Presence of Bradyrhizobium sp. under Continental Conditions in Central Europe
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Samples and Experimental Design
2.2. Extraction, Purification and Sequencing of DNA from Soil Samples
2.3. Statistical Analysis
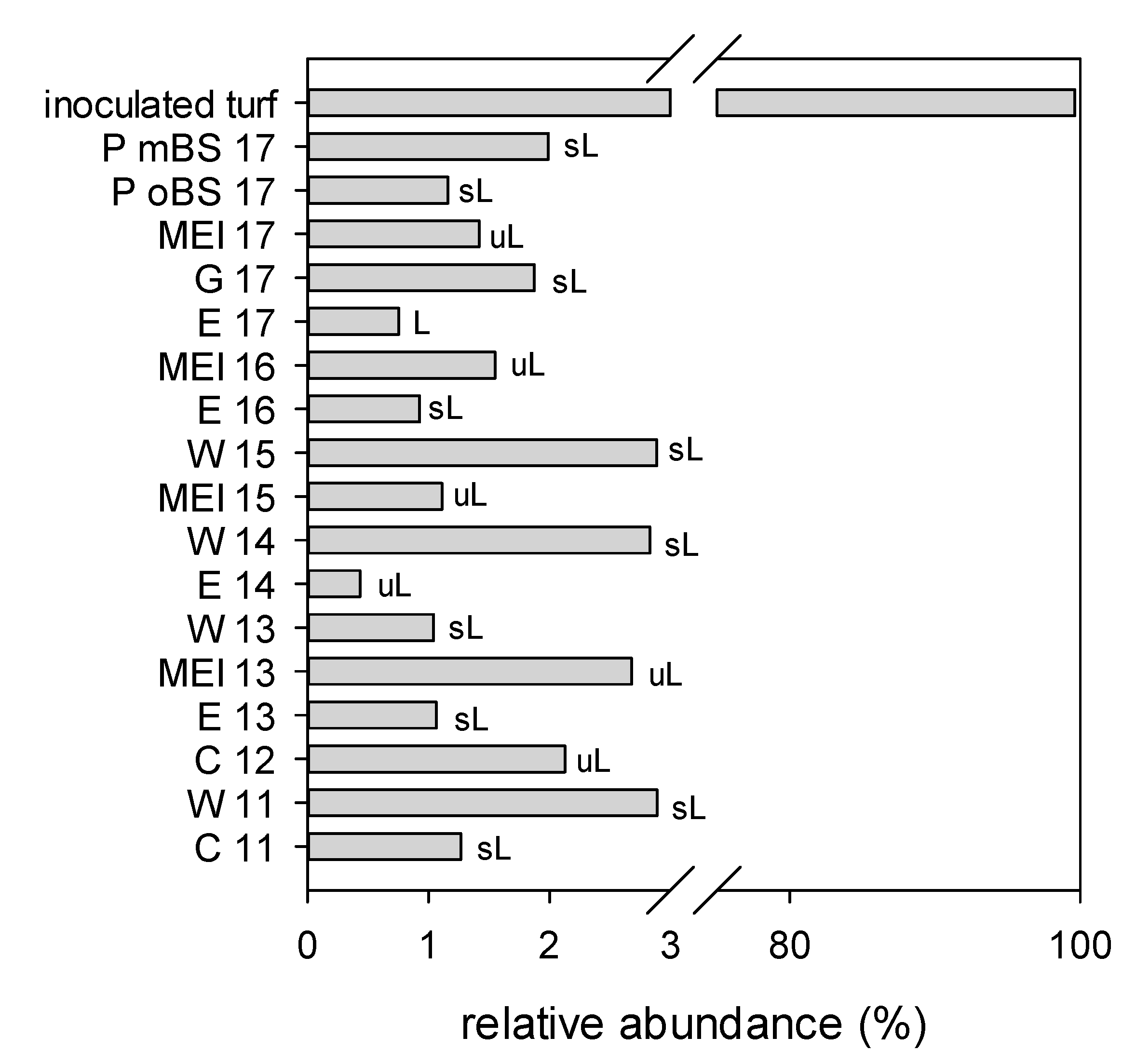
3. Results
3.1. Influence of Soil Properties on Nodule and Plant Growth
3.2. Sequencing Results
3.3. Plant Growth and Nutrient Concentration
4. Discussion
4.1. Presence of Rhizobia in Soil
4.2. Influence of Soil Properties on Nodule and Plant Growth
4.3. Plant Growth and Nutrient Concentration
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bundesministerium für Ernährung und Landwirtschaft (BMEL). Ernte 2019. Mengen und Preise; BMEL: Berlin, Germany, 2019; pp. 15–16. Available online: https://www.bmel.de/SharedDocs/Downloads/DE/_Landwirtschaft/Pflanzenbau/Ernte-Bericht/ernte-2019.html (accessed on 30 March 2020).
- Narożna, D.; Pudełko, K.; Króliczak, J.; Golińska, B.; Sugawara, M.; Mądrzak, C.J.; Sadowsky, M.J. Survival and competitiveness of Bradyrhizobium japonicum strains 20 years after introduction into field locations in Poland. Appl. Environ. Microbiol. 2015, 81, 5552–5559. [Google Scholar] [CrossRef] [PubMed]
- Brunel, B.; Cleyet-Marel, J.C.; Normand, P.; Bardin, R. Stability of Bradyrhizobium japonicum inoculants introduced into soil. In Nitrogen Fixation: Hundred Years After; Bothe, H., de Bruijn, F.J., Newton, W.E., Eds.; Fischer: Stuttgart, Germany, 1988; p. 780. [Google Scholar]
- Obaton, M.; Bouniols, A.; Guillaume, P.; Guillaume, P.; Vadez, V. Are Bradyrhizobium japonicum stable during a long stay in soil? Plant Soil 2002, 245, 315–326. [Google Scholar] [CrossRef]
- Triplett, E.W.; Albrecht, K.A.; Oplinger, E.S. Crop rotation effects on populations of Bradyrhizobium japonicum and Rhizobium meliloti. Soil Biol. Biochem. 1992, 25, 781–784. [Google Scholar] [CrossRef]
- Revellin, C.; Pinochet, X.; Beauclair, P.; Catroux, G. Influence of soil properties and soybean cropping history on the Bradyrhizobium japonicum population in some French Soils. Eur. J. Soil Sci. 1996, 47, 505–510. [Google Scholar] [CrossRef]
- Buendía-Clavería, A.; Rodriguez-Navarro, D.; Santamaría-Linaza, C.; Ruiz-Saínza, J.; Temprano-Vera, F. Evaluation of the symbiotic properties of Rhizobium fredii in European soils. System. Appl. Microbiol. 1994, 17, 155–160. [Google Scholar] [CrossRef]
- Weaver, R.W.; Frederick, L.R.; Dumenil, L.C. Effect of soybean cropping and soil properties on numbers of Rhizobium japonicum in Iowa soils. Soil Sci. 1972, 114, 137–141. [Google Scholar] [CrossRef]
- Zhang, H.; Prithiviraj, B.; Charles, T.C.; Driscoll, B.T.; Smith, D.L. Low temperature tolerant Bradyrhizobium japonicum.strains allowing improved nodulation and nitrogen fixation of soybean in a short season (cool spring) area. Eur. J. Agron. 2003, 19, 205–213. [Google Scholar] [CrossRef]
- Suzuki, Y.; Adhikari, D.; Itoh, K.; Suyama, K. Effects of temperature on competition and relative dominance of Bradyrhizobium japonicum and Bradyrhizobium elkanii in the process of soybean nodulation. Plant Soil 2014, 374, 915–924. [Google Scholar] [CrossRef]
- Association of German Agricultural Analytic and Research Institutes e. V. (VDLUFA). Methode A 5.1.1: Bestimmung des pH-Wertes. In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch); Bd. I Die Untersuchung von Böden; 7. Teillfg. 2016; VDLUFA-Verlag: Darmstadt, Germany, 1991. [Google Scholar]
- Association of German Agricultural Analytic and Research Institutes e. V. (VDLUFA). Methode A 6.2.1.1: Bestimmung von Phosphor und Kalium im Calcium-Acetat-Lactat-Auszug. In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch); Bd. I Die Untersuchung von Böden; 6. Teillfg. 2012; VDLUFA-Verlag: Darmstadt, Germany, 1991. [Google Scholar]
- Association of German Agricultural Analytic and Research Institutes e. V. (VDLUFA). Methode A 6.2.4.1: Bestimmung des pflanzenverfügbaren Magnesiums im Calciumchlorid-Auszug. In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch); Bd. I Die Untersuchung von Böden; VDLUFA-Verlag: Darmstadt, Germany, 1991. [Google Scholar]
- DIN EN 15936:2012-11 Schlamm, Behandelter Bioabfall, Boden und Abfall—Bestimmung des Gesamten Organischen Kohlenstoffs (TOC) Mittels Trockener Verbrennung; Beuth Verlag: Berlin, Germany, 2012.
- LKS LMUAA 027 Bestimmung des Gesamtstickstoffs in Böden durch Verbrennung und Gasanalyse. In DAkkS Anlage zur Akkreditierungsurkunde D-PL-14632-01-00 nach DIN EN ISO/IEC 17025:2018; Landwirtschaftliche Kommunikations- und Service- gesellschaft mbH (LKS): Amberg, Germany, 2019.
- DIN ISO 11277:2002-08 Bodenbeschaffenheit—Bestimmung der Partikelgrößenverteilung in Mineralböden—Verfahren Mittels Siebung und Sedimentation; ISO 11277: 1998/Cor.1:2002; Beuth Verlag: Berlin, Germany, 2002.
- Sächsisches Landesamt für Umwelt und Geologie Bodenatlas des Freistaates Sachsen Teil 2: Standortkundliche Verhältnisse und Bodennutzung. Lößnitz-Druck GmbH: Radebeul, Germany, 1996. Available online: https://www.umwelt.sachsen.de/umwelt/download/boden/Bodenatlas-Teil2.pdf (accessed on 20 May 2020).
- Geologischer Dienst NRW Einteilung der Bodenarten; Geologischer Dienst Nordrhein-Westfalen Krefeld: Krefeld, Germany, 2019; pp. 3–4. Available online: https://www.landwirtschaftskammer.de/lufa/pdf/bodenarten-einteilung.pdf (accessed on 20 May 2020).
- Link, M.; Vorderbrügge, T.; Michalski, A.; Kowalkowski, A.; Harrach, T. Interpretation of German and Polish soil assessment data in order to deduce and to evaluate soil parameters and functions. Die Bodenkult. 2010, 61, 11–24. [Google Scholar]
- Mullis, K.; Faloona, F.; Scharf, S.; Saiki, R.; Horn, G.; Erlich, H. Specific enzymatic amplification of DNA in Vitro: The Polymerase Chain Reaction. Cold Spring Harb. Symp. Quant. Biol. 1986, 51, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- RTLGenomics. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 15 September 2020).
- Kamicker, B.J.; Brill, W.J. Methods to alter the recovery and nodule location of Bradyrhizobium japonicum inoculant strains on field-grown soybeans. Appl. Environ. Microbiol. 1987, 53, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Granada, C.; Vargas, L.; Hayashi Sant’Anna, F.; Balsanelli, E.; Antonio de Baura, V.; de Oliveira Pedrosa, F.; Maltempi de Souza, E.; Falcon, T.; Passaglia, L. The genomes of three Bradyrhizobium sp. isolated from root nodules of Lupinus albescens grown in extremely poor soils display important genes for resistance to environmental stress. Genet. Mol. Biol. 2018, 41, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Ehinger, M.; Mohr, T.J.; Starcevich, J.B.; Sachs, J.L.; Porter, S.; Simms, E. Specialization-generalization trade-off in a Bradyrhizobium symbiosis with wild legume hosts. BMC Ecol. 2014, 14, 8. [Google Scholar] [CrossRef]
- Velázquez, E.; Valverde, A.; Rivas, R.; Gomis, V.; Peix, A.; Gantois, I.; Igual, J.M.; León-Barrios, M.; Willems, A.; Mateos, P.F.; et al. Strains nodulating Lupinus albus on different continents belong to several new chromosomal and symbiotic lineages within Bradyrhizobium. Antonie Van Leeuwenhoek 2010, 97, 363–376. [Google Scholar] [CrossRef]
- Lawson, Y.D.; Muramatsu, K.; Nioh, I. Effect of organic matter on the growth, nodulation, and nitrogen fixation of soybean grown under acid and saline conditions. Soil Sci. Plant Nutr. 1995, 41, 721–728. [Google Scholar] [CrossRef]
- Singleton, P.W.; Abdel Magid, H.M.; Tavares, J.W. Effect of phosphorus on the effectiveness of strains of Rhizobium japonicum. Soil Sci. Soc. Am. J. 1985, 49, 613–616. [Google Scholar] [CrossRef]
- Mullen, M.D.; Israel, D.W.; Wollum, A.G. Effects of Bradyrhizobium japonicum and Soybean (Glycine max (L.) Merr.) Phosphorus Nutrition on Nodulation and Dinitrogen Fixation. Appl. Environ. Microbiol. 1988, 54, 2387–2392. [Google Scholar] [CrossRef]
- Ham, G.E.; Frederick, L.R.; Anderson, I.C. Serogroups of Rhizobium japonicum in Soybean Nodules Sampled in Iowa. Agron. J. 1971, 63, 69–72. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Growth and nutrient concentrations of common bean, lowland rice, corn, soybean, and wheat at different soil ph on an inceptisol. J. Plant Nutr. 1999, 22, 1495–1507. [Google Scholar] [CrossRef]
- Unsleber, J.; Kreikenbohm, C.; Schätzl, R.; Braun, S.; Nadler, C.; Reindl, A. Soja–Anbau und Verwertung; Bayerische Landesanstalt für Landwirtschaft (LfL): Freising-Weihenstephan, Germany, 2018. [Google Scholar]
- Weaver, R.W.; Frederick, L.R. Effect of Inoculum Rate on Competitive Nodulation of Glycine max L. Merrill. II. Field Studies. Agron. J. 1974, 66, 233. [Google Scholar] [CrossRef]
- Thies, J.E.; Singleton, P.W.; Bohlool, B.B. Influence of the Size of Indigenous Rhizobial Populations on Establishment and Symbiotic Performance of Introduced Rhizobia on Field-Grown Legumes. Appl. Environ. Microbiol. 1991, 57, 19–28. [Google Scholar] [CrossRef]
- Amann, C.; Amberger, A. Phosphorus Efficiency of Buckwheat (Fagopyrum esculentum). J. Plant Nutr. Soil Sci. 1989, 152, 181–189. [Google Scholar] [CrossRef]
- Possinger, A.R.; Byrne, L.B.; Breen, N.E. Effect of buckwheat (Fagopyrum esculentum) on soil-phosphorus availability and organic acids. J. Plant Nutr. Soil Sci. 2013, 176, 16–18. [Google Scholar] [CrossRef]
- DeMooy, C.J.; Pesek, J.; Spaldon, E. Mineral nutrition in Soybeans: Improvement, Production and Uses. Am. Soc. Agron. Madison Wis. 1973, 16, 267–352. [Google Scholar]
- Streeter, J.D. Synthesis and Accumulation of Nitrite in Soybean Nodules Supplied with Nitrate. Plant Physiol. 1982, 69, 1429–1434. [Google Scholar] [CrossRef]
- Gates, C.T.; Müller, W.J. Nodule and Plant Development in the Soybean, Glycine max (L.) Merr. Growth Response to Nitrogen, Phosphorus and Sulfur. Aust. J. Bot. 1979, 27, 203–215. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, X.; Bi, D.; Hu, F. Effects of Sulfur Fertilization on Soybean Root and Leaf Traits, and Soil Microbial Activity. J. Plant Nutr. 2008, 31, 473–483. [Google Scholar] [CrossRef]
- Lange, A. Influence of S Supply on the Biological Nitrogen Fixation of Legumes. Ph.D. Thesis, University of Bonn, Bonn, Germany, 1998. [Google Scholar]
- Ferguson, B.J. The Development and Regulation of Soybean Nodules. In A Comprehensive Survey of International Soybean Research, Genetics, Physiology, Agronomy and Nitrogen Relationships; Board, J., Ed.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Li, X.; Zheng, J.; Yang, Y.; Liao, H. Increasing Nodule Size1 Expression is Required for Normal Rhizobial Symbiosis and Nodule Development. Plant Physiol. 2018, 178, 1233–1248. [Google Scholar] [CrossRef]
- Peng, W.T.; Zhang, L.D.; Zhou, Z.; Fu, C.; Chen, Z.C.; Liao, H. Magnesium promotes root nodulation through facilitation of carbohydrate allocation in soybean. Physiol. Plant. 2018, 163, 372–385. [Google Scholar] [CrossRef]
- Ohyama, T.; Fujikake, H.; Yashima, H.; Tanabata, S.; Ishikawa, S.; Sato, T.; Fujimaki, S. Effect of Nitrate on Nodulation and Nitrogen Fixation of Soybean. In Soybean Physiology and Biochemistry; El-Shemy, H., Ed.; InTech: Rijeka, Croatia, 2011; pp. 333–364. Available online: http://www.intechopen.com/books/soybeanphysiology-and-biochemistry/effect-of-nitrate-on-nodulation-and-nitrogen-fixation-of-soybean (accessed on 20 May 2020).
- Andersson, E. Need for Seed Re-Inoculation in Swedish Soybean Cropping Sequences; Second Cycle. A2E, SLU; Department of Soil and Environment: Uppsala, Sweden, 2014; Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-s-4047 (accessed on 21 August 2017).
- Indrasumunar, A.; Menzies, N.W.; Dart, P.J. Laboratory prescreening of Bradyrhizobium japonicum for low pH, Al and Mn tolerance can be used to predict their survival in acid soils. Soil Biol. Biochem. 2012, 48, 135–141. [Google Scholar] [CrossRef]
- Mądrzak, C.J.; Golińska, B.; Króliczak, J.; Pudełko, K.; Łażewska, D.; Lampka, B.; Sadowsky, M.J. Diversity among Field Populations of Bradyrhizobium japonicum in Poland. Appl. Environ. Microbiol. 1995, 61, 1194–1200. [Google Scholar] [CrossRef] [PubMed]


| Nr. | Farm Code | s.c.y. | Type | pH | P (mg/100 g) | K (mg/100 g) | Mg (mg/100 g) | Humus (%) | Nt (%) | Soil Texture ** | Soil Index * | Grain Yield (t/ha) | CV | INO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | C | 2011 | MC | 7.0 | 17.4 | 14.8 | 5.6 | 1.6 | 0.14 | sL | 50 | 2.8 | conv. | yes |
| 2 | W | 2011 | MC | 5.4 | 3.9 | 5.4 | 13.0 | 2.5 | 0.17 | sL | 50 | 3.5 | conv. | yes |
| 3 | C | 2012 | MC | 5.9 | 17.3 | 6.6 | 8.3 | 1.8 | 0.13 | uL | 70 | 2.6 | conv. | yes |
| 4 | MEI | 2013 | MC | 6.7 | 11.1 | 20.9 | 10.6 | 3.4 | 0.18 | uL | 72 | 3.4 | conv. | yes |
| 5 | E | 2013 | MC | 7.2 | 10.5 | 18.3 | 13.8 | 2.8 | 0.06 | sL | 88 | 1.6 | org. | yes |
| 6 | G | 2013 | MC | 6.6 | 11.5 | 17.7 | 12.8 | 2.4 | 0.08 | sL | 60 | 1.6 | org. | yes |
| 7 | W | 2013 | MC | 6.4 | 16.4 | 28.4 | 16.7 | 3.3 | 0.23 | sL | 50 | 1.5 | conv. | yes |
| 8 | F | 2013 | MC | 5.4 | 3.2 | 11.4 | 12.1 | 2.2 | 0.17 | ND | 83 | 3.0 | conv. | yes |
| 9 | C | 2013 | MC | 6.6 | 20.2 | 32.2 | 10.6 | 2.0 | 0.19 | sL | 42 | 2.2 | conv. | yes |
| 10 | B | 2014 | MC | 6.2 | 7.4 | 7.6 | 10.5 | 1.9 | 0.10 | sL | 38 | 2.4 | conv. | yes |
| 11 | MEI | 2014 | MC | 6.6 | 8.2 | 30.2 | 9.8 | 9.8 | 0.22 | uL | 72 | 3.6 | conv. | yes |
| 12 | E | 2014 | MC | 7.5 | 15.4 | 19.8 | 21.7 | 3.0 | 0.14 | uL | 82 | 3.8 | org. | yes |
| 13 | W | 2014 | MC | 5.7 | 6.3 | 10.6 | 15.3 | 3.2 | 0.20 | sL | 50 | 1.8 | conv. | yes |
| 14 | K | 2014 | MC | 5.8 | 2.8 | 11.7 | 12.8 | 1.3 | 0.21 | uL | 70 | 2.9 | conv. | yes |
| 15 | BP | 2014 | MC | 7.2 | 5.7 | 18.8 | 11.0 | 2.0 | 0.09 | ND | 70 | 2.9 | conv. | yes |
| 16 | C | 2014 | MC | 6.4 | 12.8 | 8.0 | 5.8 | 1.3 | 0.12 | uL | 70 | 3.1 | conv. | yes |
| 17 | J | 2015 | MC | 6.5 | 11.2 | 10.6 | 17.6 | 3.5 | 0.13 | sU | 31 | 1.5 | conv. | yes |
| 18 | B | 2015 | MC | 6.4 | 4.5 | 4.3 | 17.2 | 2.0 | 0.08 | sL | 40 | 1.8 | conv. | yes |
| 19 | MEI | 2015 | MC | 6.6 | 10.2 | 19.9 | 9.4 | 2.7 | 0.17 | uL | 72 | 2.7 | conv. | yes |
| 20 | E | 2015 | MC | 7.1 | 11.6 | 17.7 | 15.6 | 3.0 | 0.14 | uL | 88 | 1.0 | org. | yes |
| 21 | G | 2015 | MC | 5.9 | 9.2 | 8.0 | 8.7 | 1.5 | 0.12 | sL | 53 | 1.3 | org. | yes |
| 22 | W | 2015 | MC | 5.4 | 8.7 | 8.5 | 15.2 | 2.6 | 0.14 | sL | 50 | 2.0 | conv. | yes |
| 23 | K | 2015 | MC | 6.2 | 7.4 | 9.4 | 12.0 | 1.6 | 0.26 | sL | 83 | 3.0 | conv. | yes |
| 24 | A | 2015 | MC | 6.3 | 6.6 | 11.8 | 11.5 | 1.0 | 0.21 | ND | 55 | 1.5 | conv. | yes |
| 25 | BP | 2015 | MC | 6.3 | 3.6 | 15.1 | 13.4 | 2.4 | 0.23 | ND | 70 | 2.2 | conv. | yes |
| 26 | C | 2015 | MC | 6.4 | 16.4 | 11.0 | 11.2 | 1.3 | 0.15 | uL | 70 | 2.7 | conv. | yes |
| 27 | J | 2016 | MC | 6.0 | 12.1 | 8.9 | 7.9 | 2.6 | 0.09 | sU | 28 | 3.4 | conv. | yes |
| 28 | B | 2016 | MC | 6.1 | 5.9 | 11.5 | 14.3 | 2.2 | 0.12 | sL | 42 | 2.9 | conv. | yes |
| 29 | MEI | 2016 | MC | 6.6 | 8.0 | 28.6 | 9.8 | 2.3 | 0.18 | uL | 72 | 3.2 | conv. | yes |
| 30 | E | 2016 | MC | 7.5 | 14.0 | 13.6 | 11.2 | 2.1 | 0.10 | sL | 88 | 2.0 | org. | yes |
| 31 | K | 2016 | MC | 6.6 | 3.0 | 12.1 | 15.8 | 1.3 | 0.21 | sL | 92 | 3.0 | conv. | yes |
| 32 | A | 2016 | MC | 6.2 | 6.9 | 22.0 | 14.0 | 1.3 | 0.27 | ND | 55 | 3.3 | conv. | yes |
| 33 | BP | 2016 | MC | 7.6 | 29.2 | 7.3 | 13.3 | 3.1 | 0.35 | ND | 70 | 2.7 | conv. | yes |
| 34 | C | 2016 | MC | 6.2 | 8.9 | 12.8 | 9.4 | 1.6 | 0.17 | uL | 70 | 2.7 | conv. | yes |
| 35 | J | 2017 | MC | 6.7 | 13.1 | 15.9 | 10.4 | 2.9 | 0.13 | sU | 28 | 3.5 | conv. | yes |
| 36 | P | 2017 | MC | 6.7 | 4.3 | 6.3 | 15.1 | 2.1 | 0.10 | tL | 60 | 4.2 | conv. | yes |
| 37 | B | 2017 | MC | 6.6 | 5.7 | 5.3 | 11.2 | 2.1 | 0.15 | sL | 42 | 3.5 | conv. | yes |
| 38 | MEI | 2017 | MC | 7.1 | 9.9 | 22.1 | 9.1 | 1.6 | 0.08 | uL | 72 | 3.2 | conv. | yes |
| 39 | E | 2017 | MC | 7.5 | 18.2 | 13.0 | 24.1 | 3.5 | 0.19 | L | 88 | 1.6 | org. | yes |
| 40 | G | 2017 | MC | 6.2 | 13.0 | 27.6 | 11.7 | 2.2 | 0.11 | sL | 50 | 1.5 | org. | yes |
| 41 | W | 2017 | MC | 6.0 | 5.0 | 9.0 | 18.5 | 2.5 | 0.17 | sL | 50 | 4.0 | conv. | yes |
| 42 | K | 2017 | MC | 7.1 | 5.6 | 8.5 | 11.7 | 1.3 | 0.14 | sL | 57 | 2.9 | conv. | yes |
| 43 | A | 2017 | MC | 6.0 | 7.6 | 13.8 | 11.7 | 1.0 | 0.02 | ND | 60 | 2.4 | conv. | yes |
| 44 | F | 2017 | MC | 7.1 | 7.0 | 13.4 | 10.2 | 3.3 | 0.33 | ND | 83 | 2.8 | conv. | yes |
| 45 | BP | 2017 | MC | 6.8 | 15.1 | 21.7 | 3.3 | 2.1 | 0.08 | ND | 50 | 2.9 | conv. | yes |
| 46 | C | 2017 | MC | 5.7 | 20.0 | 7.8 | 11.1 | 1.3 | 0.20 | uL | 70 | 2.4 | conv. | yes |
| 47 | bp | 0 | c | 5.9 | 2.5 | 7.5 | 16.1 | 10.7 | 0.16 | ND | ND | − | conv. | no |
| 48 | Wr | 17/18 | IC | 5.8 | 4.8 | 10.6 | 11.7 | 5.8 | 0.09 | sL | 70 | − | conv. | yes/no |
| 49 | Pi | 17/18 | IC | 6.9 | 6.7 | 12.3 | 13.9 | 2.6 | 0.12 | sL | 65 | − | org. | yes/no |
| 50 | W | 17/18 | IC | 5.5 | 5.6 | 12.7 | 14.9 | 5.2 | 0.49 | sL | 50 | − | conv. | yes |
| pH | P | K | Mg | Humus | Nt | |
|---|---|---|---|---|---|---|
| Number of nodules | −0.15 | −0.06 | 0.00 | −0.19 | 0.12 | −0.30 * |
| Nodular mass | 0.03 | 0.27 | 0.01 | −0.11 | 0.29 | −0.31 * |
| N concentration | 0.12 | 0.08 | 0.04 | 0.01 | 0.32 * | −0.25 |
| Sample | Nodule Weight (g plant−1) | Nodules (Number plant−1) | Root Weight (g plant−1) | Shoot Weight (g plant−1) |
|---|---|---|---|---|
| control | ||||
| C uI | 0.0799 (±0.03) | 12.0 (±7.3) | 0.4 (±0.35) | 2.3 (±0.68) |
| C I | 0.0919 (±0.02) | 57.7 (±22.8) | 0.4 (±0.12) | 4.6 (±1.12) |
| C BW | 0.0037 (±0.01) | 0.2 (±0.7) | 0.8 (±0.32) | 6.3 (±1.88) |
| year | ||||
| 2011 [N = 2] | 0.0785 (±0.02) | 33.2 (±12.4) | 0.5 (±0.17) | 3.7 (±0.53) |
| 2012 [N = 1] | 0.1565 (±0.02) | 32.7 (±19.8) | 0.6 (±0.24) | 5.0 (±0.63) |
| 2013 [N = 6] | 0.1061 (±0.03) | 29.7 (±11.0) | 0,4 (±0.08) | 4.1 (±0.64) |
| 2014 [N = 7] | 0.0910 (±0.03) | 21.0 (±7.5) | 0.5 (±0.12) | 4.1 (±0.64) |
| 2015 [N = 9] | 0.0918 (±0.04) | 28.9 (±13.5) | 0.4 (±0.13) | 3.7 (±0.66) |
| 2016 [N = 9] | 0.0877 (±0.03) | 24.3 (±10.2) | 0.4 (±0.14) | 4.0 (±0.59) |
| 2017 [N = 12] | 0.0929 (±0.03) | 36.7 (±13.4) | 0.4 (±0.15) | 4.2 (±0.97) |
| intercrop | ||||
| Wr oS | 0.0942 (±0.06) | 9.3 (±10.1) | 0.7 (±0.31) | 3.8 (±0.42) |
| Wr mS | 0.1210 (±0.01) | 29.1 (±1.9) | 0.3 (±0.11) | 4.1 (±0.50) |
| P oS | 0.0533 (±0.04) | 13.5 (±12.2) | 0.7 (±0.33) | 5.0 (±2.00) |
| P mS | 0.1745 (±0.11) | 56.2 (±53.6) | 1.0 (±0.61) | 7.1 (±2.31) |
| P oBS | 0.0632 (±0.04) | 13.2 (±9.9) | 0.8 (±0.43) | 6.4 (±2.87) |
| P mBS | 0.0862 (±0.05) | 13.3 (±6.2) | 0.6 (±0.24) | 6.4 (±1.38) |
| W IC | 0.1391 (±0.03) | 18.8 (±8.2) | 0.7 (±0.15) | 5.1 (±0.99) |
| DMroot (g plant−1) | DMshoot (g plant−1) | Nroot (%) | Nshoot (%) | Nnodules (%) | |
|---|---|---|---|---|---|
| number of nodules | 0.18 | 0.57 *** | −0.25 | 0.29 | 0.29 |
| nodular mass | 0.54 *** | 0.88 *** | −0.36 * | 0.21 | 0.44 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griebsch, A.; Matschiavelli, N.; Lewandowska, S.; Schmidtke, K. Presence of Bradyrhizobium sp. under Continental Conditions in Central Europe. Agriculture 2020, 10, 446. https://doi.org/10.3390/agriculture10100446
Griebsch A, Matschiavelli N, Lewandowska S, Schmidtke K. Presence of Bradyrhizobium sp. under Continental Conditions in Central Europe. Agriculture. 2020; 10(10):446. https://doi.org/10.3390/agriculture10100446
Chicago/Turabian StyleGriebsch, Anne, Nicole Matschiavelli, Sylwia Lewandowska, and Knut Schmidtke. 2020. "Presence of Bradyrhizobium sp. under Continental Conditions in Central Europe" Agriculture 10, no. 10: 446. https://doi.org/10.3390/agriculture10100446
APA StyleGriebsch, A., Matschiavelli, N., Lewandowska, S., & Schmidtke, K. (2020). Presence of Bradyrhizobium sp. under Continental Conditions in Central Europe. Agriculture, 10(10), 446. https://doi.org/10.3390/agriculture10100446





