In Vivo Pollen Tube Growth and Evidence of Self-Pollination and Prefloral Anthesis in cv. Macabeo (Vitis vinifera L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Treatments
2.3. Seed Number
2.4. Flower Sampling for Pollination Experiments and Floral Structure Analysis
2.5. Statistical Analysis
3. Results
3.1. Analysis of the Mean Seed Number per Grape and Fruit Set
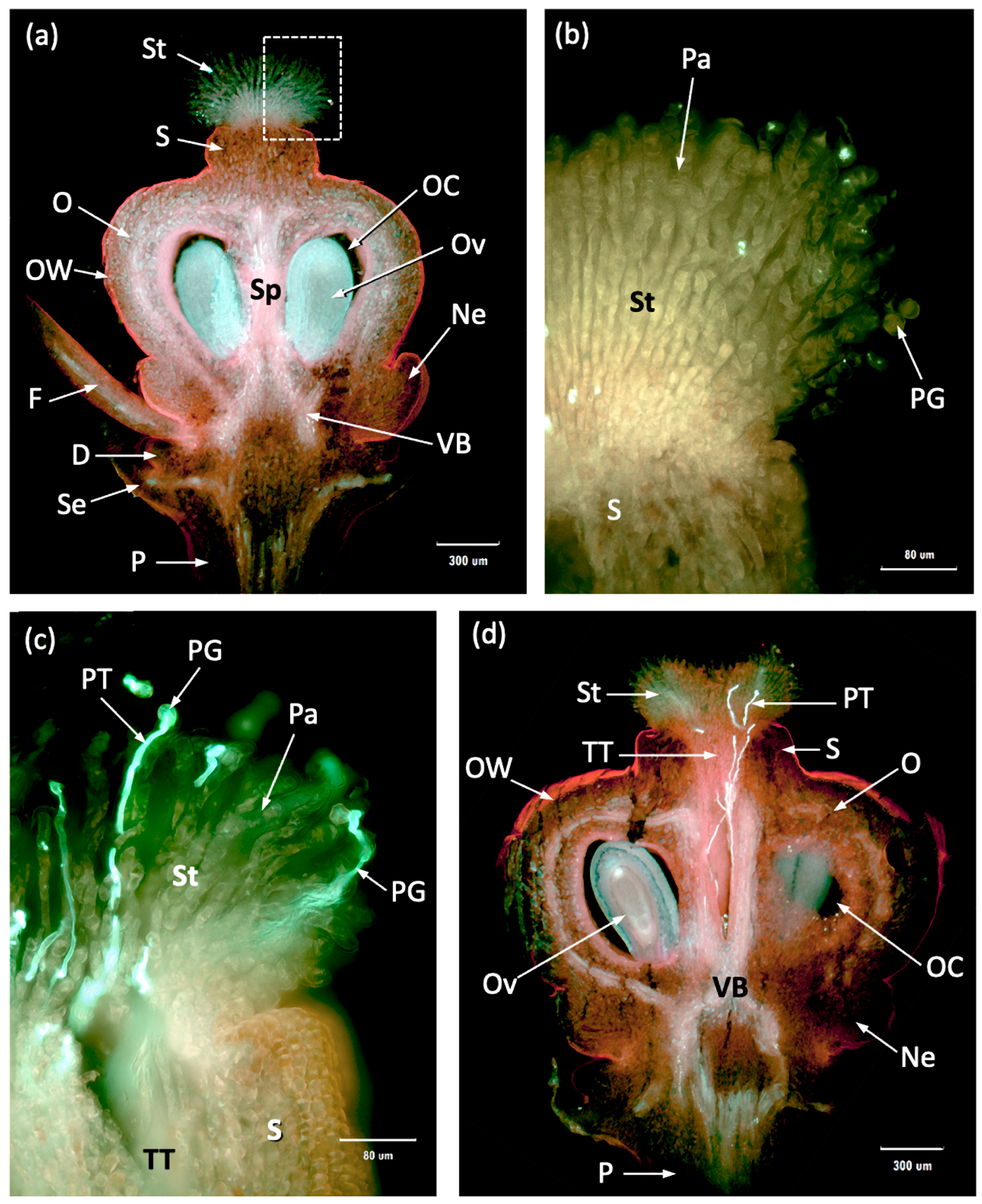
3.2. Macabeo Flower Structure
3.3. In Vivo Pollen Tube Growth and Ovule Fertilization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO Resource Database, Crops. Available online: http://www.fao.org/faostat/en/#data/ (accessed on 26 October 2020).
- Robinson, J. Macabeo. In Oxford Companion to Wine; Oxford University Press: Oxford, UK, 2006; p. 414. ISBN 0-19-860990-6. [Google Scholar]
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, Including Their Origins and Flavours; Penguin: London, UK, 2013. [Google Scholar]
- Longbottom, M.L.; Dry, P.R.; Sedgley, M. Observations on the morphology and development of star flowers of Vitis vinifera L. cvs Chardonnay and Shiraz. Aust. J. Grape Wine Res. 2008, 14, 203–210. [Google Scholar]
- Vasconcelos, M.C.; Greven, M.; Winefield, C.S.; Trought, M.C.; Raw, V. The flowering process of Vitis vinifera: A review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar]
- Coito, J.L.; Silva, H.G.; Ramos, M.J.; Cunha, J.; Eiras-Dias, J.; Amâncio, S.; Costa, M.M.; Rocheta, M. Vitis flower types: From the wild to crop plants. PeerJ 2019, 7, e7879. [Google Scholar] [CrossRef]
- Bordeu, E.; Gil, G. Fructificación de la vid, cv. Moscatel Rosado, sometida a polinización artificial y eliminación manual de caliptras. Int. J. Agric. Nat. Resour. 1983, 10, 279–281. [Google Scholar] [CrossRef]
- Sampson, B.; Noffsinger, S.; Gupton, C.; Magee, J. Pollination biology of the muscadine grape. HortScience 2001, 36, 120–124. [Google Scholar] [CrossRef]
- Chkhartishvili, N.; Vashakidze, L.; Gurasashvili, V.; Maghradze, D. Type of pollination and indices of fruit set of some Georgian grapevine varieties. Vitis-Geilweilerhof- 2006, 45, 153. [Google Scholar]
- Kearns, C.A.; Inouye, D.W. Techniques for Pollination Biologists; University Press of Colorado: Boulder, CO, USA, 1993. [Google Scholar]
- McGregor, S.E. Insect Pollination of Cultivated Crop Plants; Agricultural Research Service, US Department of Agriculture: Washington, DC, USA, 1976; Volume 496.
- Muñoz-Rodríguez, A.F.; Tormo, R.; Silva, M.I. Pollination Dynamics in Vitis vinifera L. Am. J. Enol. Vitic. 2011, 62, 113–117. [Google Scholar] [CrossRef]
- Pratt, C. Reproductive anatomy in cultivated grapes-a review. Am. J. Enol. Vitic. 1971, 22, 92–109. [Google Scholar]
- Mullins, L. Biology of the Grapevine; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Zhang, T.; Luan, L.; Zhang, L.; Meng, L.; Pei, G.; Li, J. Flowering information of wine grape affects its flower visitors. In Proceedings of the BIBE 2019, The Third International Conference on Biological Information and Biomedical Engineering; VDE, Hangzhou, China, 20–22 July 2019; pp. 1–5. [Google Scholar]
- Meneghetti, S.; Calò, A.; Gardiman, M. Flower Biology of Grapevine. A Review; Firenze University Press: Florence, Italy, 2006. [Google Scholar]
- Petrie, P.R.; Clingeleffer, P.R. Effects of temperature and light (before and after budburst) on inflorescence morphology and flower number of Chardonnay grapevines (Vitis vinifera L.). Aust. J. Grape Wine Res. 2005, 11, 59–65. [Google Scholar] [CrossRef]
- Eltom, M.; Trought, M.C.; Agnew, R.; Parker, A.; Winefield, C.S. Pre-budburst temperature influences the inner and outer arm morphology, phenology, flower number, fruitset, TSS accumulation and variability of Vitis vinifera L. Sauvignon Blanc bunches. Aust. J. Grape Wine Res. 2017, 23, 280–286. [Google Scholar] [CrossRef]
- Culley, T.M.; Klooster, M.R. The cleistogamous breeding system: A review of its frequency, evolution, and ecology in angiosperms. Bot. Rev. 2007, 73, 1. [Google Scholar] [CrossRef]
- Lord, E.M. Cleistogamy: A tool for the study of floral morphogenesis, function and evolution. Bot. Rev. 1981, 47, 421–449. [Google Scholar] [CrossRef]
- Staudt, G. Opening of flowers and time of anthesis in grapevines, Vitis vinifera L. Vitis 1999, 38, 15–20. [Google Scholar]
- Miaja, M.L.; Porporato, M.; Caramiello, R.; Vallania, R. Pollen-stigma interactions in Vitis vinifera L. cv. Barbera. Allionia 1999, 36, 35–40. [Google Scholar]
- Heazlewood, J.E.; Wilson, S. Anthesis, pollination and fruitset in Pinot Noir. Vitis-Geilweilerhof- 2004, 43, 65–68. [Google Scholar]
- Jovanovic-Cvetkovic, T.; Micic, N.; Djuric, G.; Cvetkovic, M. Pollen Morphology and Germination of Indigenous Grapevine Cultivars Žilavka and Blatina (Vitis vinifera L.). Agrolife Sci. J. 2016, 5, 105–109. [Google Scholar]
- Pereira, M.R.; Ribeiro, H.; Cunha, M.; Abreu, I. Comparison of pollen quality in Vitis vinifera L. cultivars. Sci. Hortic. 2018, 227, 112–116. [Google Scholar] [CrossRef]
- Sharafi, Y.; Bahmani, A. Pollen germination, tube growth and longevity in some cultivars of Vitis vinifera L. Afr. J. Microbiol. Res. 2011, 5, 1102–1107. [Google Scholar]
- Sheehan, D.; Hrapchak, B. Theory and Practice of Histotechnology, 1980; Battelle Press: Columbus, OH, USA, 1980. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2016. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. 2020. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 26 October 2020).
- Wickham, H. ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org/ (accessed on 26 October 2020).
- Heslop-Harrison, Y.; Shivanna, K.R. The receptive surface of the angiosperm stigma. Ann. Bot. 1977, 41, 1233–1258. [Google Scholar] [CrossRef]
- Bouard, J. Tissus et organes de la vigne. In Ribereau Gayon, Jean Traite d’Ampelologie; Sciences e Techniques de la Vigne; Academic Publishers: Dordrecht, The Netherlands, 1971. [Google Scholar]
- Kimura, P.H.; Okamoto, G.; Hirano, K. Artificial pollination in Vitis coignetiae Pulliat. Vitis 1998, 37, 83–86. [Google Scholar]
- Mesejo, C.; Martínez-Fuentes, A.; Reig, C.; Agustí, M. The effective pollination period in ‘Clemenules’ mandarin, ‘Owari’Satsuma mandarin and ‘Valencia’sweet orange. Plant Sci. 2007, 173, 223–230. [Google Scholar] [CrossRef]







| Treatment | N | Mean | HSD | KW | SE | Skew | Kurtosis |
|---|---|---|---|---|---|---|---|
| Emasculation | 87 | 1.43 | a | a | 0.08 | 0.45 | −0.03 |
| Pollination | 101 | 1.39 | a | a | 0.07 | 0.25 | −0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Breijo, F.; Armiñana, J.R.; Garmendia, A.; Cebrián, N.; Beltrán, R.; Merle, H. In Vivo Pollen Tube Growth and Evidence of Self-Pollination and Prefloral Anthesis in cv. Macabeo (Vitis vinifera L.). Agriculture 2020, 10, 647. https://doi.org/10.3390/agriculture10120647
García-Breijo F, Armiñana JR, Garmendia A, Cebrián N, Beltrán R, Merle H. In Vivo Pollen Tube Growth and Evidence of Self-Pollination and Prefloral Anthesis in cv. Macabeo (Vitis vinifera L.). Agriculture. 2020; 10(12):647. https://doi.org/10.3390/agriculture10120647
Chicago/Turabian StyleGarcía-Breijo, Francisco, José Reig Armiñana, Alfonso Garmendia, Nuria Cebrián, Roberto Beltrán, and Hugo Merle. 2020. "In Vivo Pollen Tube Growth and Evidence of Self-Pollination and Prefloral Anthesis in cv. Macabeo (Vitis vinifera L.)" Agriculture 10, no. 12: 647. https://doi.org/10.3390/agriculture10120647
APA StyleGarcía-Breijo, F., Armiñana, J. R., Garmendia, A., Cebrián, N., Beltrán, R., & Merle, H. (2020). In Vivo Pollen Tube Growth and Evidence of Self-Pollination and Prefloral Anthesis in cv. Macabeo (Vitis vinifera L.). Agriculture, 10(12), 647. https://doi.org/10.3390/agriculture10120647







