Influence of Spray Technology and Application Rate on Leaf Deposit and Ground Losses in Mountain Viticulture
Abstract
1. Introduction
2. Materials and Methods
2.1. Spray Application Equipment
- A High-Volume Sprayer (HVS) with a spray gun (Honda GX 120, Hamamatsu, Japan) equipped with a 4.0 HP engine, with a hose length of 100 m, calibrated at a nominal volume of 1000 L ha−1.
- A conventional Orchard Sprayer (OS) equipped with a vertical tower (Arcadia Terra, Model Cronos, Greece) calibrated at 500 L ha−1 (OS500).
- The same conventional Orchard Sprayer calibrated at 250 L ha−1 (OS250).
- A Motorized air-assisted knapsack sprayer (CIFARELLI Mist Blower M1200, CIFARELLI, Voghera, Italy) adapted for Low Volume Spray (LVS) calibrated at 200 L ha−1.
2.2. Experimental Design and Spraying Technique
2.3. Determination of the Relationship between Leaf Weight-Area and Estimation of the Leaf Area Index
2.4. Characterization of the Canopy
2.5. Leaf Sampling Procedure
2.6. Quantification of Spray Deposition on Leaves
2.7. Data Normalization
2.8. Evaluation of Spray Losses to the Ground
2.9. Statistical Analyses
3. Results
3.1. Relationship between Leaf Weight-Area and Estimation of the Leaf Area Index
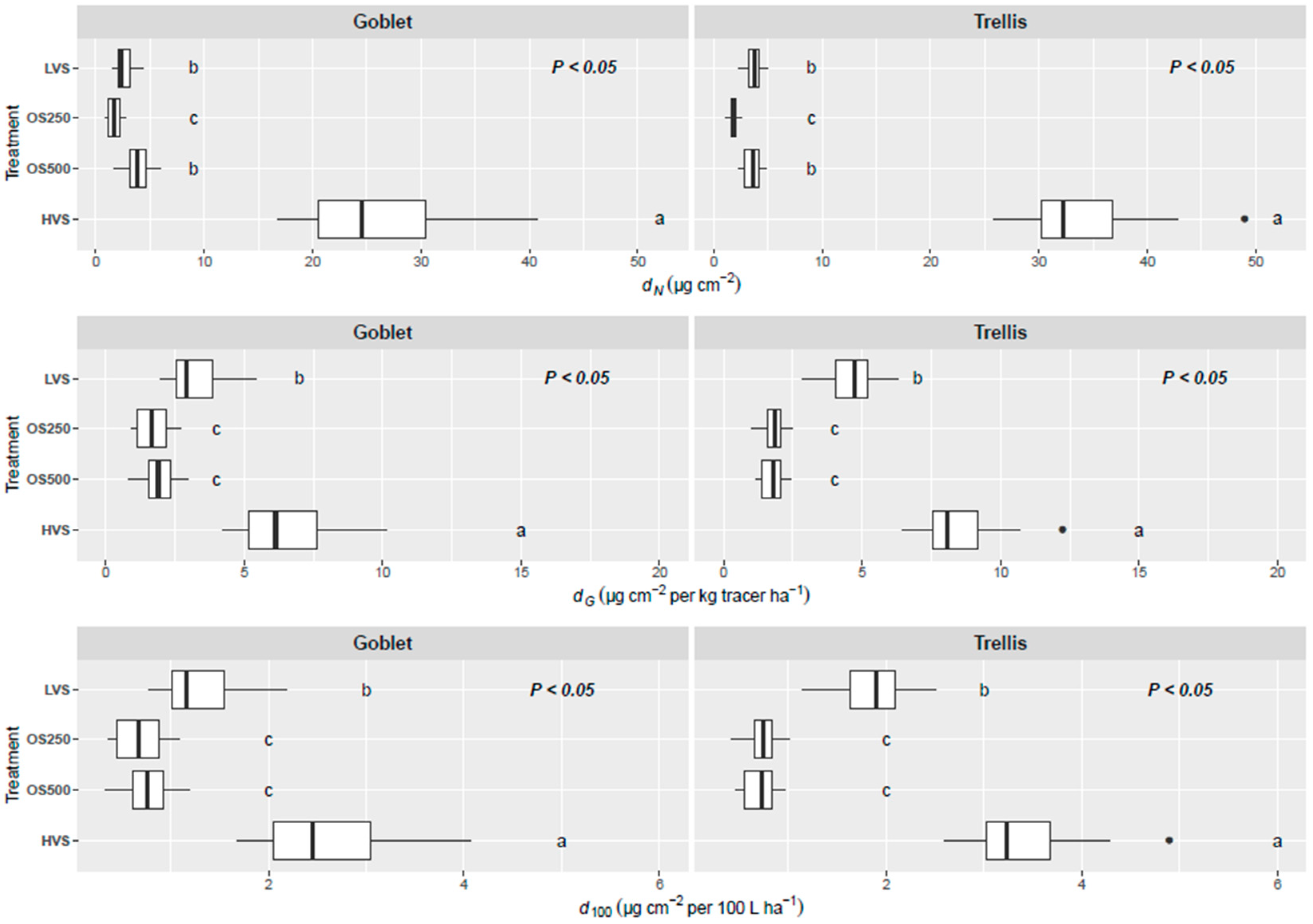
3.2. Quantification of Spray Deposition on Leaves
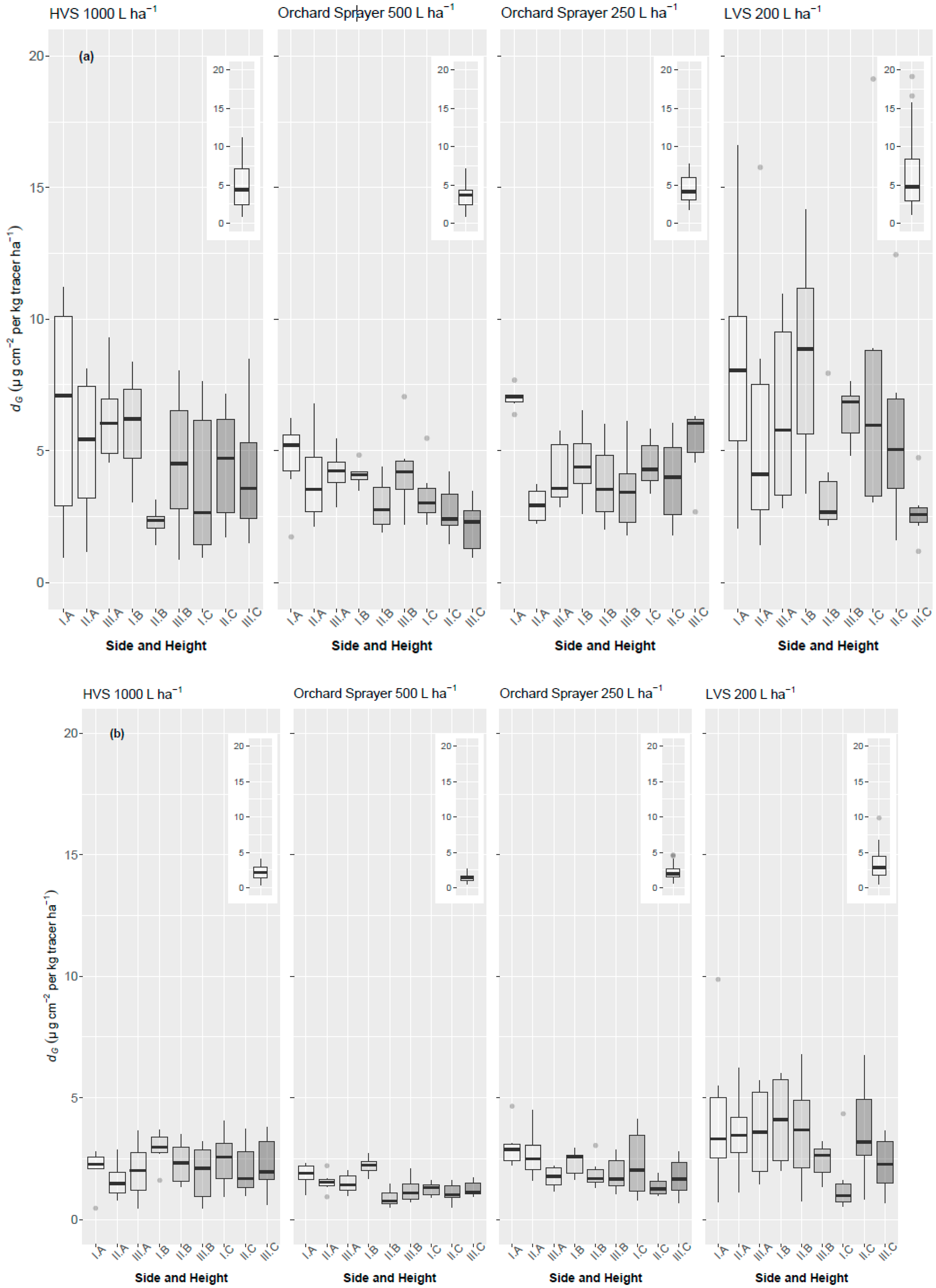
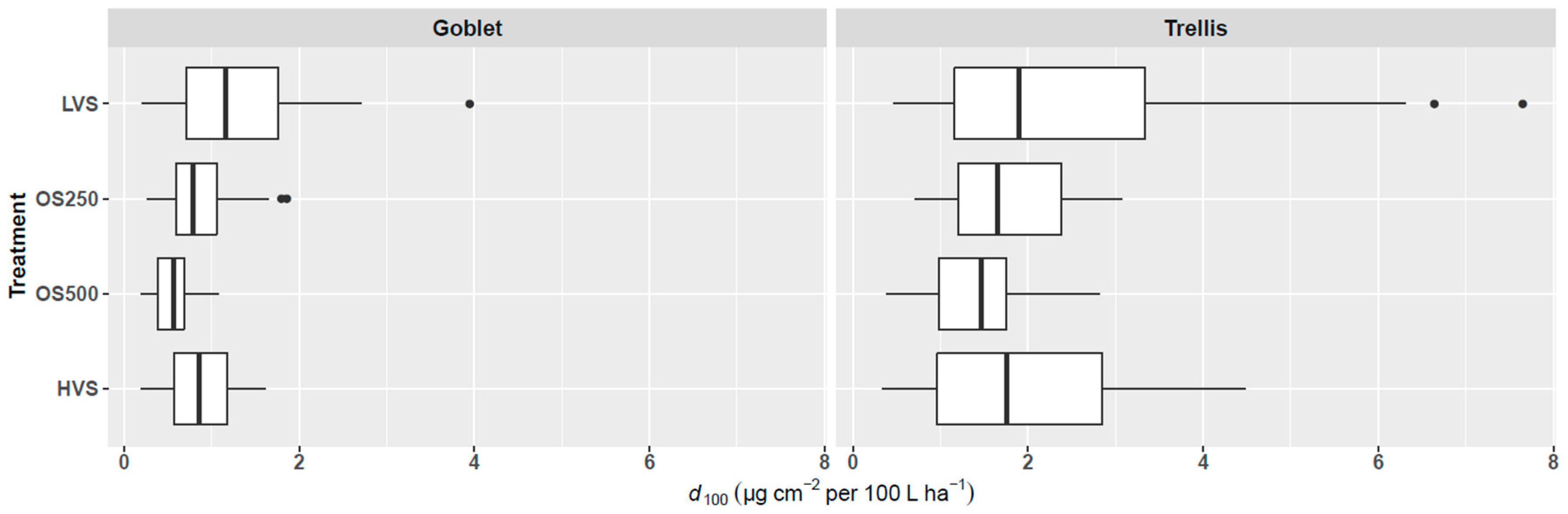
3.3. Losses to the Ground
4. Discussion
4.1. Deposition on Leaves
4.2. Losses to the Ground
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. OJ L 309. 24 November 2009, pp. 71–86. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 15 October 2018).
- BTSF. Available online: https://ec.europa.eu/chafea/food/index_en.htm (accessed on 30 July 2019).
- Matthews, G.; Bateman, R.; Miller, P. Pesticide Application Methods, 4th ed.; Wiley & Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Balsari, P.; Gil, E.; Marucco, P.; van de Zande, J.C.; Nuyttens, D.; Herbst, A.; Gallart, M. Field-crop-sprayer potential drift measured using test bench: Effects of boom height and nozzle type. Biosyst. Eng. 2017, 154, 3–13. [Google Scholar] [CrossRef]
- Lefrancq, M.; Payraudeau, S.; Verdú, A.J.G.; Maillard, E.; Millet, M.; Imfeld, G. Fungicides transport in runoff from vineyard plot and catchment: Contribution of non-target areas. ESPR 2014, 21, 4871–4882. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.V.; Walklate, P.J.; Murray, R.A.; Richardson, G.M. Spray deposits and losses in different sized apple trees from an axial fan orchard sprayer: 1. Effects of spray liquid flow rate. Crop Prot. 2001, 20, 13–30. [Google Scholar] [CrossRef]
- Miranda-Fuentes, A.; Llorens, J.; Rodríguez-Lizana, A.; Cuenca, A.; Gil, E.; Blanco-Roldán, G.L.; Gil-Ribes, J.A. Assessing the optimal liquid volume to be sprayed on isolated olive trees according to their canopy volumes. Sci. Total Environ. 2016, 568, 296–305. [Google Scholar] [CrossRef]
- Arvidsson, T.; Bergström, L.; Kreuger, J. Spray drift as influenced by meteorological and technical factors. Pest Manag. Sci. 2011, 67, 586–598. [Google Scholar] [CrossRef]
- Gil, E.; Llorens, J.; Llop, J.; Fàbregas, X.; Gallart, M. Use of a terrestrial LIDAR sensor for drift detection in vineyard spraying. Sensors 2013, 13, 516–534. [Google Scholar] [CrossRef]
- Gregorio, E.; Rosell-Polo, J.R.; Sanz, R.; Rocadenbosch, F.; Solanelles, F.; Garcerá, C.; Chueca, P.; Arnó, J.; del Moral, I.; Masip, J.; et al. LIDAR as an alternative to passive collectors to measure pesticide spray drift. Atmosp. Environ. 2014, 82, 83–93. [Google Scholar] [CrossRef]
- Nuyttens, D.; De Schampheleire, M.; Verboven, P.; Sonck, B. Comparison between indirect and direct spray drift assessment methods. Biosyst. Eng. 2010, 105, 2–12. [Google Scholar] [CrossRef]
- Landers, A.J. Developments towards an Automatic Precision Sprayer for Fruit Crop Canopies. In Proceedings of the ASABE Annual International Meeting, Pittsburg, PA, USA, 20–23 June 2010. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- OIV-International Organization of Vine and Wine 2018; OIV Statistical Report on World Vitiviniculture; World Vitiviniculture: Paris, France, 2018.
- Michael, C.; Gil, E.; Gallart, M.; Kanetis, L.; Stavirinides, M.C. Evaluating the effectiveness of low volume spray application using air-assisted knapsack sprayers in wine vineyards. Int. J. Pest Manag. 2020. [Google Scholar] [CrossRef]
- Pergher, G.; Gubiani, R.; Tonetto, G. Foliar deposition and pesticide losses from three air-assisted sprayers in a hedgerow vineyard. Crop Prot. 1997, 16, 25–33. [Google Scholar] [CrossRef]
- Koch, H. How to achieve conformity with the dose expression and sprayer function in high crops. Bayer Cropsci. J. 2007, 60, 71–84. [Google Scholar]
- Pivato, A.; Barausse, A.; Zecchinato, F.; Palmeri, L.; Raga, R.; Lavagnolo, M.C.; Cossu, R. An integrated model-based approach to the risk assessment of pesticide drift from vineyards. Atmos. Environ. 2015, 111, 136–150. [Google Scholar] [CrossRef]
- Miranda-Fuentes, A.; Rodríguez-Lizana, A.; Gil, E.; Agüera-Vega, J.; Gil-Ribes, J.A. Influence of liquid-volume and airflow rates on spray application quality and homogeneity in super-intensive olive tree canopies. Sci. Total Environ. 2015, 537, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Viret, O.; Siegfried, W.; Holliger, E.; Raisigl, U. Comparison of spray deposits and efficacy against powdery mildew of aerial and ground-based spraying equipment in viticulture. Crop Prot. 2003, 22, 1023–1032. [Google Scholar] [CrossRef]
- Baldoin, C.; Zanche, C.; De, B.D. Field testing of a prototype recycling sprayer in a vineyard: Spray distribution and loss. Agric. Eng. Int. CIGR J. 2008, X, 1–10. [Google Scholar]
- Gil, E.; Escola, A.; Rosell, J.R.; Planas, S.; Val, L. Variable rate application of plant protection products in vineyard using ultrasonic sensors. Crop Prot. 2007, 26, 1287–1297. [Google Scholar] [CrossRef]
- Sarri, D.; Martelloni, L.; Rimediotti, M.; Lisci, R.; Lombardo, S.; Vieri, M. Testing a multi-rotor unmanned aerial vehicle for spray application in high slope terraced vineyard. J. Agric. Eng. 2019, 50, 38–47. [Google Scholar] [CrossRef]
- Wise, J.C.; Jenkins, P.E.; Schilder, A.M.C.; Vandervoort, C.; Isaacs, R. Sprayer type and water volume influence pesticide deposition and control of insect pests and diseases in juice grapes. Crop Prot. 2010, 29, 378–385. [Google Scholar] [CrossRef]
- Naud, O.; Verges, A.; Herbard, O.; Codis, S.; Douzals, J.-P.; Ruelle, B. Comparative assessment of agro-environmental perfor-mance of vineyard sprayers using a physical full scale model of a vineyard row. In Proceedings of the AgEng 2014, Zurich, Switzerland, 6–10 July 2014. [Google Scholar] [CrossRef]
- Pergher, G. Recovery rate of tracer dyes used for spray deposit assessment. Trans. ASAE 2001, 44, 787. [Google Scholar] [CrossRef]
- TOPPS. Available online: http://www.topps-life.org/topps-prowadis-project.html (accessed on 29 October 2019).
- Rasband, W.S. ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA, 1997–2008. Available online: https://imagej.nih.gov/ij/ (accessed on 16 October 2018).
- Llorens, J.; Gil, E.; Llop, J.; Escola, A. Variable rate dosing in precision viticulture: Use of electronic devices to improve application efficiency. Crop Prot. 2010, 29, 239–248. [Google Scholar] [CrossRef]
- Gil, E.; Llorens, J.; Landers, A.; Llop, J.; Giralt, L. Field validation of DOSAVIÑA, a decision support system to determine the optimal volume rate for pesticide application in vineyards. Eur. J. Agron. 2011, 35, 33–46. [Google Scholar] [CrossRef]
- Salcedo, R.; Llop, J.; Campos, J.; Michael, C.; Gallart, M.; Ortega, P.; Gil, E. Evaluation of leaf deposit quality between electrostatic and conventional multi-row sprayers in a trellised vineyard. Crop Prot. 2020, 127, 104964. [Google Scholar] [CrossRef]
- Codis, S.; Carra, M.; Delpuech, X.; Montegano, P.; Nicot, H.; Ruelle, B.; Ribeyrolles, X.; Savajols, B.; Vergès, A.; Naud, O. Dataset of spray deposit distribution in vine canopy for two contrasted performance sprayers during a vegetative cycle associated with crop indicators (LWA and TRV). Data Brief. 2018, 18, 415–421. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 16 October 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 16 October 2020)ISBN 978-3-319-24277-4.
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 8, 1–26. [Google Scholar] [CrossRef]
- Manktelow, D.W.; Gurnsey, S.J.; MacGregor, A.M. Deposit variability and prediction in fruit crops: What use are label rates anyway? Asp. Appl. Biol. 2004, 71, 269–278. [Google Scholar]
- Solanelles, F.; Escola, A.; Planas, S.; Rosell, J.R.; Camp, F.; Gracia, F. An electronic control system for pesticide application proportional to the canopy width of tree crops. Biosyst. Eng. 2006, 95, 473–481. [Google Scholar] [CrossRef]
- Molto, E.; Martin, B.; Gutierrez, A. Design and testing of an automatic machine for spraying at a constant distance from the tree canopy. J. Agric. Eng. Res. 2000, 77, 379–384. [Google Scholar] [CrossRef]
- Landers, A. Improving spray deposition and reducing drift—Air flow adjustment is the answer. N. Y. Fruit Q. 2011, 19, 1–6. [Google Scholar]
- Pergher, G.; Petris, R. The effect of air flow rate on spray deposition in a guyot-trained vineyard. Agric. Eng. Int. CIGR J. 2008. [Google Scholar] [CrossRef]
- Pergher, G.; Gubiani, R. The effect of spray application rate and airflow rate on foliar deposition in a hedgerow vineyard. J. Agric. Eng. Res. 1995, 61, 205–216. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Heuvel, J.E.V. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar]
- Deloire, A. A few thoughts on grapevine training systems. Wineland Mag. 2012, 274, 82–86. [Google Scholar]





| Treatment—Nominal Volume Rate (VR) | Forward Speed (km h−1) | Actual Volume Rate (L ha−1) | Flow Rate (L min−1) | Number of Nozzles |
|---|---|---|---|---|
| HVS (High Volume Sprayer—1000 L ha−1) | 1.5 | 1077 | 10.00 | 1 |
| OS500 (Orchard Sprayer—500 L ha−1) | 4.0 | 524 | 12.96 | 6 |
| OS250 (Orchard Sprayer 250 L ha−1) | 4.0 | 283 | 7.00 | 6 |
| LVS (Low Volume Sprayer—200 L ha−1) | 1.5 | 188 | 1.75 | 1 |
| Vineyard | Row Distance (m) | Distance Between Plants (m) | Canopy Height (m) | Canopy Width (m) | LAI |
|---|---|---|---|---|---|
| Trellis system | 2.25 | 1.65 | 1.18 | 0.85 | 2.21 |
| Goblet system | 2.25 | 1.65 | 0.98 | 1.05 | 1.00 |
| Fixed Effects | df | F-Value | p-Value | F-Value | p-Value |
|---|---|---|---|---|---|
| Trellis | Goblet | ||||
| Sprayer | 3, 20 | 58.17 | 0.02 | 63.32 | <0.001 |
| Side | 2, 160 | 8.96 | <0.001 | 3.71 | 0.03 |
| Height | 2, 160 | 5.74 | 0.004 | 5.05 | 0.01 |
| Sprayer: Side | 6, 160 | 0.72 | 0.63 | 1.62 | 0.15 |
| Sprayer: Height | 6, 160 | 1.32 | 0.25 | 1.99 | 0.07 |
| Side: Height | 4, 160 | 2.31 | 0.06 | 2.12 | 0.08 |
| Sprayer: Side: Height | 12, 160 | 1.74 | 0.06 | 1.06 | 0.40 |
| Random Effect | Vine | 0.114 | 0.102 | ||
| (standard deviation) | Residual | 0.510 | 0.503 | ||
| Fixed Effects | df | F-Value | p-Value | F-Value | p-Value |
|---|---|---|---|---|---|
| Trellis | Goblet | ||||
| Sprayer | 3, 20 | 4.17 | 0.02 | 11.95 | <0.001 |
| Side | 2, 160 | 8.96 | <0.001 | 3.70 | 0.03 |
| Height | 2, 160 | 5.74 | 0.004 | 5.05 | 0.01 |
| Sprayer: Side | 6, 160 | 0.72 | 0.63 | 1.61 | 0.15 |
| Sprayer: Height | 6, 160 | 1.32 | 0.25 | 1.99 | 0.07 |
| Side: Height | 4, 160 | 2.31 | 0.06 | 2.11 | 0.08 |
| Sprayer: Side: Height | 12, 160 | 1.74 | 0.06 | 1.06 | 0.40 |
| Random Effect | Vine | 0.114 | 0.102 | ||
| (standard deviation) | Residual | 0.510 | 0.503 | ||
| df | F-Value | p-Value | F-Value | p-Value | |
|---|---|---|---|---|---|
| Trellis | Goblet | ||||
| dN (Fixed effect) | |||||
| Sprayer | 3, 20 | 253.79 | <0.001 | 79.641 | <0.001 |
| Random effect | Vine id | 0.143 | 0.298 | ||
| (standard deviation) | Residual | 0.192 | 0.216 | ||
| dG or d100 | |||||
| Sprayer (Fixed effect) | 3, 20 | 92.28 | <0.001 | 21.41 | <0.001 |
| Random effect | Vine id | 0.143 | 0.298 | ||
| (standard deviation) | Residual | 0.192 | 0.216 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michael, C.; Gil, E.; Gallart, M.; Stavrinides, M.C. Influence of Spray Technology and Application Rate on Leaf Deposit and Ground Losses in Mountain Viticulture. Agriculture 2020, 10, 615. https://doi.org/10.3390/agriculture10120615
Michael C, Gil E, Gallart M, Stavrinides MC. Influence of Spray Technology and Application Rate on Leaf Deposit and Ground Losses in Mountain Viticulture. Agriculture. 2020; 10(12):615. https://doi.org/10.3390/agriculture10120615
Chicago/Turabian StyleMichael, Costas, Emilio Gil, Montserrat Gallart, and Menelaos C. Stavrinides. 2020. "Influence of Spray Technology and Application Rate on Leaf Deposit and Ground Losses in Mountain Viticulture" Agriculture 10, no. 12: 615. https://doi.org/10.3390/agriculture10120615
APA StyleMichael, C., Gil, E., Gallart, M., & Stavrinides, M. C. (2020). Influence of Spray Technology and Application Rate on Leaf Deposit and Ground Losses in Mountain Viticulture. Agriculture, 10(12), 615. https://doi.org/10.3390/agriculture10120615






