Growth Potential of Yellow Mealworm Reared on Industrial Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Insects
2.2. Diet Preparation, Larval Growth and Measurements during the Experiment
2.3. Feed Conversion Efficiency Experiment
2.4. Statistical Analysis
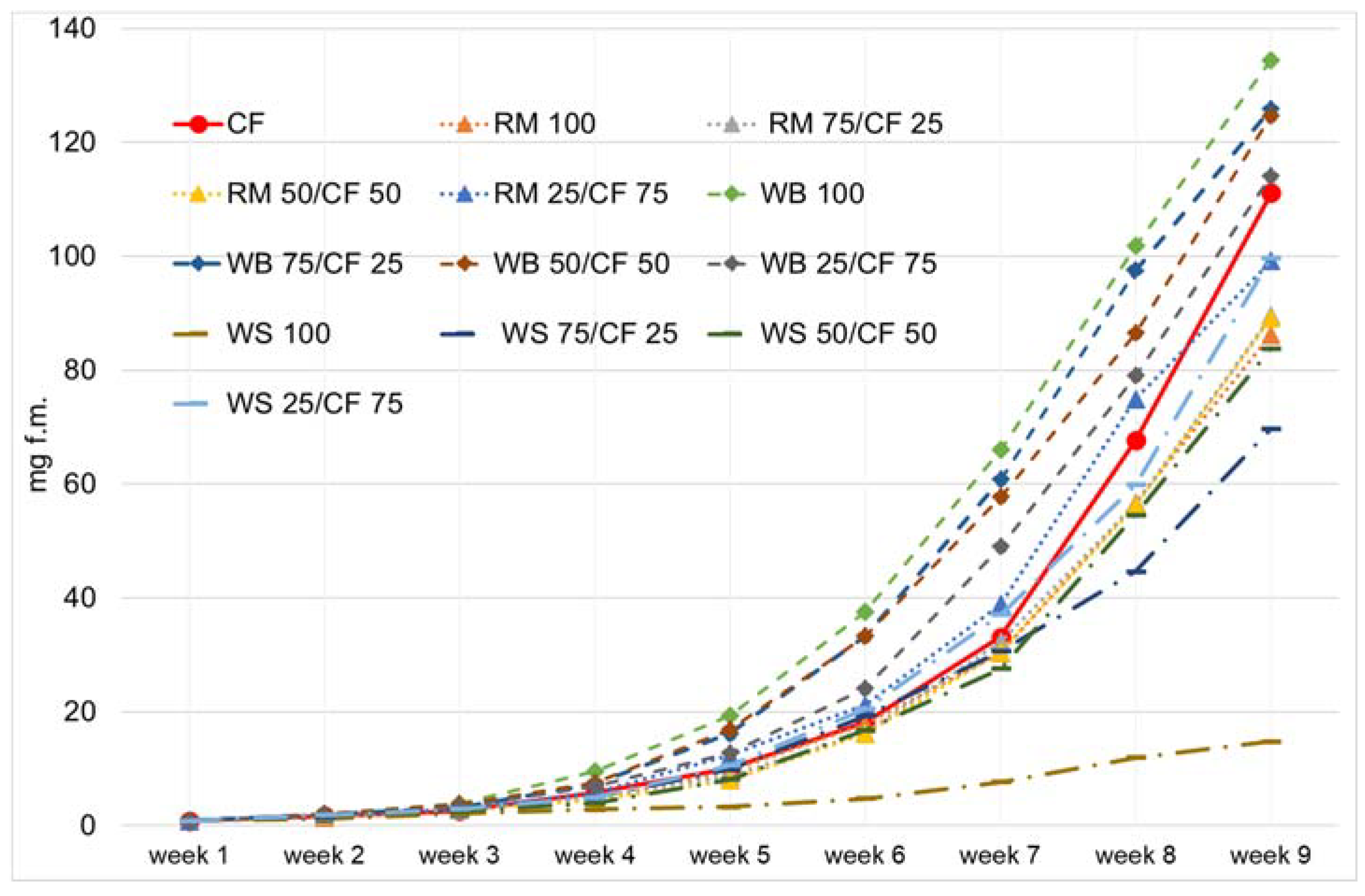
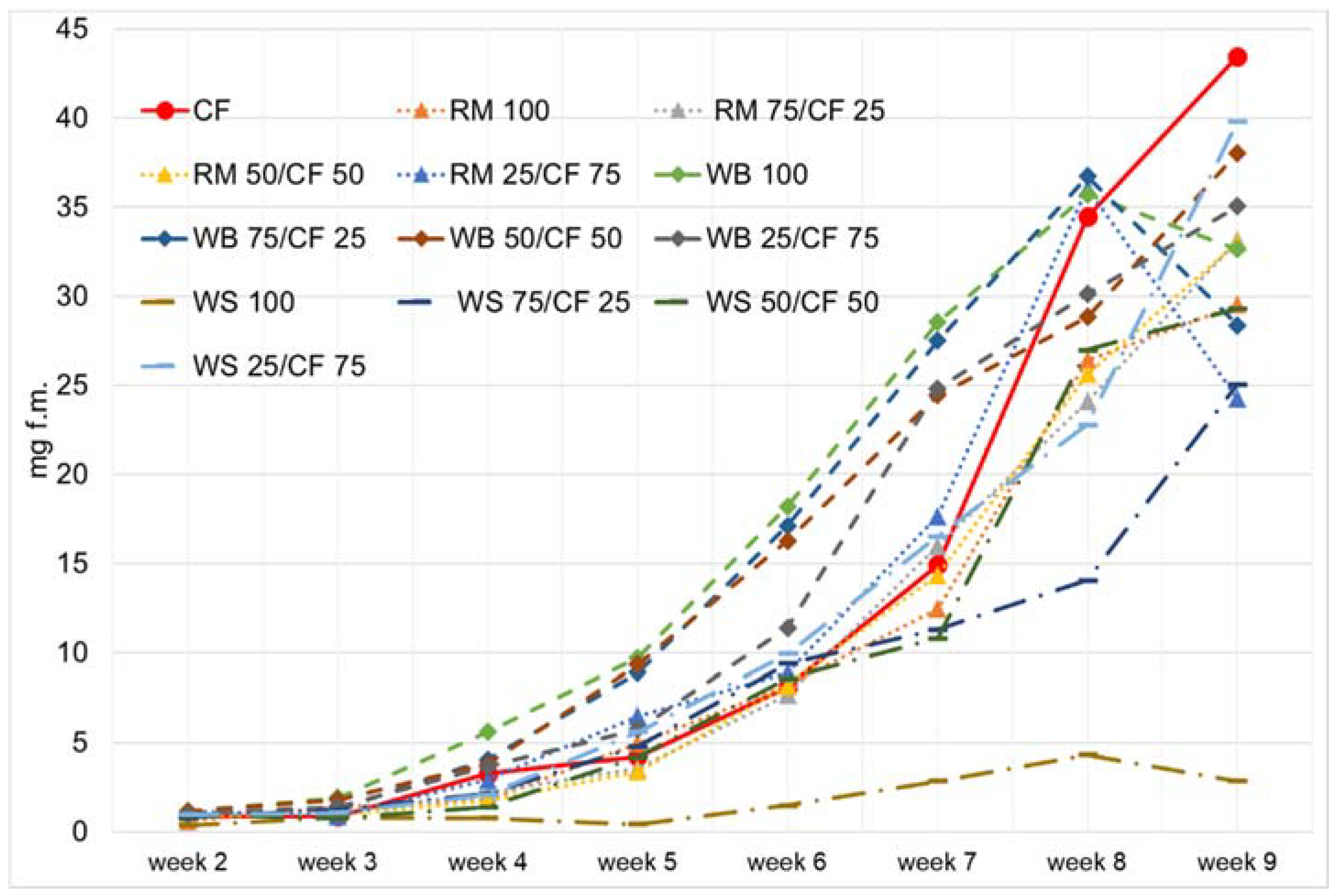
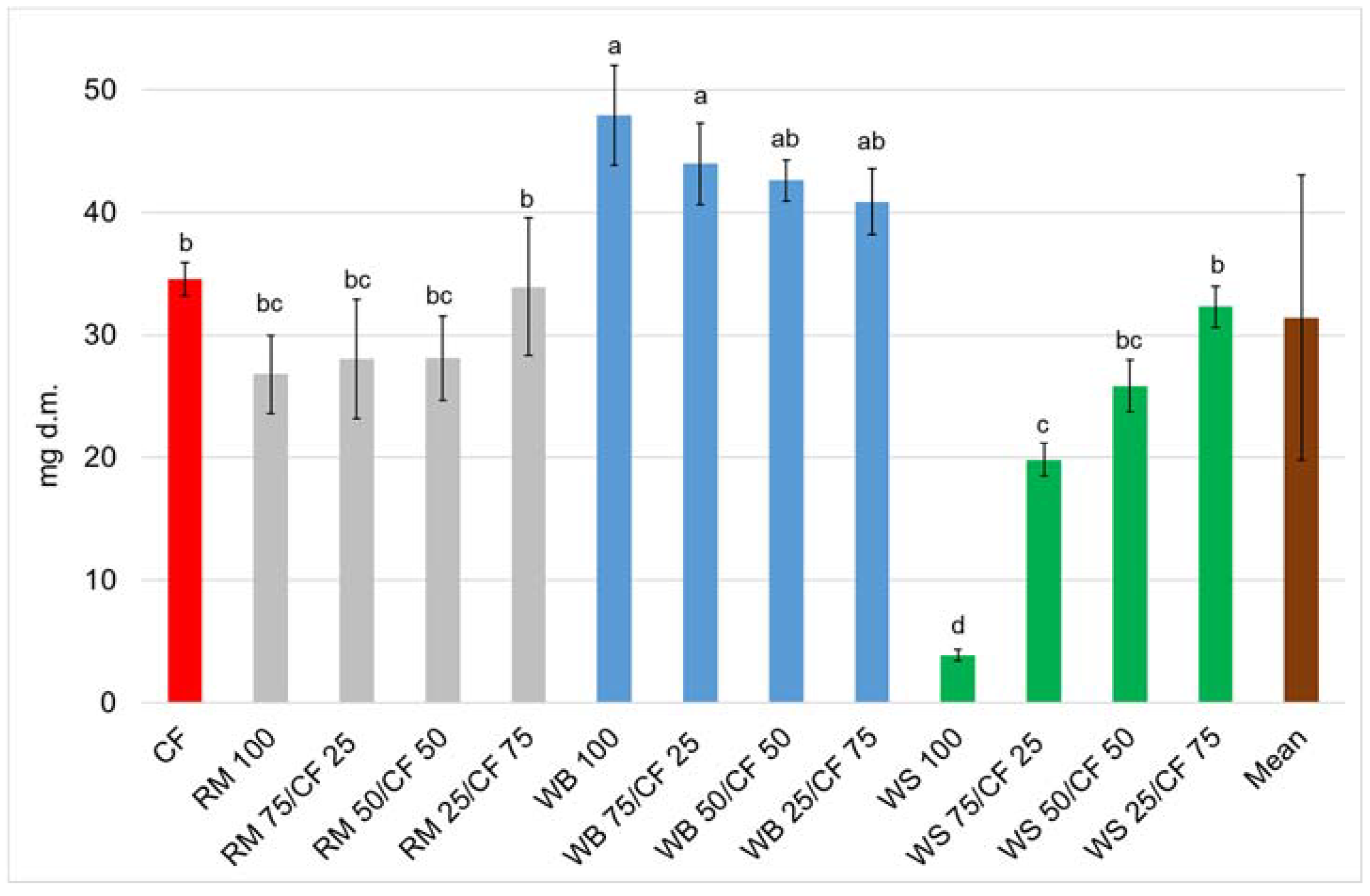
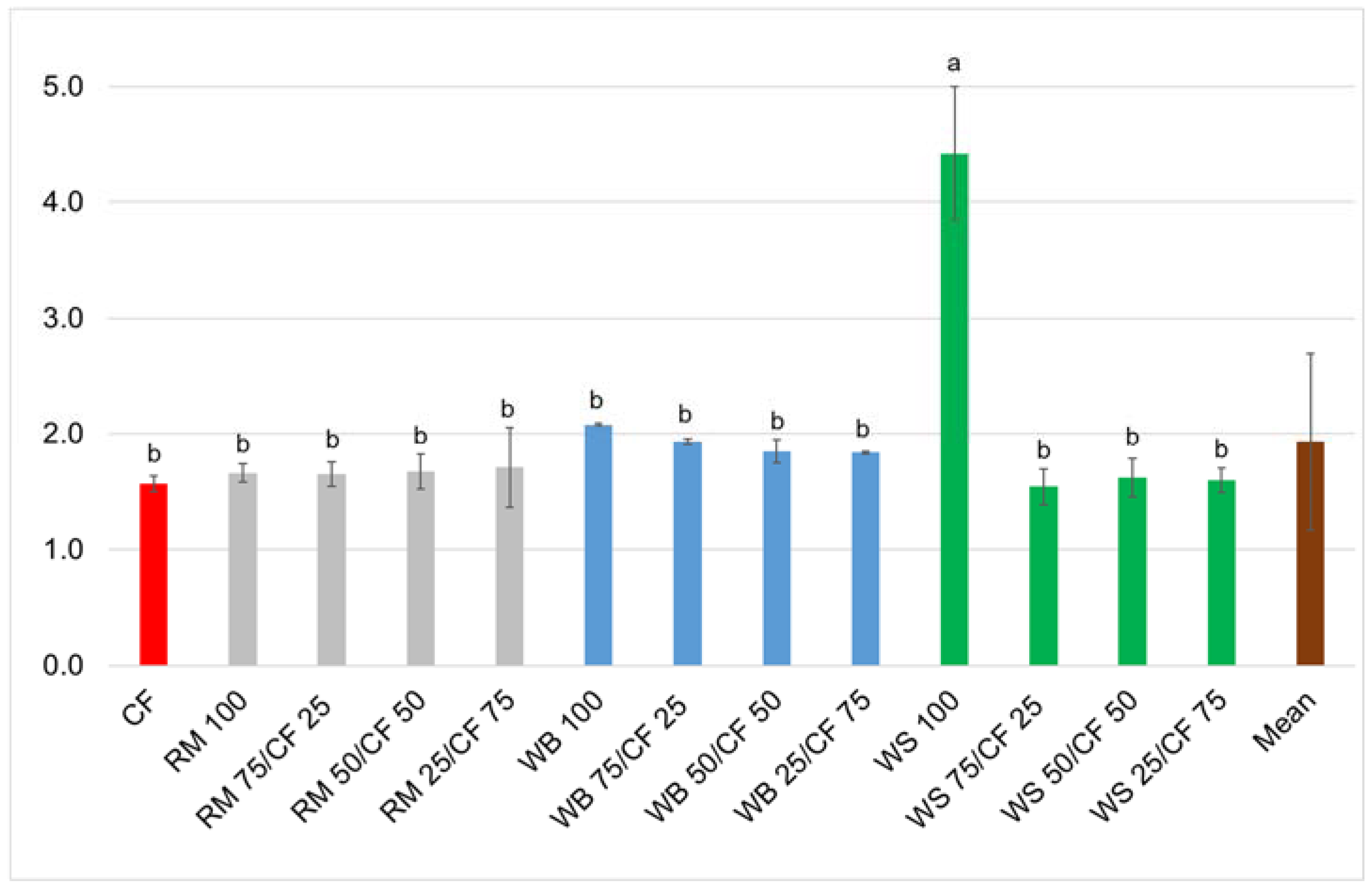
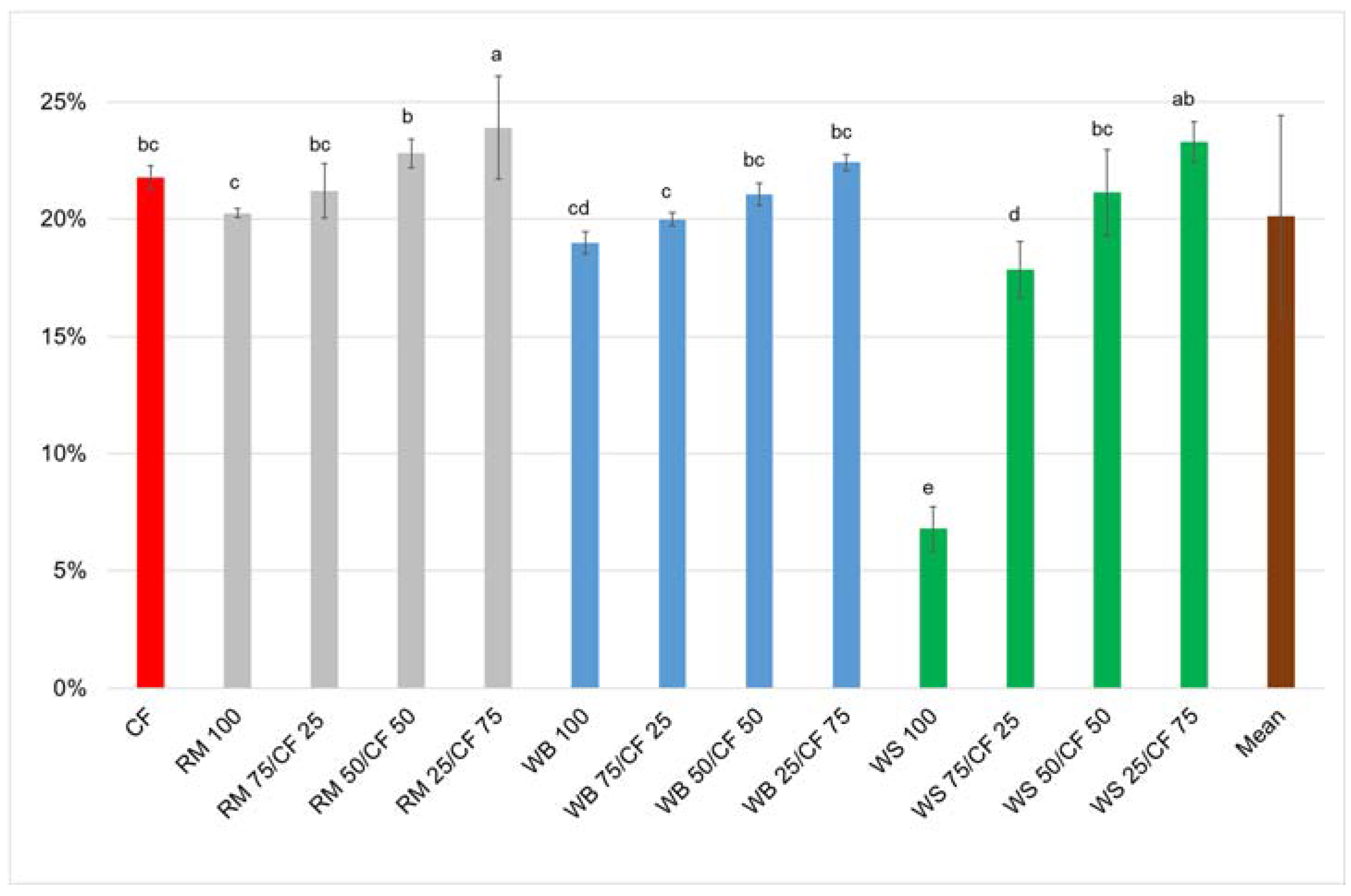
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture–Trends and Challenges; Food and Agriculture Organization: Rome, Italy, 2017. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Food Losses and Food Waste—Extent, Causes and Prevention; Food and Agriculture Organization: Rome, Italy, 2011. [Google Scholar]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; De Haan, C. Livestock’s Long Shadow: Environmental Issues and Options; Food & Agriculture Organization: Rome, Italy, 2006. [Google Scholar]
- World Health Organization. The State of Food Security and Nutrition in the World 2018: Building Climate Resilience for Food Security and Nutrition; Food & Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Losses, inefficiencies and waste in the global food system. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Stehfest, E.; Bouwman, L.; Van Vuuren, D.P.; Den Elzen, M.G.J.; Eickhout, B.; Kabat, P. Climate benefits of changing diet. Clim. Chang. 2009, 95, 83–102. [Google Scholar] [CrossRef]
- De Vries, M.; De Boer, I.J.M. Comparing environmental impacts for livestock products: A review of life cycle assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Wegier, A.; Alavez, V.; Pérez-López, J.; Calzada, L.; Cerritos, R. Beef or grasshopper hamburgers: The ecological implications of choosing one over the other. Basic Appl. Ecol. 2018, 26, 89–100. [Google Scholar] [CrossRef]
- Józefiak, D.; Józefiak, A.; Kierończyk, B.; Rawski, M.; Świątkiewicz, S.; Długosz, J.; Engberg, R.M. 1. Insects–a natural nutrient source for poultry—A review. Ann. Anim. Sci. 2016, 16, 297–313. [Google Scholar] [CrossRef]
- Wijkstrom, U. Future demand for, and supply of, fish and shellfish as food. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 2004; Volume 42, pp. 9–23. [Google Scholar]
- Chemello, G.; Renna, M.; Caimi, C.; Guerreiro, I.; Oliva-Teles, A.; Enes, P.; Biasato, I.; Schiavone, A.; Gai, F.; Gasco, L. Partially Defatted Tenebrio molitor Larva Meal in Diets for Grow-Out Rainbow Trout, Oncorhynchus mykiss (Walbaum): Effects on Growth Performance, Diet Digestibility and Metabolic Responses. Animals 2020, 10, 229. [Google Scholar] [CrossRef]
- Beretta, C.; Stoessel, F.; Baier, U.; Hellweg, S. Quantifying food losses and the potential for reduction in Switzerland. Waste Manag. 2013, 33, 764–773. [Google Scholar] [CrossRef]
- Secondi, L.; Principato, L.; Laureti, T. Household food waste behaviour in EU-27 countries: A multilevel analysis. Food Policy 2015, 56, 25–40. [Google Scholar] [CrossRef]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef]
- FAO. Food Wastage Footprint: Impacts on Natural Resources: Summary Report; Food and Agriculture Organization: Rome, Italy, 2013. [Google Scholar]
- Monier, V.; Shailendra, M.; Escalon, V.; O’Connor, C.; Gibon, T.; Anderson, G.; Hortense, M.; Reisinger, H. Preparatory Study on Food Waste across EU 27—European Commission (DG ENV) Directorate C-Industry; Final Report; European Commission: Brussels, Belgium, 2010; ISBN 978-92-79-22138-5. [Google Scholar]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming insect biomass into consumer wellness foods: A review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A. Did Early Humans Consume Insects? J. Insects Food Feed. 2017, 3, 161–163. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World, 2017; Wageningen University: Wageningen, The Netherlands, 2018. [Google Scholar]
- EFSA Scientific Committee. Scientific Opinion on a risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J. Anthropo-entomophagy: Cultures, evolution and sustainability. Entomol. Res. 2009, 39, 271–288. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Tran, G.; Heuzé, V.; Makkar, H.P.S. Insects in fish diets. Anim. Front. 2015, 5, 37–44. [Google Scholar] [CrossRef]
- Loponte, R.; Nizza, S.; Bovera, F.; De Riu, N.; Fliegerova, K.; Lombardi, P.; Vassalotti, G.; Mastellone, V.; Nizza, A.; Moniello, G. Growth performance, blood profiles and carcass traits of Barbary partridge (Alectoris barbara) fed two different insect larvae meals (Tenebrio molitor and Hermetia illucens). Res. Vet. Sci. 2017, 115, 183–188. [Google Scholar] [CrossRef]
- Bovera, F.; Piccolo, G.; Gasco, L.; Marono, S.; Loponte, R.; Vassalotti, G.; Mastellone, V.; Lombardi, P.; Attia, Y.A.; Nizza, A. Yellow mealworm larvae (Tenebrio molitor L.) as a possible alternative to soybean meal in broiler diets. Br. Poult. Sci. 2015, 56, 569–575. [Google Scholar] [CrossRef]
- Gasco, L.; Dabbou, S.; Trocino, A.; Xiccato, G.; Capucchio, M.T.; Biasato, I.; Dezzutto, D.; Birolo, M.; Meneguz, M.; Schiavone, A. Effect of dietary supplementation with insect fats on growth performance, digestive efficiency and health of rabbits. J. Anim. Sci. Biotechnol. 2019, 10, 4. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Czachorowski, S.; Peni, D. Will Yellow Mealworm Become A Source of Safe Proteins for Europe? Agriculture 2020, 10, 233. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Caparros Megido, R.; Sablon, L.; Geuens, M.; Brostaux, Y.; Alabi, T.; Blecker, C.; Drugmand, D.; Haubruge, É.; Francis, F. Edible Insects Acceptance by Belgian Consumers: Promising Attitude for Entomophagy Development. J. Sens. Stud. 2014, 29, 14–20. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; Van Huis, A.; Van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; Van Broekhoven, S.; Van Huis, A.; Van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- European Commission. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 November and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, L327, 1–12. [Google Scholar]
- European Commission. European Commission Regulation (EU) 2017/893 of 23 May 2017 amending Annexes I and IV of Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as regards the provisions on processed animal protein. Off. J. Eur. Union 2017, L138, 92–116. [Google Scholar]
- Waldbauer, G.P. The consumption and utilization of food by insects. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 1968; Volume 5, pp. 229–288. [Google Scholar]
- Miech, P.; Berggren, Å.; Lindberg, J.E.; Chhay, T.; Khieu, B.; Jansson, A. Growth and survival of reared Cambodian field crickets (Teleogryllus testaceus) fed weeds, agricultural and food industry by-products. J. Insects Food Feed 2016, 2, 285–292. [Google Scholar] [CrossRef]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth Performance and Nutrient Composition of Mealworms (Tenebrio Molitor) Fed on Fresh Plant Materials-Supplemented Diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Self-selection of two diet components by Tenebrio molitor (Coleoptera: Tenebrionidae) larvae and its impact on fitness. Environ. Entomol. 2011, 40, 1285–1294. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Liu, H. Feasibility of feeding yellow mealworm (Tenebrio molitor L.) in bioregenerative life support systems as a source of animal protein for humans. Acta Astronaut. 2013, 92, 103–109. [Google Scholar] [CrossRef]
- Harsányi, E.; Juhász, C.; Kovács, E.; Huzsvai, L.; Pintér, R.; Fekete, G.; Varga, Z.I.; Aleksza, L.; Gyuricza, C. Evaluation of organic wastes as substrates for rearing Zophobas morio, Tenebrio molitor, and Acheta domesticus larvae as alternative feed supplements. Insects 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Urrejola, S.; Nespolo, R.; Lardies, M.A. Diet-induced developmental plasticity in life histories and energy metabolism in a beetle. Rev. Chil. Hist. Nat. 2011, 84, 523–533. [Google Scholar] [CrossRef]
- Weaver, D.K.; McFarlane, J.E. The effect of larval density on growth and development of Tenebrio molitor. J. Insect Physiol. 1990, 36, 531–536. [Google Scholar] [CrossRef]
- Waldbauer, G.P.; Bhattacharya, A.K. Self-selection of an optimum diet from a mixture of wheat fractions by the larvae of Tribolium confusum. J. Insect Physiol. 1973, 19, 407–418. [Google Scholar] [CrossRef]
- House, H.L. Effects of low levels of the nutrient content of a food and of nutrient imbalance on the feeding and the nutrition of a phytophagous larva, Celerio euphorbiae (Linnaeus) (Lepidoptera: Sphingidae). Can. Entomol. 1965, 97, 62–68. [Google Scholar] [CrossRef]
- Smil, V. Eating meat: Evolution, patterns, and consequences. Popul. Dev. Rev. 2002, 28, 599–639. [Google Scholar] [CrossRef]
- Van Huis, A. Edible insects contributing to food security? Agric. Food Secur. 2015, 4. [Google Scholar] [CrossRef]
- IPIFF (International Platform of Insects for Food and Feed). Guide on Good Hygiene Practices for European Union (EU) Producers of Insects as Food and Feed 2019. Available online: https://ipiff.org/good-hygiene-practices/ (accessed on 29 November 2020).
- Collavo, A.; Glew, R.H.; Huang, Y.-S.; Chuang, L.-T.; Bosse, R.; Paoletti, M.G. House cricket small-scale farming. In Ecological Implications of Minilivestock: Potential of Insects, Rodents, Frogs and Snails; Science Publisher: Enfield, NH, USA, 2005; Volume 27, pp. 515–540. [Google Scholar]
| Substrate | Ash (% d.m.) | Fibre Content (% d.m.) | Crude Fat (% d.m.) | Protein (% d.m.) |
|---|---|---|---|---|
| Chicken feed (CF) | 12.89 | 3.23 | 2.38 | 20.11 |
| Rapeseed meal (RM) | 7.39 | 11.12 | 1.78 | 35.52 |
| Wheat bran (WB) | 5.82 | 8.68 | 2.9 | 18.63 |
| Willowleaf sunflower (WS) | 9.49 | 22.64 | 0.76 | 10.56 |
| No. | Diet | Residual Feed Proportion (%) | Chicken Feed Proportion (%) |
|---|---|---|---|
| 1. | CF (control) | - | 100 |
| 2. | RM 100 | 100 | - |
| 3. | RM 75/CF 25 | 75 | 25 |
| 4. | RM 50/CF 50 | 50 | 50 |
| 5. | RM 25/CF 75 | 25 | 75 |
| 6. | WB 100 | 100 | - |
| 7. | WB 75/CF 25 | 75 | 25 |
| 8. | WB 50/CF 50 | 50 | 50 |
| 9. | WB 25/CF 75 | 25 | 75 |
| 10. | WS 100 | 100 | - |
| 11. | WS 75/CF 25 | 75 | 25 |
| 12. | WS 50/CF 50 | 50 | 50 |
| 13. | WS 25/CF 75 | 25 | 75 |
| Source of Variation | Individual Weight | Weight Gain | ||||
|---|---|---|---|---|---|---|
| df | F | p | df | F | p | |
| Week | 8 | 640.6 | <0.001 | 7 | 721.3 | <0.001 |
| Week×Feed | 98 | 3.3 | <0.001 | 84 | 3.7 | <0.001 |
| Source of Variation | Survival | Dry Weight | FCR | ECI |
|---|---|---|---|---|
| df | 12 | |||
| F | 0.80 | 41.9 | 39.7 | 53.0 |
| p | 0.65 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Peni, D. Growth Potential of Yellow Mealworm Reared on Industrial Residues. Agriculture 2020, 10, 599. https://doi.org/10.3390/agriculture10120599
Bordiean A, Krzyżaniak M, Stolarski MJ, Peni D. Growth Potential of Yellow Mealworm Reared on Industrial Residues. Agriculture. 2020; 10(12):599. https://doi.org/10.3390/agriculture10120599
Chicago/Turabian StyleBordiean, Anna, Michał Krzyżaniak, Mariusz J. Stolarski, and Dumitru Peni. 2020. "Growth Potential of Yellow Mealworm Reared on Industrial Residues" Agriculture 10, no. 12: 599. https://doi.org/10.3390/agriculture10120599
APA StyleBordiean, A., Krzyżaniak, M., Stolarski, M. J., & Peni, D. (2020). Growth Potential of Yellow Mealworm Reared on Industrial Residues. Agriculture, 10(12), 599. https://doi.org/10.3390/agriculture10120599







