Organic Matter Composition and Phosphorus Speciation of Solid Waste from an African Catfish Recirculating Aquaculture System
Abstract
1. Introduction
2. Materials and Methods
2.1. Solid Waste Samples
2.2. Determination of Elemental Concentrations
2.3. Pyrolysis-Field Ionization Mass Spectrometry (Py-FIMS)
2.4. P K-edge X-ray Absorption Near Edge (XANES) Spectroscopy
2.5. Statistical Analyses
3. Results
3.1. Elemental Composition
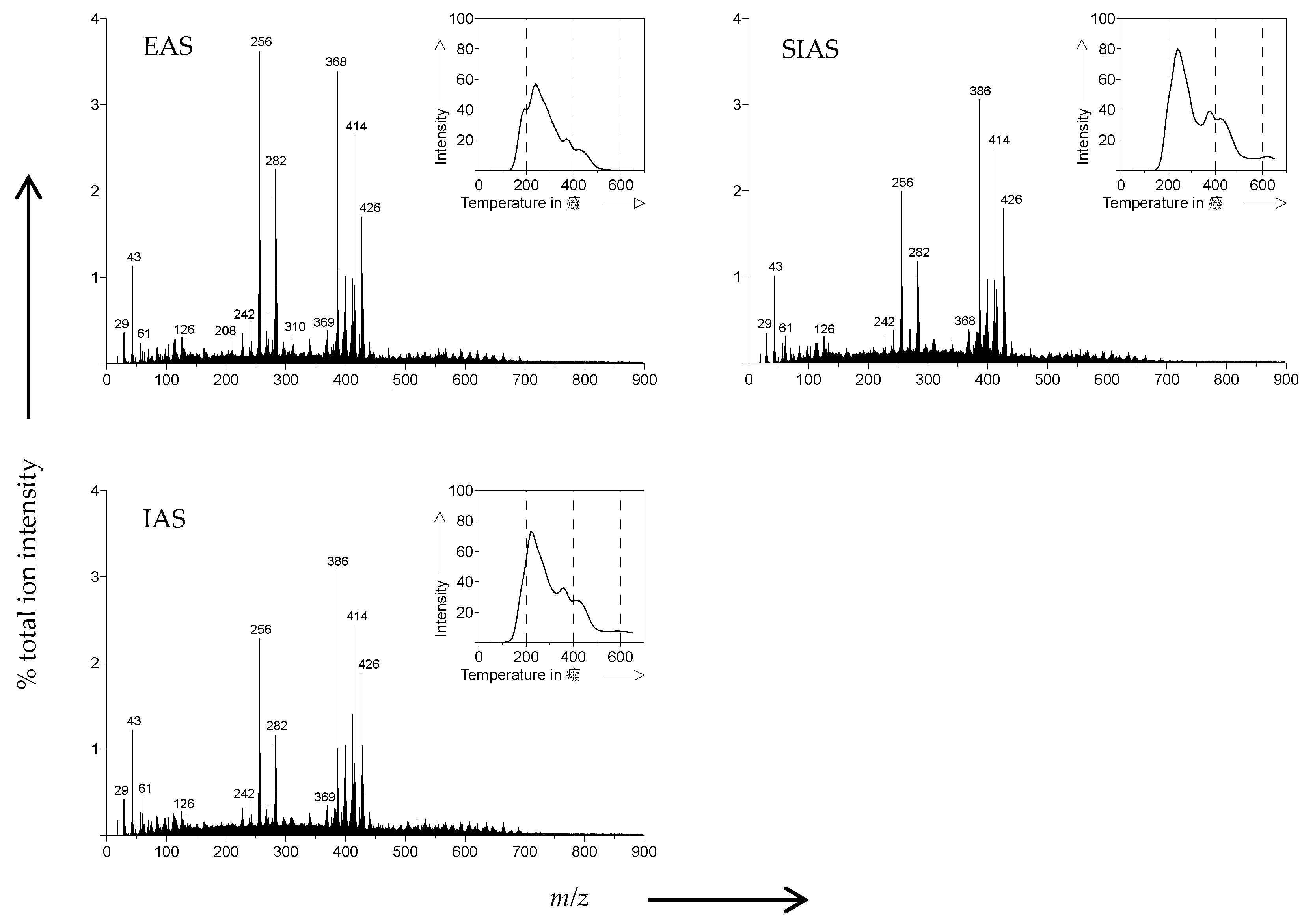
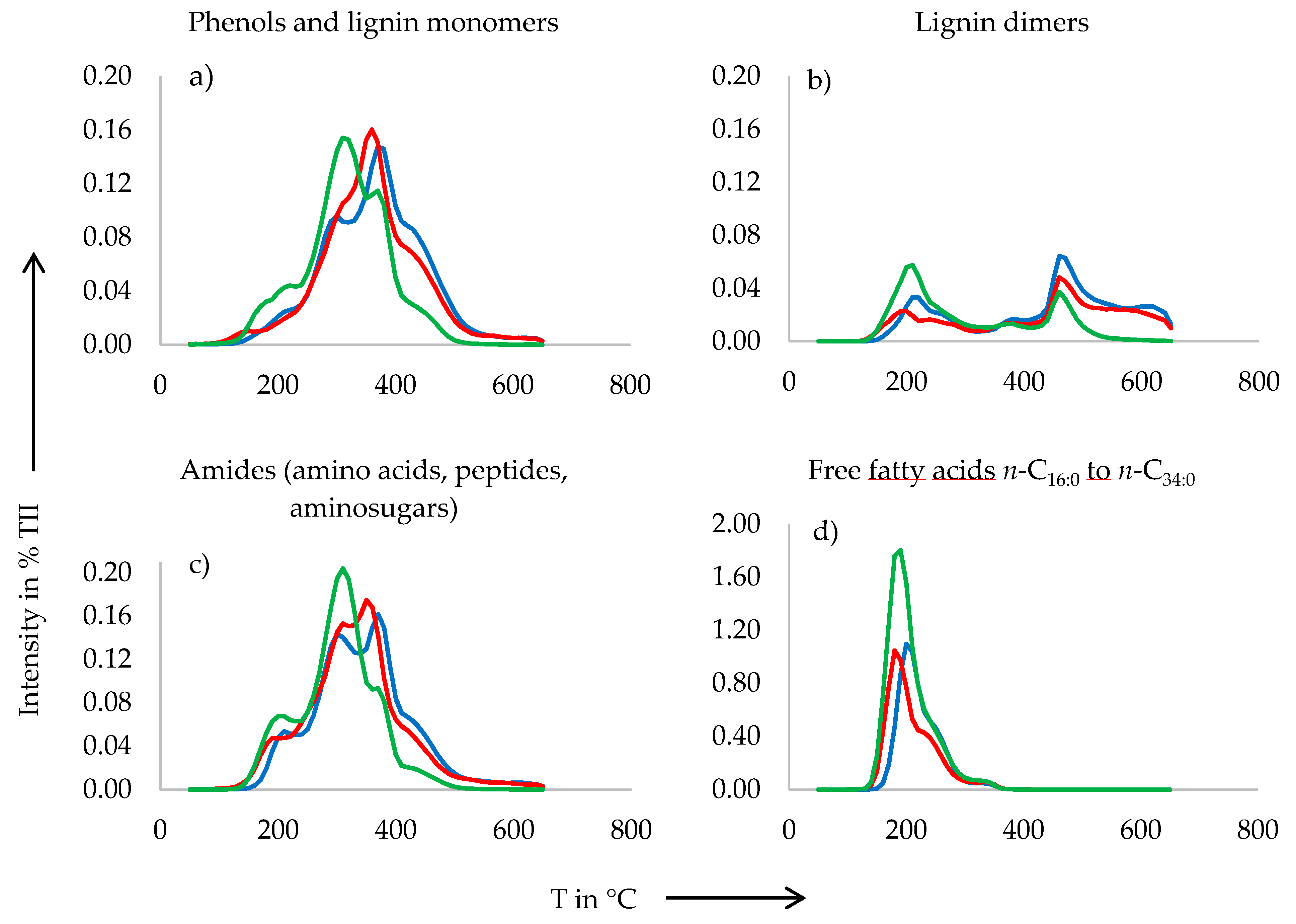
3.2. Pyrolysis-Field Ionization Mass Spectrometry (Py-FIMS)
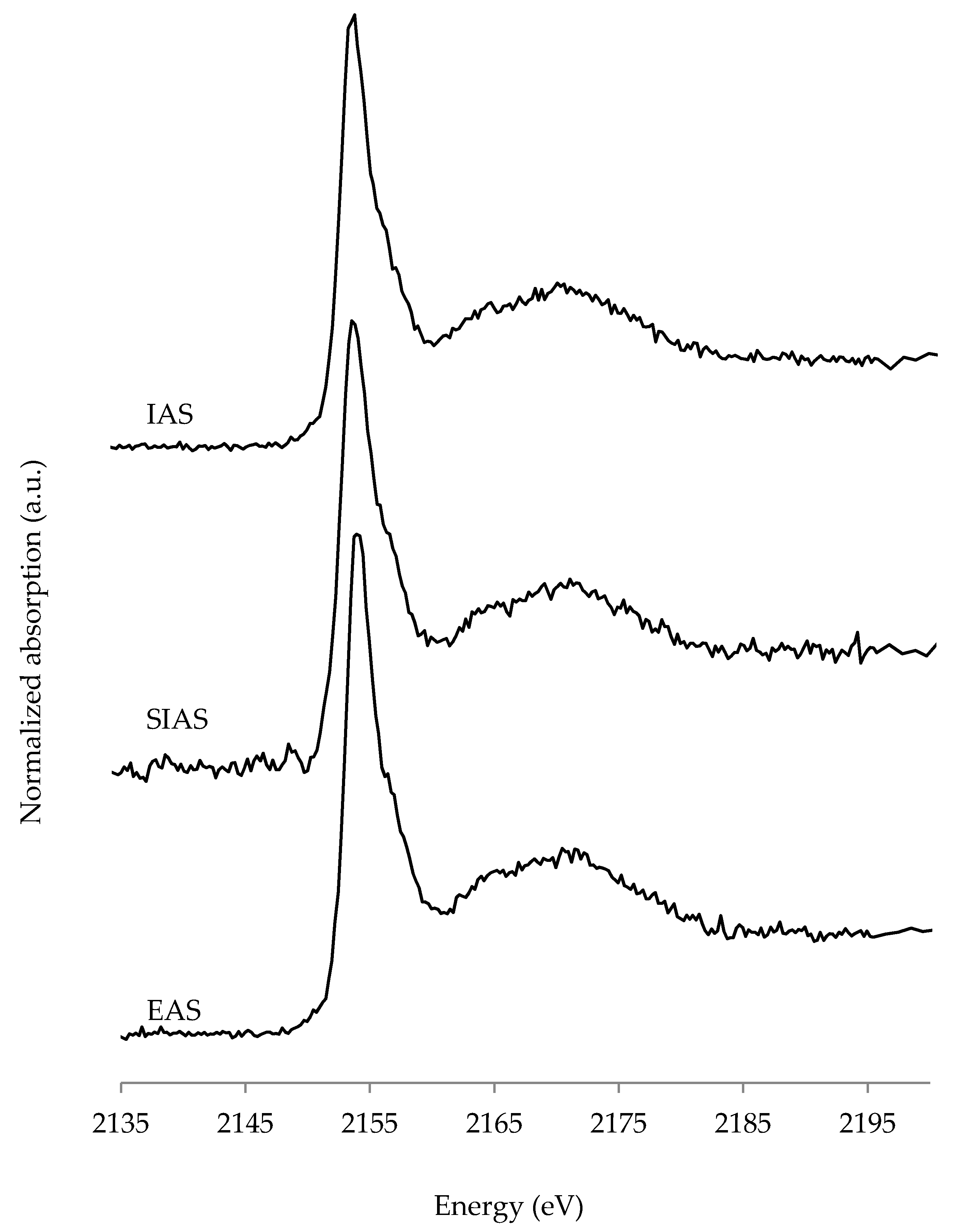
3.3. P K-edge X-ray Absorption Near Edge (XANES) Spectroscopy
4. Discussion
4.1. Organic Matter Composition
4.2. P XANES Spectroscopy
5. Conclusions
- The methodological approach of using Py-FIMS and XANES spectroscopy as methods to determine organic matter composition and P speciation of solid African catfish RAS waste samples was appropriate. It revealed insight into the distribution of organic matter compound classes in solid waste of three different fish stocking densities and provided evidence for the occurrence of exclusively Ca-bound P compounds in African catfish RAS waste.
- The high amounts of sterols, fatty acids and alkylaromatics in the solid waste of all three stocking densities of African catfish RAS determined by Py-FIMS reflect the plant-based feed of the fish. To assess the suitability of African catfish RAS solid waste as organic soil amendment and to prevent possible negative effects of sterols on the N-cycle in soil, further research is needed, especially on soils that have been amended by solid waste from African catfish RAS. Alternatively to direct land application of this waste, some pretreatments such as anaerobic digestion for biogas production or vermifiltration, should be tested.
- The stocking density had an influence on feed input, water exchange rates and total oxygen concentrations in the tested African catfish RAS systems. These three factors, alone and in combination, alter solid waste composition, and its applicability as soil amendment if originating from extensive or (semi)intensive catfish aquaculture.
- XANES spectroscopy detected exclusively Ca-associated P compounds in solid African catfish RAS waste of three different fish stocking densities. Ca-phosphate minerals as a major constituent of many bio-waste material P fertilizers have beneficial properties when applied on soils. Thus, solid African catfish RAS waste can be considered as possible addition to traditional organic P fertilizers. However, this first investigation of African catfish RAS waste with P XANES spectroscopy would benefit from the application of more different complementary techniques, such as solution 31P nuclear magnetic resonance (NMR) spectroscopy and sequential P fractionation to get a more comprehensive view on P speciation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Leinweber, P.; Bathmann, U.; Buczko, U.; Douhaire, C.; Eichler-Löbermann, B.; Frossard, E.; Ekardt, F.; Jarvie, H.; Krämer, I.; Kabbe, C.; et al. Handling the phosphorus paradox in agriculture and natural ecosystems: Scarcity, necessity, and burden of P. Ambio 2018, 47, 3–19. [Google Scholar] [CrossRef]
- Withers, P.; Doody, D.; Sylvester-Bradley, R. Achieving sustainable phosphorus use in food systems through circularisation. Sustainability 2018, 10, 1804. [Google Scholar] [CrossRef]
- Schroder, J. Revisiting the agronomic benefits of manure: A correct assessment and exploitation of its fertilizer value spares the environment. Bioresour. Technol. 2005, 6, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Env. Sci. Tec. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Eding, E.H.; Verdegem, M.C.; Heinbroek, L.T.; Schneider, O.; Blancheton, J.P.; Roques d’Orbcastel, E.; Verreth, J.A.J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Hall, O.J. Chemical flux and mass balances in a marine fish cage farm. IV. Nitrogen. Mar. Ecol. Prog. Ser. 1992, 89, 81–91. [Google Scholar] [CrossRef]
- Holby, O.; Hall, O.J. Chemical fluxes and mass balances in a marine fish cage farm. II. Phosphorus. Mar. Ecol. Prog. Ser. 1991, 70, 263–272. [Google Scholar] [CrossRef]
- Galasso, H.L.; Callier, M.D.; Bastianelli, D.; Blancheton, J.-P.; Aliaume, C. The potential of near infrared spectroscopy (NIRS) to measure the chemical composition of aquaculture solid waste. Aquaculture 2017, 476, 134–140. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Juárez, M.F.-D.; Zangerle, M.; Isam, H. Effects of digestate on soil chemical and microbiological properties: A comparative study with compost and vermicompost. J. Hazard. Mater. 2016, 302, 267–274. [Google Scholar] [CrossRef]
- Case, S.D.C.; Oelofse, M.; Hou, Y.; Oenema, O.; Jensen, L.S. Farmer perceptions and use of organic waste products as fertilisers–A survey study of potential benefits and barriers. Agric. Syst. 2016, 151, 84–95. [Google Scholar] [CrossRef]
- Köster, J.R.; Cárdenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Senbayram, M.; Well, R.; Giesemann, A.; Dittert, K. Anaerobic digestates lower N2O emissions compared to cattle slurry by affecting rate and product stoichiometry of denitrification–An N2O isotopomer case study. Soil. Biol. Biochem. 2015, 84, 65–74. [Google Scholar] [CrossRef]
- Adani, F.; Tambone, F. Long-term effect of sewage sludge application on soil humic acids. Chemosphere 2005, 60, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Hupfauf, S.; Bachmann, S.; Fernández-Delgado Juárez, M.; Insam, H.; Eichler-Löbermann, B. Biogas digestates affect crop P uptake and soil microbial community composition. Sci. Total Environ. 2016, 542, 1144–1154. [Google Scholar] [CrossRef]
- Strauch, S.; Wenzel, L.; Bischoff, A.; Dellwig, O.; Klein, J.; Schüch, A.; Wasenitz, B.; Palm, H.W. Commercial African catfish (Clarias gariepinus) recirculating aquaculture systems: Assessment of element and energy pathways with special focus on the phosphorus cycle. Sustainability 2018, 10, 1805. [Google Scholar] [CrossRef]
- Eshetu, B.; Jandl, G.; Leinweber, P. Compost changed soil organic matter molecular composition: A study by Py-GC/MS and Py-FIMS. Compos. Sci. Util. 2012, 20, 230–238. [Google Scholar] [CrossRef]
- Franke, M.; Jandl, G.; Leinweber, P. Organic compounds in re-circulated leachates of aerobic biological treated municipal solid waste. Biodegradation 2006, 17, 473–485. [Google Scholar] [CrossRef]
- Negassa, W.; Baum, C.; Leinweber, P. Soil amendment with agro-industrial byproducts: Molecular-chemical compositions and effects on soil biochemical activities and phosphorus fractions. J. Plant. Nutr. Soil Sci. 2011, 174, 113–120. [Google Scholar] [CrossRef]
- Jegajeevagan, K.; Mabilde, L.; Gebremikael, M.T.; Ameloot, N.; De Neve, S.; Leinweber, P.; Sleutel, S. Artisanal and controlled pyrolysis-based biochars differ in biochemical composition, thermal recalcitrance, and biodegradability in soil. Biomass Bioenerg. 2016, 84, 1–11. [Google Scholar] [CrossRef]
- Aust, M.-O.; Thiele-Bruhn, S.; Eckhardt, K.-U.; Leinweber, P. Composition of organic matter in particle size fractioned pig slurry. Bioresour. Technol. 2009, 100, 5736–5743. [Google Scholar] [CrossRef]
- Kazi, Z.H.; Schnitzer, M.I.; Monreal, C.; Mayer, P. Separation and identification of heterocyclic nitrogen compounds in biooil derived by fast pyrolysis of chicken manure. J. Environ. Sci. Heal. B 2011, 46, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Acksel, A.; Amelung, W.; Kühn, P.; Gehrt, E.; Regier, T.; Leinweber, P. Soil organic matter characteristics as indicator of Chernozem genesis in the Baltic Sea region. Geoderma Reg. 2016, 7, 187–200. [Google Scholar] [CrossRef]
- Hesterberg, D.; Zhou, W.; Hutchison, J.; Beauchemin, S.; Sayers, D.E. XAFS study of adsorbed and mineral forms of phosphate. J. Synchrotron Rad. 1999, 6, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Kruse, J.; Eichler-Löbermann, B.; Zimmer, D.; Willbold, S.; Leinweber, P.; Siebers, N. Phosphorus stocks and speciation in soil profiles of a long-term fertilizer experiment: Evidence from sequential fractionation, P K-edge XANES, and 31P NMR spectroscopy. Geoderma 2018, 316, 115–126. [Google Scholar] [CrossRef]
- Morshedizad, M.; Panten, K.; Klysubun, W.; Leinweber, P. Bone char effects on soil: Sequential fractionations and XANES spectroscopy. Soil 2018, 4, 23–35. [Google Scholar] [CrossRef]
- Prietzel, J.; Dümig, A.; Wu, Y.; Zhou, J.; Klysubun, W. Synchrotron-based P K-edge XANES spectroscopy reveals rapid changes of phosphorus speciation in the topsoil of two glacier foreland chronosequences. Geochim. Cosmochim. Acta. 2013, 108, 154–171. [Google Scholar] [CrossRef]
- Ajiboye, B.; Akinremi, O.O.; Hu, Y.; Jürgensen, A. XANES speciation of phosphorus in organically amended and fertilized vertisol and mollisol. Soil Sci. Soc. Am. J. 2008, 72, 1256–1262. [Google Scholar] [CrossRef]
- Kar, G.; Hundal, L.S.; Schoenau, J.J.; Peak, D. Direct chemical speciation of P in sequential chemical extraction residues using P K-edge X-ray absorption near-edge structure spectroscopy. Soil Sci. 2011, 176, 589–595. [Google Scholar] [CrossRef]
- Peak, D.; Sims, J.T.; Sparks, D.L. Solid-state speciation of natural and alum-amended poultry litter using XANES spectroscopy. Environ. Sci. Technol. 2002, 36, 4253–4261. [Google Scholar] [CrossRef]
- Sato, S.; Solomon, D.; Hyland, C.; Ketterings, Q.M.; Lehmann, J. Phosphorus speciation in manure and manure-amended soils using XANES spectroscopy. Environ. Sci. Technol. 2005, 39, 7485–7491. [Google Scholar] [CrossRef]
- Kraal, P.; Bostick, B.C.; Behrends, T.; Reichart, G.-J.; Slomp, C. Characterization of phosphorus species in sediments from the Arabian Sea oxygen minimum zone: Combining sequential extractions and X-ray spectroscopy. Mar. Chem. 2015, 168, 1–8. [Google Scholar] [CrossRef]
- Li, W.; Joshi, S.R.; Hou, G.; Burdige, D.J.; Sparks, D.L.; Jaisi, D.P. Characterizing phosphorus speciation of Chesapeake Bay sediments using chemical extraction, 31P NMR, and X-ray absorption fine structure spectroscopy. Environ. Sci. Technol. 2015, 49, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Prüter, J.; Leipe, T.; Michalik, D.; Klysubun, W.; Leinweber, P. Phosphorus speciation in sediments from the Baltic Sea, evaluated by a multi-method approach. J. Soils Sediments 2020, 20, 1676–1691. [Google Scholar] [CrossRef]
- Zhang, J.; Kitazawa, D. Numerical analysis of particulate organic waste diffusion in an aquaculture area of Gokasho Bay, Japan. Mar. Pollut. Bull. 2015, 93, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Palm, H.W.; Knaus, U.; Wasenitz, B.; Bischoff, A.A.; Strauch, S.M. Proportional up scaling of African catfish (Clarias gariepinus Burchell, 1822) commercial recirculating aquaculture systems disproportionally affects nutrient dynamics. Aquaculture 2018, 491, 155–168. [Google Scholar] [CrossRef]
- Hempfling, R.; Zech, W.; Schulten, H.-R. Chemical composition of the organic matter in forest soils: 2. Moder Profile. Soil Sci. 1988, 146, 262–276. [Google Scholar] [CrossRef]
- Schnitzer, M.; Schulten, H.-M. The analysis of soil organic matter by pyrolysis-field ionization mass spectrometry. Soil Sci. Soc. Am. J. 1992, 56, 1811. [Google Scholar] [CrossRef]
- Schulten, H.-R.; Leinweber, P. Characterization of humic and soil particles by analytical pyrolysis and computer modelling. J. Anal. Appl. Pyrolysis 1996, 38, 1–53. [Google Scholar] [CrossRef]
- Leinweber, P.; Jandl, G.; Eckhardt, K.-U.; Schulten, H.-R.; Schlichting, A.; Hoffman, D. Analytical pyrolysis and soft-ionization mass spectrometry. In Biophysico-Chemical Processes Involving Natural Nonliving Organic Matter in Environmental Systems, 1st ed.; Senesi, N., Xing, B., Huang, P.M., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 2009; pp. 539–588. [Google Scholar]
- Leinweber, P.; Kruse, J.; Baum, C.; Arcand, M.; Knight, J.D.; Farrell, R.; Eckhardt, K.-U.; Kiersch, K.; Jandl, G. Advances in understanding organic nitrogen chemistry in soils using state-of-the-art analytical techniques. Adv. Agron. 2013, 119, 83–151. [Google Scholar]
- Klysubun, W.; Tarawarakarn, P.; Thamsanong, N.; Amonpattaratkit, P.; Cholsuk, C.; Lapboonrueng, S.; Chaichuay, S.; Wongtepa, W. Upgrade of SLRI BL8 beamline for XFAS spectroscopy in a photon energy range of 1 keV to 13 keV. Radiat. Phys. Chem. 2020, 175, 108145. [Google Scholar] [CrossRef]
- Klysubun, W.; Sombunchoo, P.; Deenan, W.; Kongmark, C. Performance and status of beamline BL8 at SLRI for X-ray absorption spectroscopy. J. Synchrotron Radiat. 2012, 19, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Prietzel, J.; Harrington, G.; Häusler, W.; Heister, K.; Werner, F.; Klysubun, W. Reference spectra of important adsorbed organic and inorganic phosphate binding forms for soil P speciation using synchrotron-based K-edge XANES spectroscopy. J. Synchrotron Radiat. 2016, 23, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Werner, F.; Prietzel, J. Standard protocol and quality assessment of soil phosphorus speciation by P K-Edge XANES spectroscopy. Environ. Sci. Technol. 2015, 49, 10521–10528. [Google Scholar] [CrossRef]
- Calvin, S. XAFS for Everyone, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; p. 427. [Google Scholar]
- Both, A.; Parrish, C.C.; Penney, R.W.; Thompson, R.J. Physical and biochemical properties of effluent leaving an onshore Atlantic cod (Gadus morhua, Linnaeus 1758; Gadiformes: Gadiae) aquaculture facility and potential use in integrated multi-trophic aquaculture. Aquac. Res. 2012, 44, 1–12. [Google Scholar] [CrossRef]
- Heumann, S.; Schlichting, A.; Böttcher, J.; Leinweber, P. Sterols in soil organic matter relation to nitrogen mineralization in sandy arable soils. J. Plant. Nutr. Soil Sci. 2011, 174, 576–586. [Google Scholar] [CrossRef]
- Leite, S.P.; Vieira, J.R.C.; de Medeiros, P.L.; Leite, R.M.P.; Lima, V.M.; Xavier, H.S.; Lima, E.O. Antimicrobial activity of Indigofera suffruticosa. Evid. Based Complementray Altern. Med. 2006, 3, 261–265. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition and Reactions, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1994; pp. 166–187. [Google Scholar]
- Jandl, G.; Leinweber, P.; Schulten, H.-R.; Eusterhues, K. The concentration of fatty acids in organo-mineral particle-size fraction of a Chernozem. Eur. J. Soil Sci. 2004, 55, 459–469. [Google Scholar] [CrossRef]
- Wiedow, D.; Baum, C.; Leinweber, P. Inoculation with Trichoderma saturnisporum accelerates wheat straw decomposition on soil. Arch. Agron. Soil Sci. 2007, 53, 1–12. [Google Scholar] [CrossRef]
- Leue, M.; Eckhardt, K.-U.; Ellerbrock, R.H.; Gerke, H.H.; Leinweber, P. Analyzing organic matter composition at intact biopore and crack surfaces by combining DRFIT spectroscopy and Pyrolysis-Field Ionization Mass Spectrometry. J. Plant. Nutr. Soil Sci. 2016, 179, 5–17. [Google Scholar] [CrossRef]
- Sleutel, S.; Leinweber, P.; Begum, S.A.; Kader, M.A.; Van Oostveldt, P.; De Neve, S. Composition of organic matter in sandy relict and cultivated heathlands as examined by pyrolysis-field ionization MS. Biogeochemistry 2008, 89, 253–271. [Google Scholar] [CrossRef]
- Schulten, H.-R.; Schnitzer, M. A state of the art structural concept for humic substances. Naturwissenschaften 1993, 80, 29–30. [Google Scholar] [CrossRef]
- Negassa, W.; Acksel, A.; Eckhardt, K.-U.; Regier, T.; Leinweber, P. Soil organic matter characteristics in drained and rewetted peatlands of northern Germany: Chemical and spectroscopic analyses. Geoderma 2019, 353, 468–481. [Google Scholar] [CrossRef]
- Comeau, Y. Microbial Metabolism. In Biological Wastewater Treatment: Principles, Modelling and Design, 1st ed.; Henze, M., van Loosdrecht, M.C.M., Ekama, G.A., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2008; pp. 16–17. [Google Scholar]
- Pickering, I.J.; Brown, G.E., Jr.; Tokunaga, T.K. Quantitative speciation of selenium in soils using X-ray absorption spectroscopy. Eviron. Sci. Technol. 1995, 29, 2456–2459. [Google Scholar] [CrossRef] [PubMed]
- Beauchemin, S.; Hesterberg, D.; Chou, J.; Beauchemin, M.; Simard, R.R.; Sayers, D.E. Speciation of phosphorus in phosphorus-enriched agricultural soils using X-ray absorption near-edge structure spectroscopy and chemical fractionation. J. Environ. Qual. 2003, 32, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.P.; Braun, S.; Tuyishime, J.R.M.; Adediran, G.A.; Warrinnier, R.; Hesterberg, D. A probabilistic approach to phosphorus speciation of soils using P K-edge XANES spectroscopy with linear combination fitting. Soil Syst. 2020, 4, 26. [Google Scholar] [CrossRef]
- Kruse, J.; Leinweber, P. Phosphorus in sequentially extracted fen peat soils: A K-edge X-ray absorption near-edge structure (XANES) spectroscopy study. J. Plant. Nutr. Soil Sci. 2008, 171, 613–620. [Google Scholar] [CrossRef]
- Eriksson, A.K.; Hillier, S.; Hesterberg, D.; Klysubun, W.; Ulén, B.; Gustafsson, J.P. Evolution of phosphorus speciation with depth in an agricultural soil profile. Geoderma 2016, 280, 29–37. [Google Scholar] [CrossRef]
- Darn, S.M.; Sodi, R.; Ranganath, L.R.; Roberts, N.B.; Duffield, J.R. Experimental and computer modelling speciation studies of the effect of pH and phosphate on the precipitation of calcium and magnesium salts in urine. Clin. Chem. Lab. Med. 2006, 44, 185–191. [Google Scholar] [CrossRef]
- Frankowski, L.; Bolałek, J.; Szostek, A. Phosphorus in bottom sediments of Pomeranian Bay (Southern Baltic–Poland). Estuar. Coast. Shelf Sci. 2002, 54, 1027–1038. [Google Scholar] [CrossRef]
- Robinson, J.S.; Baumann, K.; Hu, Y.; Hagemann, P.; Kebelmann, L.; Leinweber, P. Phosphorus tranformation in plant-based and bio-waste materials induced by pyrolysis. Ambio 2018, 47, S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Shober, A.; Hesterberg, D.L.; Sims, J.T.; Gardner, S. Characterization of phosphorus species in biosolids and manures using XANES spectroscopy. J. Environ. Qual. 2006, 35, 1983–1993. [Google Scholar] [CrossRef] [PubMed]
- Maguire, R.O.; Hesterberg, D.; Gernat, A.; Anderson, K.; Wineland, M.; Grimes, J. Liming poultry manures to decrease soluble phosphorus and suppress the bacteria population. J. Environ. Qual. 2006, 35, 849–857. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Unit | EAS | SIAS | IAS |
|---|---|---|---|---|
| N | % | 5.2 ± 0.2 | 5.4 ± 0.2 | 5.1 ± 0.3 |
| S | % | 0.9 ± 0.0 | 0.9 ± 0.0 | 1.0 ± 0.1 |
| C:N | ratio | 7.9 ± 0.2 | 7.4 ± 0.2 | 7.8 ± 0.2 |
| P | % | 1.4 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.1 |
| Al | % | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 |
| Fe | % | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 |
| Ca | % | 3.4 ± 0.1 | 4.2 ± 0.2 | 3.9 ± 0.1 |
| Mg | % | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| K | % | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 |
| Sample | VM | TII | %TII From Compound Classes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (106 counts mg−1) | CHYDR | PHLM | LDIM | LIPID | ALKYL | NCOMP | STEROL | AMID | SUBER | FATTY | |
| EAS | 78.5 a | 958.5 a | 2.3 a | 2.3 a | 0.8 a | 3.6 a | 6.1 a | 1.1 a | 14.4 a | 2.6 a | 0.7 a | 12.2 a |
| SIAS | 77.2 a | 1467.8 b | 2.5 a | 2.5 a | 1.2 c | 3.7 a | 6.2 a | 1.3 b | 14.1 a | 2.8 a | 0.8 b | 7.4 b |
| IAS | 85.8 b | 1396.3 b | 2.7 a | 2.4 a | 1.0 b | 3.7 a | 6.0 a | 1.4 b | 14.2 a | 2.9 a | 0.8 b | 7.2 b |
| Sample | Ca Phytate | CaHPO4 | Ca Hydroxyapatite | R-Factor |
|---|---|---|---|---|
| in % | ||||
| EAS | 87 | 6 | 7 | 0.0094 |
| SIAS | 53 | 47 | 0.0267 | |
| IAS | 76 | 24 | 0.0102 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prüter, J.; Strauch, S.M.; Wenzel, L.C.; Klysubun, W.; Palm, H.W.; Leinweber, P. Organic Matter Composition and Phosphorus Speciation of Solid Waste from an African Catfish Recirculating Aquaculture System. Agriculture 2020, 10, 466. https://doi.org/10.3390/agriculture10100466
Prüter J, Strauch SM, Wenzel LC, Klysubun W, Palm HW, Leinweber P. Organic Matter Composition and Phosphorus Speciation of Solid Waste from an African Catfish Recirculating Aquaculture System. Agriculture. 2020; 10(10):466. https://doi.org/10.3390/agriculture10100466
Chicago/Turabian StylePrüter, Julia, Sebastian Marcus Strauch, Lisa Carolina Wenzel, Wantana Klysubun, Harry Wilhelm Palm, and Peter Leinweber. 2020. "Organic Matter Composition and Phosphorus Speciation of Solid Waste from an African Catfish Recirculating Aquaculture System" Agriculture 10, no. 10: 466. https://doi.org/10.3390/agriculture10100466
APA StylePrüter, J., Strauch, S. M., Wenzel, L. C., Klysubun, W., Palm, H. W., & Leinweber, P. (2020). Organic Matter Composition and Phosphorus Speciation of Solid Waste from an African Catfish Recirculating Aquaculture System. Agriculture, 10(10), 466. https://doi.org/10.3390/agriculture10100466








