in major wine-producing areas. In the last decade, participating vineyards received, on average < 1.5 insecticidal treatment and no acaricidal treatment per year. In the last two decades, several new chemicals were registered as fungicides and insecticides. However, few ecotoxicological studies have been published, notably on natural enemies. Likewise, systematic studies concerning the effect of flower strips, especially native plant species, are required to manage arthropod pests in a sustainable manner.
The area devoted to viticulture increased dramatically in Quebec over the years, allowing reservoirs of hosts plants much greater than in early 1980s. Several new rustic cultivars issued from North American hybrids were planted recently by Quebec viticulturists. They are generally sensitive to the form gallicoles of phylloxera, notably Oceola muscat. As increasing world trade and climate change scenarios will exert pressure, biovigilance is in order to keep Quebec viticulture as sustainable as possible.
Author Contributions
Conceptualization, C.V. and J.L.; writing—original draft preparation, C.V.; writing—review and editing, C.V. and J.L.; photographs, J.L.; project administration, C.V.; funding acquisition, C.V. All authors have read and agreed to the published version of the manuscript.
Funding
Agriculture and Agri-Food Canada.
Acknowledgments
We thank Charles-Henri de Coussergues and the late Victor Dietrich for their involvement over the years, Karine Bergeron for agronomic information, and Jérémie Côté for technical input. We thank our colleague taxonomists who added much value to our research projects.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Daane, K.M.; Vincent, C.; Isaacs, R.; Ioriatti, C. Entomological Opportunities and Challenges of Sustainable Viticulture in a Global Market. Annu. Rev. Entomol. 2018, 63, 193–214. [Google Scholar] [CrossRef] [PubMed]
- OIV. Statistical Report on World Vitiviniculture. 2019. Available online: http://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 9 January 2020).
- Jones, G.V.; Alves, F. Impact of climate change on wine production: A global overview and regional assessment in the Douro Valley of Portugal. Int. J. Glob. Warm. 2012, 4, 383–406. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Canadian Vintners Association. Industry Statistics. 2019. Available online: http://www.canadianvintners.com/industry-statistics/ (accessed on 13 December 2019).
- Conseil des Vins du Québec. Available online: https://vinsduquebec.com/a-propos/ (accessed on 15 January 2020).
- RACJQ. Registre des Titulaires de Permis de Production Artisanale en Vigueur en Date du 2020-01-16. Régie des Alcools des Jeux et des Courses du Québec. 2019. Available online: https://www.racj.gouv.qc.ca/fileadmin/documents/Accueil/Registre_publique/RIF_Artisan.pdf (accessed on 21 January 2020).
- Isaacs, R.; Vincent, C.; Bostanian, N.J. Vineyard IPM in a changing world: Adapting to new pests, tactics and challenges. In Arthropod Management in Vineyards: Pests Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 475–484. [Google Scholar]
- Hannah, L.; Roehrdanz, P.R.; Ikegami, M.; Shepard, A.M.; Shaw, M.R.; Tabor, G.; Zhi, L.; Marquet, P.A.; Hijmans, R.J. Climate change, wine, and conservation. Proc. Nat. Acad. Sci. USA 2013, 110, 6907–6912. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, F. L’essor du vignoble au Québec. Histoire de climats et de goûts. Cybergeo Eur. J. Geogr. 2001, 190. Available online: http://www.cybergeo.eu/index3747.html (accessed on 11 February 2020). [CrossRef]
- Atlas Agroclimatique du Québec. Moyenne de la Longueur de la Saison Sans Gel (Seuil 0 °C). Available online: http://www.agrometeo.org/index.php/atlas/map/moyenne17/M0/1979-2008/false (accessed on 9 January 2020).
- Jolivet, Y.; Dubois, J.-M. Essais de Protection Hivernale de la Vigne Hybride au Moyen d’une Toile Isolante avec un fil Chauffant; Research Bulletin 180; Université de Sherbrooke: Sherbrooke, QC, Canada, 2006; p. 15. Available online: https://www.usherbrooke.ca/geomatique/fileadmin/sites/flsh/geomatique/bulletin_180.pdf (accessed on 9 January 2020).
- Vincent, C.; Isaacs, R.; Bostanian, N.J.; Lasnier, J. Principles of Arthropod Pest Management in Vineyards. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–16. [Google Scholar]
- Carisse, O.; Lasnier, J. Les vignobles du Québec face aux maladies. Phytoma 2016, 698, 11–14. [Google Scholar]
- Bostanian, N.J.; Vincent, C.; Goulet, H.; Lesage, L.; Lasnier, J.; Bellemare, J.; Mauffette, Y. The arthropod fauna of Quebec vineyards with particular reference to phytophagous arthropods. J. Econ. Entomol. 2003, 96, 1221–1229. [Google Scholar] [CrossRef]
- Vincent, C.; Lasnier, J.; Bostanian, N.J. La Viticulture au Québec; Technical Bulletin: Saint-Jean-sur-Richelieu, QC, Canada, 2002; Volume 1, p. 42. Available online: http://eduportfolio.org/6644 (accessed on 11 February 2020).
- Vincent, C.; Bostanian, N.J.; Lasnier, J. La Viticulture au Québec; Technical Bulletin: Saint-Jean-sur-Richelieu, QC, Canada, 2005; Volume 2, p. 53. Available online: http://eduportfolio.org/6644 (accessed on 11 February 2020).
- Vincent, C.; Bostanian, N.J.; Lasnier, J. Biodiversity and Management of Arthropods in Cool-Climate Vineyards. In Proceedings of the 2nd International Conference on Northern Viticulture, Saint-Hyacinthe, QC, Canada, 9–11 November 2009; pp. 189–199. Available online: http://eduportfolio.org/6644 (accessed on 11 February 2020).
- Vincent, C.; Lasnier, J. Les arthropodes des vignobles québécois. Phytoma 2016, 697, 45–48. [Google Scholar]
- Vincent, C.; Lowery, T.; Parent, J.-P. The Entomology of Vineyards in Canada. Can. Entomol. 2018, 150, 697–715. [Google Scholar] [CrossRef]
- Lasnier, J.; Vincent, C.; de Coussergues, C.-H. Evolution de la viticulture québécoise depuis trente ans. Phytoma 2016, 696, 37–40. [Google Scholar]
- Lasnier, J.; McFadden-Smith, W.; Moreau, D.; Bouchard, P.; Vincent, C. Guide to the Key Arthropods of Vineyards of Eastern Canada; Catalogue No. A59-72/2019E-PDF, Technical Bulletin; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2019; p. 114. Available online: http://publications.gc.ca/site/eng/9.868732/publication.html (accessed on 22 January 2020).
- Bloesch, B.; Viret, O. Stades phénologiques repères de la vigne. Rev. Suisse Vitic. Arboric. Hortic. 2008, 40, 6–10. [Google Scholar]
- Kreiter, S. Pest Management in Organic Grape Production. pp. 173-217. In Handbook of Pest Management in Organic Farming; Vacante, V., Kreiter, S., Eds.; CABI: Wallingford, UK, 2018; p. 559. [Google Scholar]
- Lesage, L. Flea beetles of the genus Altica found on grape in northeastern North America (Coleoptera: Chrysomelidae). J. Entomol. Soc. Ont. 2002, 133, 3–46. [Google Scholar]
- Lesage, L.; Bouchard, P.; Goulet, H. Leaf beetle diversity and abundance in two Quebec vineyards (Coleoptera, Chrysomelidae). Nouv. Revue Entomol. 2008, 25, 3–16. [Google Scholar]
- Fleury, D.; Mauffette, Y.; Méthot, S.; Vincent, C. Population activity of Lygus lineolaris (Heteroptera: Miridae) adults at the periphery and inside a commercial vineyard. Eur. J. Entomol. 2010, 107, 527–534. [Google Scholar] [CrossRef]
- Bostanian, N.J.; Bourgeois, G.; Plouffe, D.; Vincent, C. Modelling phytophagous mirid nymphs in cool-climate vineyards. Phytoparasitica 2014, 42, 13–22. [Google Scholar] [CrossRef]
- Fleury, D.; Paré, J.; Vincent, C. Identification histologique des lésions causées sur l’inflorescence de Vitis vinifera L. (Vitacées) par Lygus lineolaris (Palisot de Beauvois) (Heteroptera: Miridae). Rev. Cytol. Biol. Vég. 2003, 26, 8–18. [Google Scholar]
- Fleury, D.; Paré, J.; Vincent, C.; Mauffette, Y. Feeding impact of Lygus lineolaris (Heteroptera: Miridae) on Vitis vinifera: A behavioural and histological study. Can. J. Bot. 2006, 84, 493–500. [Google Scholar] [CrossRef]
- Fleury, D.; Bostanian, N.J.; Vincent, C.; Mauffette, Y. The intrinsic residual toxicity of two insecticides on three field populations of Lygus lineolaris collected along the St. Laurence valley in eastern Canada. Pest Man. Sci. 2007, 63, 495–499. [Google Scholar] [CrossRef]
- Saguez, J.; Olivier, C.; Lasnier, J.; Hamilton, A.; Stobbs, L.W.; Vincent, C. Biology and Integrated Management of Leafhoppers and Phytoplasma Diseases in Vineyards of Eastern Canada; Technical Bulletin No. 12429E, Catalogue No. A59-32/2015E-PDF; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 2015; p. 67.
- Pfeiffer, D.G. Japanese Beetle and Other Coleoptera Feeding on Grapevines in Eastern North America. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 403–429. [Google Scholar]
- Potter, D.A.; Held, D.W. Biology and management of the Japanese beetle. Annu. Rev. Entomol. 2002, 47, 175–205. [Google Scholar] [CrossRef]
- Saguez, J.; Lasnier, J.; Vincent, C. First record of Drosophila suzukii in Quebec vineyards. J. Intern. Sci. Vigne Vin 2013, 47, 69–72. [Google Scholar] [CrossRef]
- Rott, M.; Johnson, R.; Masters, C.; Green, M. First report of Bois Noir phytoplasma in grapevine in Canada. Plant Dis. 2007, 91, 1682. [Google Scholar] [CrossRef]
- Olivier, C.; Vincent, C.; Saguez, J.; Galka, B.; Weintraub, P.; Maixner, M. Leafhoppers and planthoppers: Their bionomics, pathogen transmission and management in vineyards. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 253–270. [Google Scholar]
- Olivier, C.Y.; Saguez, J.; Stobbs, L.W.; Lowery, D.T.; Galka, B.; Whybourne, K.; Bittner, L.; Xiensheng, C.; Vincent, C. Occurrence of phytoplasmas in leafhoppers and cultivated grapevines in Canada. Agric. Ecosyst. Environ. 2014, 195, 91–97. [Google Scholar] [CrossRef]
- Vincent, C.; Olivier, C.; Lasnier, J.; Saguez, J. Dix questions sur le système cicadelles/phytoplasmes/vignes. Antennae 2015, 22, 3–6. [Google Scholar]
- Saguez, J.; Vincent, C. A method for continuous rearing of grapevine leafhoppers, Erythroneura spp. (Hemiptera: Cicadellidae). Can. Entomol. 2011, 143, 102–104. [Google Scholar] [CrossRef]
- Saguez, J.; Lemoyne, P.; Giordanengo, P.; Olivier, C.; Lasnier, J.; Mauffette, Y.; Vincent, C. Characterization of the feeding behavior of three Erythroneura species on grapevine by histological and DC-electrical penetration techniques. Entomol. Exp. Appl. 2015, 157, 227–240. [Google Scholar] [CrossRef]
- Saguez, J.; Olivier, C.; Hamilton, A.; Lowery, D.T.; Stobbs, L.W.; Lasnier, J.; Galka, B.; Chen, X.; Mauffette, Y.; Vincent, C. Diversity and abundance of leafhoppers (Hemiptera: Cicadellidae) in Canadian vineyards. J. Insect Sci. 2014, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Bostanian, N.J.; Bourgeois, G.; Vincent, C.; Plouffe, D.; Trudeau, M.; Lasnier, J. Modeling Leafhopper Nymphs in Temperate Vineyards for Optimal Sampling. Environ. Entomol. 2006, 35, 1477–1482. [Google Scholar] [CrossRef][Green Version]
- Brouillet, L.; Coursol, F.; Meades, S.J.; Favreau, M.; Anions, M.; Bélisle, P.; Desmet, P. Vitis, In VASCAN, the Database of Vascular Plants of Canada. Available online: http://data.canadensys.net/vascan/name/Vitis (accessed on 11 February 2020).
- Lasnier, J.; Vincent, C. Le phylloxera de la vigne. Antennae 2019, 26, 5–10. [Google Scholar]
- Isaacs, R.; Teixeira, L.; Jenkins, P.; Botero Neerdals, N.; Loeb, G.; Saunders, M. Biology and management of grape berry moth in North American vineyard ecosystems. In Arthropod Management in Vineyards: Pests Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 361–381. [Google Scholar]
- Carisse, O.; Fall, M.L.; Vincent, C. Using a biovigilance approach for pest and disease management in Quebec vineyards. Can. J. Plant Pathol. 2017, 39, 393–404. [Google Scholar] [CrossRef]
- Vincent, C.; Dumont, S.; de Tonnancour, P. The grape rootworm, Fidia viticida (Coleoptera: Chrysomelidae), newly recorded from Quebec. Phytoprotection 2017, 91, 17–19. [Google Scholar] [CrossRef]
- Jubb, G.L. History of entomological research on grapes and other crops in Erie County, Pennsylvania. Melsheimer Entomol. Ser. 1977, 22, 7–11. [Google Scholar]
- Leskey, T.C.; Nielsen, A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Fogain, R.; Graff, S. First record of the invasive pest, Halyomorpha halys (Hemiptera: Pentatomidae), in Ontario and Quebec. J. Entomol. Soc. Ont. 2011, 142, 45–48. [Google Scholar]
- Chouinard, G.; Larose, M.; Légaré, J.-P.; Bourgeois, G.; Racette, G.; Barrette, M. Interceptions and captures of Halyomorpha halys (Hemiptera: Pentatomidae) in Quebec from 2008 to 2018. Phytoprotection 2018, 98, 46–50. [Google Scholar] [CrossRef]
- Pfeiffer, D.G.; Leskey, T.C.; Burrack, H.J. Threatening the harvest: The threat from three invasive insects in late season vineyards. In Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Bostanian, N.J., Vincent, C., Isaacs, R., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 449–474. [Google Scholar]
- Mohekar, P.; Lapis, T.J.; Wiman, N.G.; Lim, J.; Tomasino, E. Brown marmorated stink bug taint in Pinot noir: Detection and consumer rejection thresholds of trans-2-decenal. Am. J. Enol. Vitic. 2017, 68, 120–126. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, Y.-L.; Leskey, T.C. A review of biology and management of Lycorma delicatula (Hemiptera: Fulgoridae), an emerging global invasive species. J. Asia-Pac. Entomol. 2019, 22, 589–596. [Google Scholar] [CrossRef]
- Canadian Food Inspection Agency. Spotted Lanternfly (Lycorma Delicatula). 2019. Available online: https://www.inspection.gc.ca/plant-health/plant-pests-invasive-species/insects/spotted-lanternfly/eng/1433365581428/1433365581959 (accessed on 10 January 2020).
- Wakie, T.T.; Neven, L.G.; Yee, W.L.; Lu, Z. The establishment risk of Lycorma delicatula (Hemiptera: Fulgoridae) in the United States and globally. J. Econ. Entomol. 2020, 113, 306–314. [Google Scholar] [CrossRef]
- Wallingford, A.K.; Fuchs, M.F.; Martinson, T.; Hesler, S.; Loeb, G.M. Slowing the Spread of Grapevine Leafroll-Associated Viruses in Commercial Vineyards With Insecticide Control of the Vector, Pseudococcus maritimus (Hemiptera: Pseudococcidae). J. Insect Sci. 2015, 15, 112. [Google Scholar] [CrossRef]
- Bostanian, N.J.; Goulet, H.; O’Hara, J.; Masner, L.; Racette, G. Towards Insecticide Free Apple Orchards: Flowering Plants to Attract Beneficial Arthropods. Biocontrol Sci. Technol. 2004, 14, 25–37. [Google Scholar] [CrossRef]
- Bolduc, E.; Buddle, C.M.; Bostanian, N.J.; Vincent, C. The Ground-Dwelling Spiders (Aranae) of two vineyards in Southern Quebec. Environ. Entomol. 2005, 34, 635–645. [Google Scholar] [CrossRef]
- Laurin, M.-C.; Bostanian, N.J. Laboratory studies to elucidate the residual toxicity of eight insecticides to Anystis baccarum (Acari: Anystidae). J. Econ. Entomol. 2007, 100, 1210–1214. [Google Scholar] [CrossRef]
- Goulet, H.; LeSage, L.; Bostanian, N.J.; Vincent, C.; Lasnier, J. Diversity and seasonal activity of ground beetles (Coleoptera: Carabidae) from two vineyards in southern Quebec. Ann. Entomol. Soc. Am. 2004, 97, 1263–1272. [Google Scholar] [CrossRef][Green Version]
- Lucas, E.; Vincent, C.; Labrie, G.; Chouinard, G.; Fournier, F.; Pelletier, F.; Bostanian, N.J.; Coderre, D.; Mignault, M.P.; Lafontaine, P. The multicolored Asian ladybeetle Harmonia axyridis (Coleoptera: Coccinellidae) in Quebec agroecosystems ten years after its arrival. Eur. J. Entomol. 2007, 104, 737–743. [Google Scholar] [CrossRef]
- Lucas, E.; Labrie, G.; Vincent, C.; Kovach, J. The Multicoloured Asian Ladybeetle Harmonia axyridis—Beneficial or nuisance organism. In Biological Control: A Global Perspective. Case Histories from Around the World; Vincent, C., Goettel, M., Lazarovits, G., Eds.; CABI Publishing: Wallingford, UK, 2007; pp. 38–52. [Google Scholar]
- Vincent, C.; Pickering, G. Multicolored Asian ladybeetle, Harmonia axyridis (Coleoptera: Coccinellidae). In Biological Control Programmes in Canada 2001–2012; Mason, P.G., Gillespie, D.R., Eds.; CABI: Wallingford, UK, 2013; pp. 192–198. [Google Scholar]
- Coderre, D.; Lucas, E.; Gagné, I. The occurrence of Harmonia axyridis (Pallas) (Coleoptera, Coccinellidae) in Canada. Can. Entomol. 1995, 127, 609–611. [Google Scholar] [CrossRef]
- O’Hara, J.E. New tachinid records for the United States and Canada. Tachinid Times 2014, 27, 34–40. [Google Scholar]
- Bostanian, N.J.; Vincent, C.; Isaacs, R. Arthropod Management in Vineyards: Pests, Approaches, and Future Directions; Springer: Dordrecht, The Netherlands, 2012; p. 505. [Google Scholar]
- MAPAQ. Agri-Réseau Vigne et Vin. 2020. Available online: https://www.agrireseau.net/vigne-vin (accessed on 9 January 2020).
- Gerling, C. Environmentally Sustainable Viticulture. Practices and Practicality; CRC Press: Boca Raton, FL, USA, 2015; p. 414. [Google Scholar]
- van Leeuwen, C.; Darriet, P. The Impact of Climate Change on Viticulture and Wine Quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).