Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Source
2.2. Seed Priming Treatments
2.3. Experimental Site and Conditions
2.4. Seedling Establishment
2.5. Seedling Vigor
2.6. Physiological Variables
2.7. Biochemical Variables
2.8. Statistical Analysis
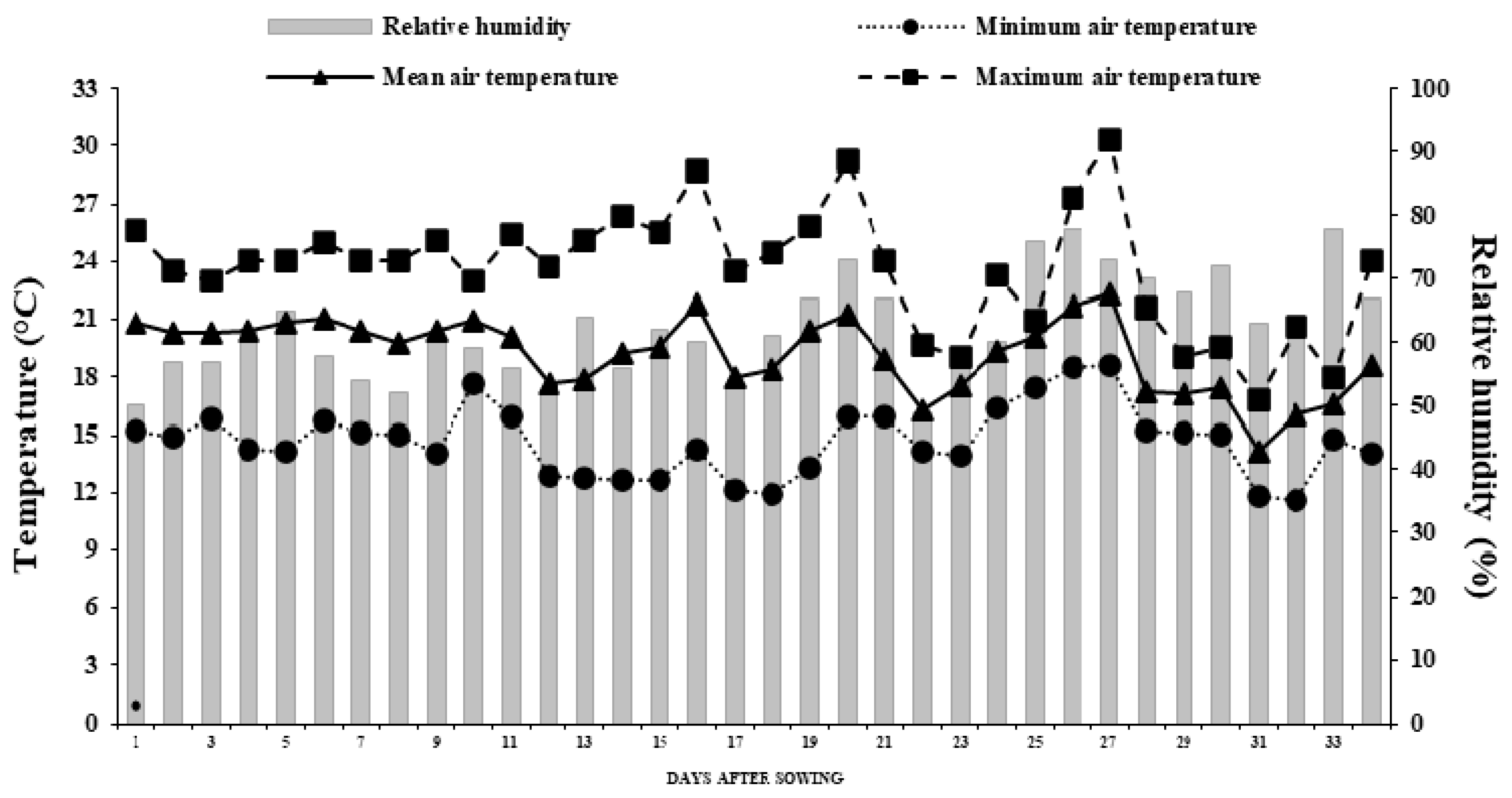
2.9. Greenhouse Microclimate Conditions During the Trial
3. Results
3.1. Growth Chamber Screening
3.1.1. Seedling Establishment
3.1.2. Seedling Vigor
3.1.3. Physiological and Biochemical Attributes
3.2. Greenhouse Screening
3.2.1. Seedling Establishment
3.2.2. Seedling Vigor
3.2.3. Physiological and Biochemical Variables
4. Discussion
4.1. Seedling Establishment
4.2. Seedling Vigor
4.3. Physiological and Biochemical Attributes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mauro, R.P.; Rizzo, V.; Leonardi, C.; Mazzaglia, A.; Muratore, G.; Distefano, M.; Sabatino, L.; Giuffrida, F. Influence of harvest stage and rootstock genotype on compositional and sensory profile of the elongated Tomato cv. “Sir Elyan”. Agriculture 2020, 10, 82. [Google Scholar] [CrossRef]
- Mauro, R.P.; Lo Monaco, A.; Lombardo, S.; Restuccia, A.; Mauromicale, G. Eradication of Orobanche/Phelipanche spp. seedbank by soil solarization and organic supplementation. Sci. Hortic. 2015, 193, 62–68. [Google Scholar] [CrossRef]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W. The tomato as a functional food. J. Nut. 2005, 135, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Tiwari, R.; Karthik, K.; Chakraborty, S.; Deb, R.; Dhama, K. Nutraceuticals from fruits and vegetables at a glance: A review. J. Biol. Sci. 2013, 13, 38–47. [Google Scholar]
- Uddain, J.; Hossain, K.M.A.; Mostafa, M.G.; Rahman, M.J. Effect of different plant growth regulators on growth and yield of tomato. Int. J. Sustain. Agric. 2009, 1, 58–63. [Google Scholar]
- Farooq, M.; Aziz, T.; Basra, S.M.A.; Cheema, M.A.; Rehman, H. Chilling tolerance in hybrid maize induced by seed priming with salicylic acid. J. Agron. Crop Sci. 2008, 194, 161–168. [Google Scholar] [CrossRef]
- Javed, T.; Afzal, I. Impact of seed pelleting on germination potential, seedling growth and storage of tomato seed. Acta Hortic. 2020, 417–424. [Google Scholar] [CrossRef]
- Afzal, I.; Hussain, B.; Basra, S.M.A.; Rehman, H. Priming with moringa leaf extract reduces imbibitional chilling injury in spring maize. Seed Sci. Technol. 2012, 40, 271–276. [Google Scholar] [CrossRef]
- Farooq, M.S.M.A.; Basra, S.M.A.; Saleem, B.A.; Nafees, M.; Chishti, S.A. Enhancement of tomato seed germination and seedling vigor by osmopriming. Pak. J. Agric. Sci. 2005, 42, 3–4. [Google Scholar]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Pre-sowing seed treatment-A shotgun approach to improve germination, plant growth, and crop yield under saline and non-saline conditions. Adv. Agron. 2005, 88, 223–271. [Google Scholar]
- Chen, K.; Arora, R.; Arora, U. Osmopriming of spinach (Spinacia oleracea L. cv. Bloomsdale) seeds and germination performance under temperature and water stress. Seed Sci. Technol. 2010, 38, 36–48. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G.; Qi, D.; Li, F.; Wang, E. Effect of PEG on germination and active oxygen metabolism in wildrye (Leymus chinensis) seeds. Acta Pratacult. Sin. 2002, 11, 59–64. [Google Scholar]
- De Castro, R.D.; van Lammeren, A.A.M.; Groot, S.P.C.; Bino, R.J.; Hilhorst, H.W.M. Cell division and subsequent radicle protrusion in tomato seeds are inhibited by osmotic stress but dna synthesis and formation of microtubular cytoskeleton are not. Plant Physiol. 2000, 122, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Aziz, T.; Wahid, A.; Lee, D.J.; Siddique, K.H.M. Chilling tolerance in maize: Agronomic and physiological approaches. Crop Past. Sci. 2009, 60, 501. [Google Scholar] [CrossRef]
- Coolbear, P.; McGill, C.R. Effects of a low-temperature pre-sowing treatment on the germination of tomato seed under temperature and osmotic stress. Sci. Hortic. 1990, 44, 43–54. [Google Scholar] [CrossRef]
- Sliwinska, E. Nuclear DNA replication and seed quality. Seed Sci. Res. 2009, 19, 15–25. [Google Scholar] [CrossRef]
- Mohammadi, G.R. The effect of seed priming on plant traits of late-spring seeded soybean (Glycine max L.). Am. Eurasian J. Agric. Environ. Sci. 2009, 5, 322–326. [Google Scholar]
- Ebrahimi, R.; Ahmadizadeh, M.; Rahbarian, P. Enhancing stand establishment of tomato cultivars under salt stress condition. Southwest J. Hortic. Biol. Environ. 2014, 5, 19–42. [Google Scholar] [CrossRef]
- Javed, T.; Ali, M.M.; Shabbir, R.; Gull, S.; Ali, A.; Khalid, E.; Abbas, A.N.; Tariq, M. Rice seedling establishment as influenced by cultivars and seed priming with potassium nitrate. J. Appl. Res. Plant Sci. 2020, 1, 65–75. [Google Scholar]
- Association of Official Seed Analysis. Rules for testing seeds. J. Seed Technol. 1990, 12, 101–112. [Google Scholar]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2015; pp. 1–276. [Google Scholar]
- Waterhouse, A.L. Determination of total phenolics. Curr. Prot. Food Anal. Chem. 2002, 6, I1-1. [Google Scholar]
- Scott, T.A.; Melvin, E.H. Determination of dextran with anthrone. Anal. Chem. 1953, 25, 1656–1661. [Google Scholar] [CrossRef]
- Bray, C.M. Biochemical processes during the osmopriming of seeds. In Seed Development and Germination; Jaime Kigel; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Singh, G.; Gill, S.S.; Sandhu, K.K. Improved performance of muskmelon (Cucumis melo) seeds with osmoconditioning. Acta Agrobot. 2013, 52, 121–137. [Google Scholar] [CrossRef][Green Version]
- Kiers, E.T.; Leakey, R.R.B.; Izac, A.M.; Heinemann, J.A.; Rosenthal, E.; Nathan, D.; Jiggins, J. Ecology: Agriculture at a crossroads. Science 2008, 320, 320–321. [Google Scholar] [CrossRef]
- Shafiei, A.M.; Ghobadi, M. The effects of source of priming and post-priming storage duration on seed germination and seedling growth characteristics in wheat (Triticum aestivem L.). J. Agric. Sci 2012, 4, 256. [Google Scholar] [CrossRef]
- Shehzad, M.; Ayub, M.; Ahmad, A.U.H.; Yaseen, M. Influence of priming techniques on emergence and seedling growth of forage sorghum (Sorghum bicolor L.). J. Anim. Plant Sci. 2012, 22, 154–158. [Google Scholar]
- Ruttanaruangboworn, A.; Chanprasert, W.; Tobunluepop, P.; Onwimol, D. Effect of seed priming with different concentrations of potassium nitrate on the pattern of seed imbibition and germination of rice (Oryza sativa L.). J. Integr. Agric. 2017, 16, 605–613. [Google Scholar] [CrossRef]
- Hadinezhad, P.; Payamenur, V.; Mohamadi, J.; Ghaderifar, F. The effect of priming on seed germination and seedling growth in Quercus castaneifolia. Seed Sci. Technol. 2013, 41, 121–124. [Google Scholar] [CrossRef]
- Demir, I.; Van De Venter, H.A. The effect of priming treatments on the performance of watermelon (Citrullus lanatus (Thunb.) Matsum. and Nakai) seeds under temperature and osmotic stress. Seed Sci. Technol. 1999, 27, 871–876. [Google Scholar]
- Mirabi, E.; Hasanabadi, M. Effect of seed priming on some characteristic of seedling and seed vigor of tomato (Lycopersicon esculentum). J. Adv. Lab. Res. Biol. 2012, 3, 237–240. [Google Scholar]
- Oliveira, C.E.D.S.; Steiner, F.; Zuffo, A.M.; Zoz, T.; Alves, C.Z.; Aguiar, V.C.B. Seed priming improves the germination and growth rate of melon seedlings under saline stress. Ciência Rural 2019, 49. [Google Scholar] [CrossRef]
- Vaktabhai, C.K.; Kumar, S. Seedling invigouration by halo priming in tomato against salt stress. J. Pharmacol. Phytochem. 2017, 6, 716–722. [Google Scholar]
- Basra, S.; Pannu, I.; Afzal, I. Evaluation of seedling vigor of hydro and matriprimed wheat (Triticum aestivum L.) seeds. Int. J. Agric. Biol. 2003, 5, 121–123. [Google Scholar]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344–353. [Google Scholar] [CrossRef]
- Mauro, R.P.; Agnello, M.; Distefano, M.; Sabatino, L.; San Bautista Primo, A.; Leonardi, C.; Giuffrida, F. Chlorophyll fluorescence, photosynthesis and growth of tomato plants as affected by long-term oxygen root zone deprivation and grafting. Agronomy 2020, 10, 137. [Google Scholar] [CrossRef]
- Mauro, R.P.; Occhipinti, A.; Longo, A.M.G.; Mauromicale, G. Effects of shading on chlorophyll content, chlorophyll fluorescence and photosynthesis of subterranean clover. J. Agron. Crop Sci. 2011, 197, 57–66. [Google Scholar] [CrossRef]
- De Castro, F.A.; Campostrini, E.; Netto, A.T.; De Menezes De Assis Gomes, M.; Ferraz, T.M.; Glenn, D.M. Portable chlorophyll meter (PCM-502) values are related to total chlorophyll concentration and photosynthetic capacity in papaya (Carica papaya L.). Exp. Plant Physiol. 2014, 26, 201–210. [Google Scholar] [CrossRef]
- Dehkordi, F.S.; Nabipour, M.; Meskarbashee, M. Effect of priming on germination and biochemical indices of chicory (Cichorium intybus L.) seed. Sci. Ser. Data Rep. 2012, 4, 24–33. [Google Scholar]
| Treatments | Final Emergence (%) | Mean Emergence Time (Days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| “Sundar” | “Ahmar” | KNO3 Mean | “Sundar” | “Ahmar” | KNO3 Mean | ||||||
| Growth chamber | Control | 82 | 84 | 83 d | 5.3 | 4.7 | 5.0 a | ||||
| 0.25% KNO3 | 84 | 90 | 87 c | 4.2 | 3.7 | 4.0 b | |||||
| 0.50% KNO3 | 89 | 93 | 91 b,c | 3.3 | 3.5 | 3.4 b,d | |||||
| 0.75% KNO3 | 97 | 99 | 98 a | 3.0 | 3.0 | 3.0 d | |||||
| 1% KNO3 | 89 | 93 | 91 b,c | 3.3 | 3.1 | 3.2 c,d | |||||
| 1.25% KNO3 | 84 | 90 | 87 c | 3.8 | 3.5 | 3.7 b,c | |||||
| Cultivar mean | 88 b | 92 a | 3.8 a | 3.6 b | |||||||
| HSDinteraction (p ≤ 0.05) | 7 | 0.7 | |||||||||
| Greenhouse | Control | 79 | 82 | 81 e | 7.2 | 6.6 | 6.9 a | ||||
| 0.25% KNO3 | 81 | 85 | 83 d | 6.3 | 5.8 | 6.0 b | |||||
| 0.50% KNO3 | 84 | 88 | 86 c | 5.3 | 4.9 | 5.1 c | |||||
| 0.75% KNO3 | 93 | 97 | 95 a | 3.9 | 3.6 | 3.7 e | |||||
| 1% KNO3 | 89 | 90 | 89 b | 4.7 | 4.1 | 4.4 d | |||||
| 1.25% KNO3 | 82 | 85 | 84 d | 5.2 | 5.3 | 5.3 c | |||||
| Cultivar mean | 85 b | 88 a | 5.4 a | 5.0 b | |||||||
| HSDinteraction (p ≤ 0.05) | 4 | 0.9 | |||||||||
| Treatments | Seedling Length (cm) | Shoot Fresh Weight (mg) | Shoot Dry Weight (mg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| “Sundar” | “Ahmar” | KNO3 Mean | “Sundar” | “Ahmar” | KNO3 Mean | “Sundar” | “Ahmar” | KNO3 Mean | ||||||||
| Growth chamber | Control | 5.2 | 5.3 | 5.2 d | 25.7 | 24.4 | 25.1 d | 10.9 | 12.9 | 11.9 d | ||||||
| 0.25% KNO3 | 6.2 | 6.1 | 6.2 c | 29.2 | 28.6 | 28.9 c | 13.8 | 15.7 | 14.7 c | |||||||
| 0.50% KNO3 | 7.2 | 7.4 | 7.3 b | 32.5 | 34.7 | 33.6 b | 16.0 | 16.6 | 16.3 b | |||||||
| 0.75% KNO3 | 8.4 | 8.4 | 8.4 a | 35.1 | 37.6 | 36.3 a | 17.9 | 19.1 | 18.5 a | |||||||
| 1% KNO3 | 7.5 | 7.8 | 7.7 b | 31.9 | 32.4 | 32.2 b | 15.4 | 16.9 | 16.2 b | |||||||
| 1.25% KNO3 | 7.3 | 7.7 | 7.5 b | 28.9 | 28.6 | 28.8 c | 13.0 | 12.4 | 12.7 d | |||||||
| Cultivar mean | 7.0 b | 7.1 a | 30.5 a | 31.1 a | 14.5 b | 15.6 a | ||||||||||
| HSDinteraction (p ≤ 0.05) | 0.7 | 2.5 | 1.9 | |||||||||||||
| Greenhouse | Control | 3.8 | 3.9 | 3.8 d | 21.8 | 25.1 | 23.4 c | 9.3 | 10.1 | 9.7 c | ||||||
| 0.25% KNO3 | 4.5 | 4.9 | 4.7 c | 24.3 | 24.1 | 24.2 c | 10.1 | 11.2 | 10.6 c | |||||||
| 0.50% KNO3 | 6.0 | 6.1 | 6.0 b | 27.6 | 26.5 | 27.1 b | 11.9 | 13.1 | 12.5 b | |||||||
| 0.75% KNO3 | 7.0 | 7.1 | 7.0 a | 30.3 | 32.1 | 31.2 a | 14.6 | 14.4 | 14.5 a | |||||||
| 1% KNO3 | 6.0 | 5.7 | 5.9 b | 26.9 | 27.7 | 27.3 b | 12.9 | 14.1 | 13.5 a,b | |||||||
| 1.25% KNO3 | 6.1 | 6.2 | 6.1 b | 23.9 | 24.9 | 24.4 c | 10.4 | 11.6 | 11.0 c | |||||||
| Cultivar mean | 5.6 a | 5.7 a | 25.8 b | 26.7 a | 11.5 b,c | 12.4 a | ||||||||||
| HSDinteraction (p ≤ 0.05) | 0.9 | 3.3 | 2.2 | |||||||||||||
| Treatments | Photosynthetic Rate (µmol CO2 m−2 s−1) | Transpiration Rate (µmol H2O m−2 s−1) | CO2 Index (µmol mol−1) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| “Sundar” | “Ahmar” | KNO3 Mean | “Sundar” | “Ahmar” | KNO3 Mean | “Sundar” | “Ahmar” | KNO3 Mean | ||||||||
| Growth chamber | Control | 9.7 | 10.7 | 10.2 e | 0.81 | 0.92 | 0.86 e | 110 | 113 | 111 e | ||||||
| 0.25% KNO3 | 11.7 | 12.3 | 12.0 d | 0.99 | 1.09 | 1.04 d | 121 | 123 | 122 d | |||||||
| 0.50% KNO3 | 13.8 | 14.8 | 14.3 b,c | 1.21 | 1.29 | 1.25 c | 138 | 141 | 139 b | |||||||
| 0.75% KNO3 | 16.7 | 17.3 | 17.0 a | 1.61 | 1.70 | 1.65 a | 162 | 164 | 163 a | |||||||
| 1% KNO3 | 14.9 | 16.3 | 15.6 a,b | 1.30 | 1.40 | 1.35 b | 144 | 145 | 145 b | |||||||
| 1.25% KNO3 | 13.0 | 13.7 | 13.3 c,d | 1.24 | 1.30 | 1.27 c | 133 | 134 | 134 c | |||||||
| Cultivar mean | 13.3 b | 14.2 a | 1.19 b | 1.28 a | 135 a | 137 a | ||||||||||
| HSDinteraction (p ≤ 0.05) | 3.0 | 0.19 | 11 | |||||||||||||
| Greenhouse | Control | 11.0 | 12.7 | 11.8 e | 1.01 | 1.12 | 1.06 e | 121 | 123 | 122 e | ||||||
| 0.25% KNO3 | 12.7 | 14.3 | 13.5 d,e | 1.19 | 1.28 | 1.23 d | 131 | 133 | 132 d | |||||||
| 0.50% KNO3 | 15.1 | 17.0 | 16.1 b,c | 1.41 | 1.49 | 1.45 c | 148 | 152 | 150 b | |||||||
| 0.75% KNO3 | 18.3 | 19.3 | 18.8 a | 1.81 | 1.88 | 1.84 a | 172 | 177 | 174 a | |||||||
| 1% KNO3 | 16.3 | 18.3 | 17.3 a,b | 1.51 | 1.60 | 1.55 b | 154 | 155 | 155 b | |||||||
| 1.25% KNO3 | 14.7 | 15.7 | 15.2 c,d | 1.44 | 1.50 | 1.47 c | 144 | 144 | 144 c | |||||||
| Cultivar mean | 14.7 b | 16.2 a | 1.39 b | 1.47 a | 145 a | 147 a | ||||||||||
| HSDinteraction (p ≤ 0.05) | 3.1 | 0.20 | 13 | |||||||||||||
| Treatments | Total Soluble Sugars (mg g−1) | Phenolics (mg g−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| “Sundar” | “Ahmar” | KNO3 Mean | “Sundar” | “Ahmar” | KNO3 Mean | ||||||
| Growth chamber | Control | 70.2 | 64.5 | 67.4 b | 1.29 | 1.27 | 1.28 c | ||||
| 0.25% KNO3 | 70.8 | 69.9 | 70.3 b | 1.45 | 1.34 | 1.39 c | |||||
| 0.50% KNO3 | 83.5 | 82.5 | 83.0 a | 1.70 | 1.65 | 1.67 a,b | |||||
| 0.75% KNO3 | 85.0 | 86.3 | 85.6 a | 1.81 | 1.74 | 1.77 a | |||||
| 1% KNO3 | 80.8 | 79.0 | 79.9 a,b | 1.75 | 1.53 | 1.64 a,b | |||||
| 1.25% KNO3 | 79.5 | 76.0 | 77.8 a,b | 1.68 | 1.50 | 1.59 b | |||||
| Cultivar mean | 78.3 a | 76.4 a | 1.61 b | 1.50 a | |||||||
| HSDinteraction (p ≤ 0.05) | 9.8 | 0.29 | |||||||||
| Greenhouse | Control | 71.3 | 65.6 | 68.4 c | 1.33 | 1.26 | 1.29 e | ||||
| 0.25% KNO3 | 71.7 | 71.2 | 71.5 b,c | 1.51 | 1.35 | 1.43 d,e | |||||
| 0.50% KNO3 | 84.5 | 83.0 | 83.7 a | 1.74 | 1.63 | 1.68 b,c | |||||
| 0.75% KNO3 | 88.8 | 87.2 | 88.0 a | 1.86 | 1.76 | 1.81 a | |||||
| 1% KNO3 | 83.8 | 80.2 | 82.0 a,b | 1.79 | 1.52 | 1.65 a,b | |||||
| 1.25% KNO3 | 79.7 | 77.3 | 78.5 a,c | 1.73 | 1.49 | 1.61 c,d | |||||
| Cultivar mean | 79.9 a | 77.4 a | 1.66 b | 1.50 a | |||||||
| HSDinteraction (p ≤ 0.05) | 11.0 | 0.24 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moaaz Ali, M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agriculture 2020, 10, 498. https://doi.org/10.3390/agriculture10110498
Moaaz Ali M, Javed T, Mauro RP, Shabbir R, Afzal I, Yousef AF. Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agriculture. 2020; 10(11):498. https://doi.org/10.3390/agriculture10110498
Chicago/Turabian StyleMoaaz Ali, Muhammad, Talha Javed, Rosario Paolo Mauro, Rubab Shabbir, Irfan Afzal, and Ahmed Fathy Yousef. 2020. "Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato" Agriculture 10, no. 11: 498. https://doi.org/10.3390/agriculture10110498
APA StyleMoaaz Ali, M., Javed, T., Mauro, R. P., Shabbir, R., Afzal, I., & Yousef, A. F. (2020). Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agriculture, 10(11), 498. https://doi.org/10.3390/agriculture10110498










