Diversity and Management Strategies of Plant Parasitic Nematodes in Moroccan Organic Farming and Their Relationship with Soil Physico-Chemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematode Survey
2.2. Nematode Extraction and Identification
2.3. Assessment of Nematode Population Densities
2.4. Physico-Chemical Analysis of Soil
2.5. Diversity of Plant Parasitic Nematodes
2.6. Statistical Analyses
3. Results
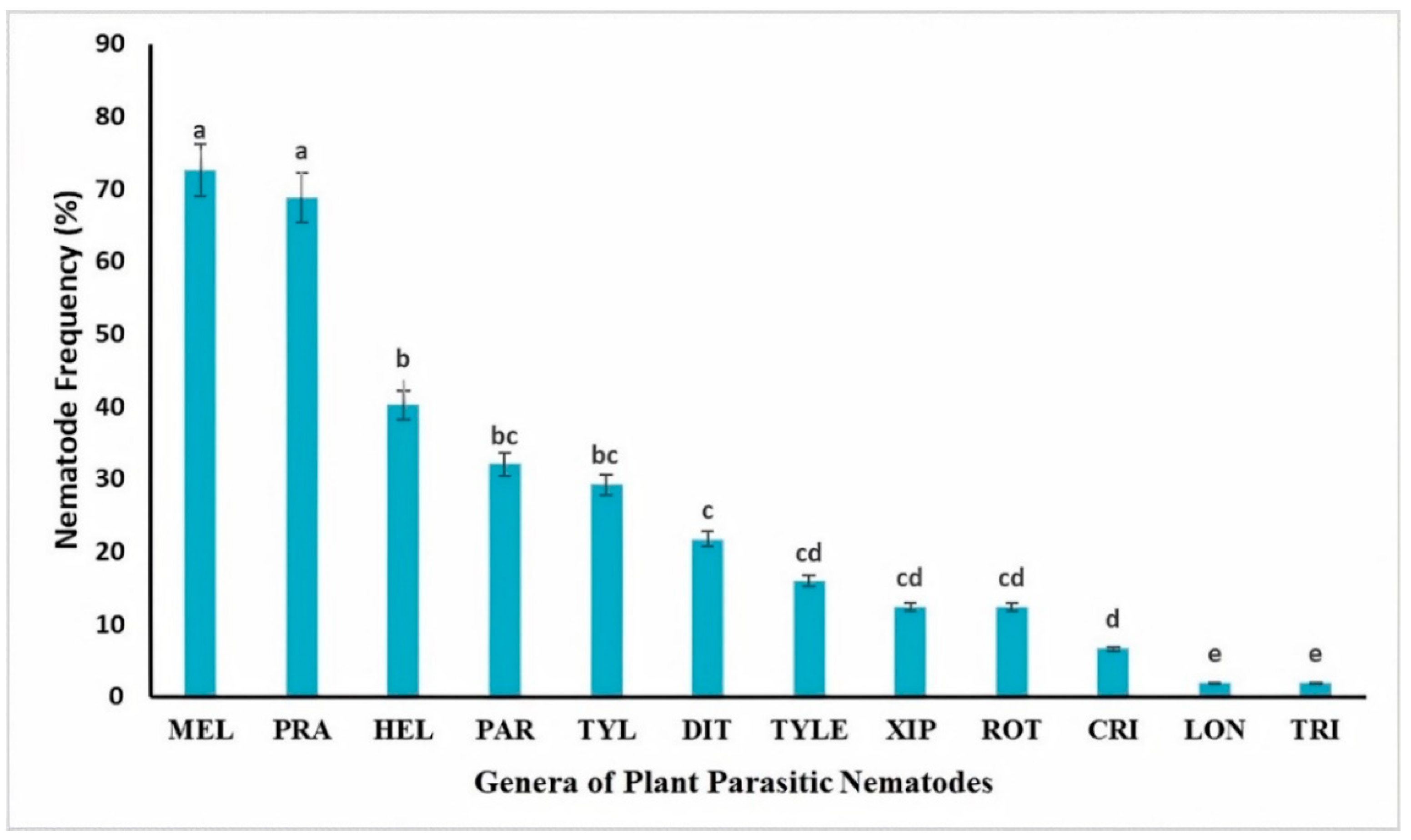
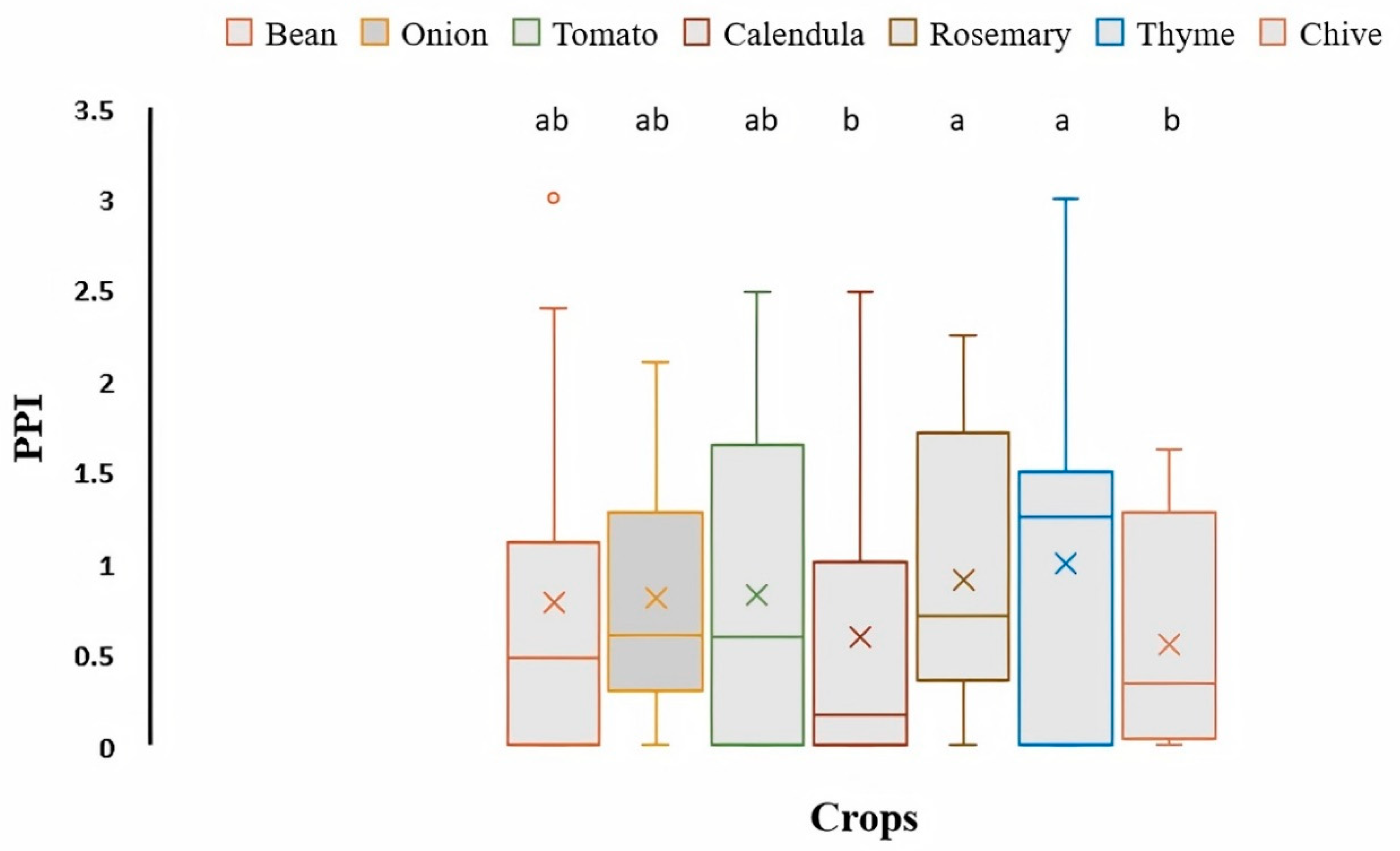
3.1. Density and Diversity of PPNs Associated with Organically Grown Vegetables, Medicinal, and Aromatic Plants
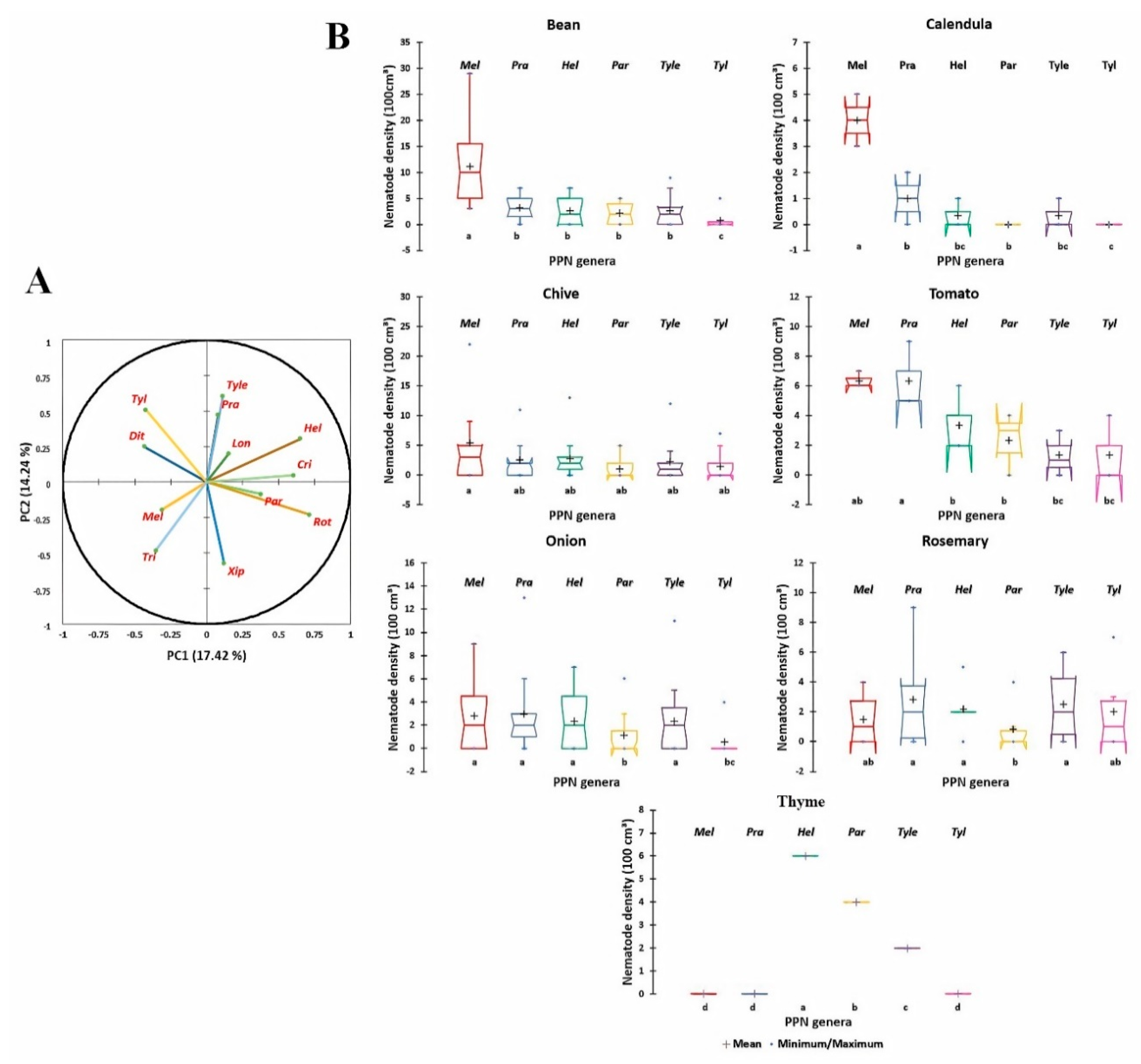
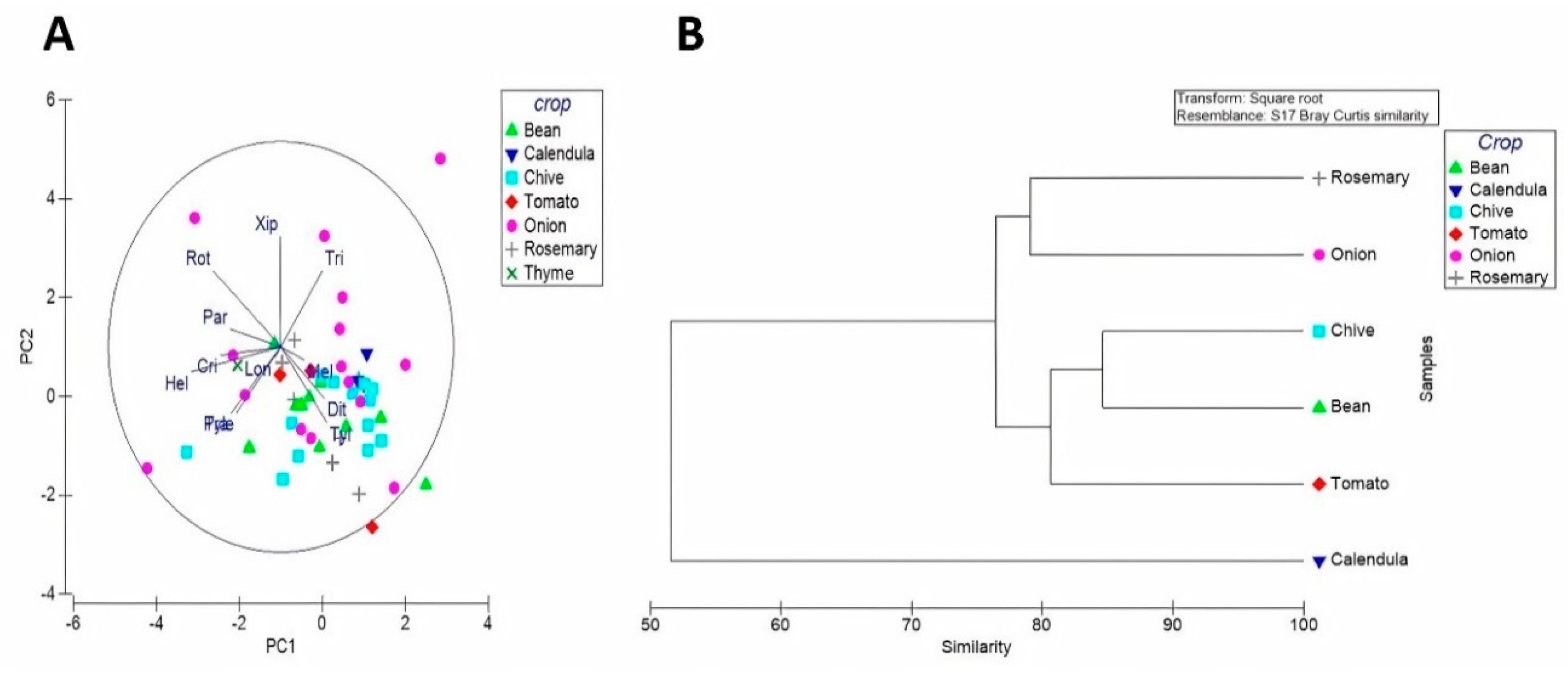
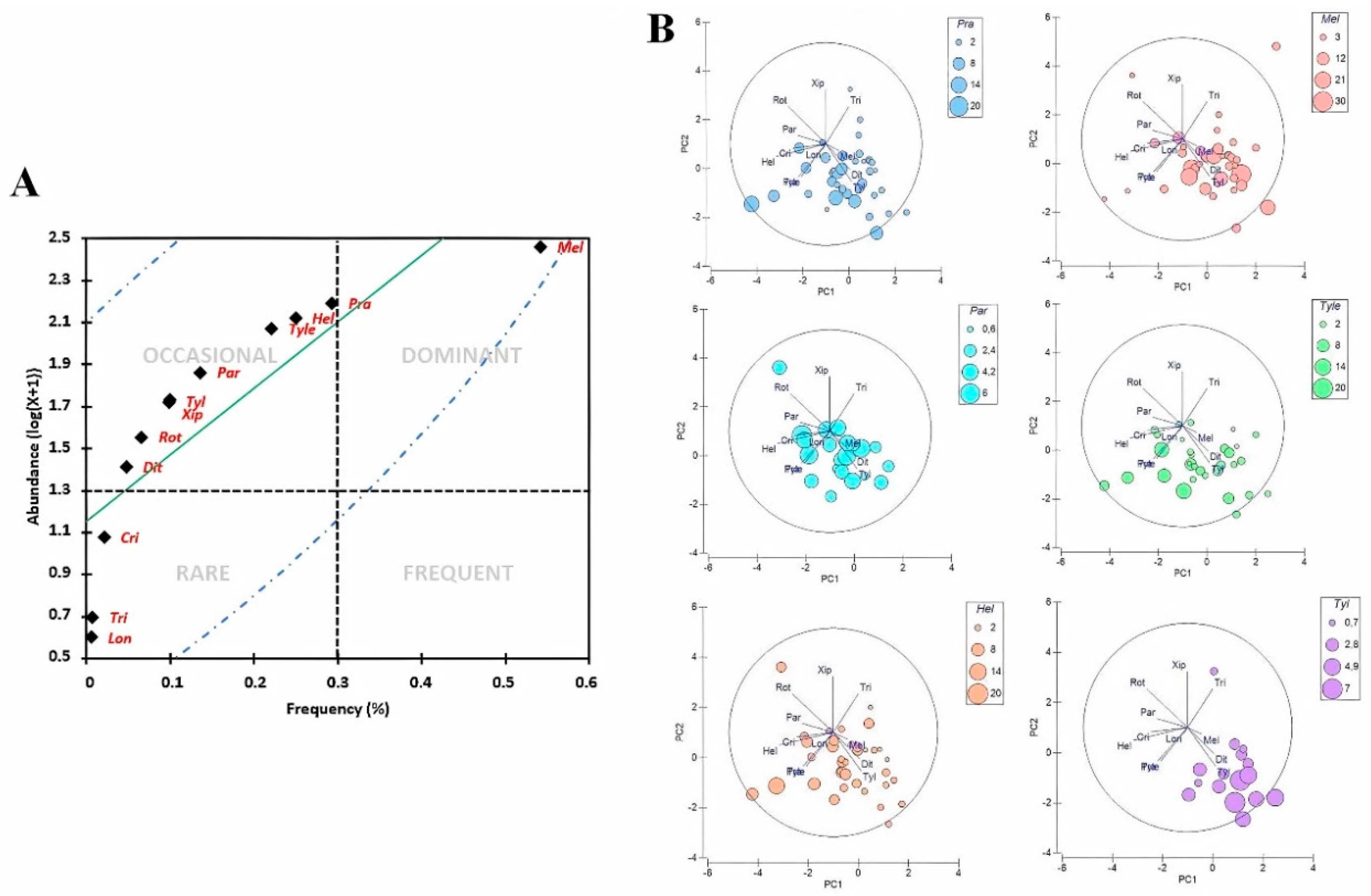
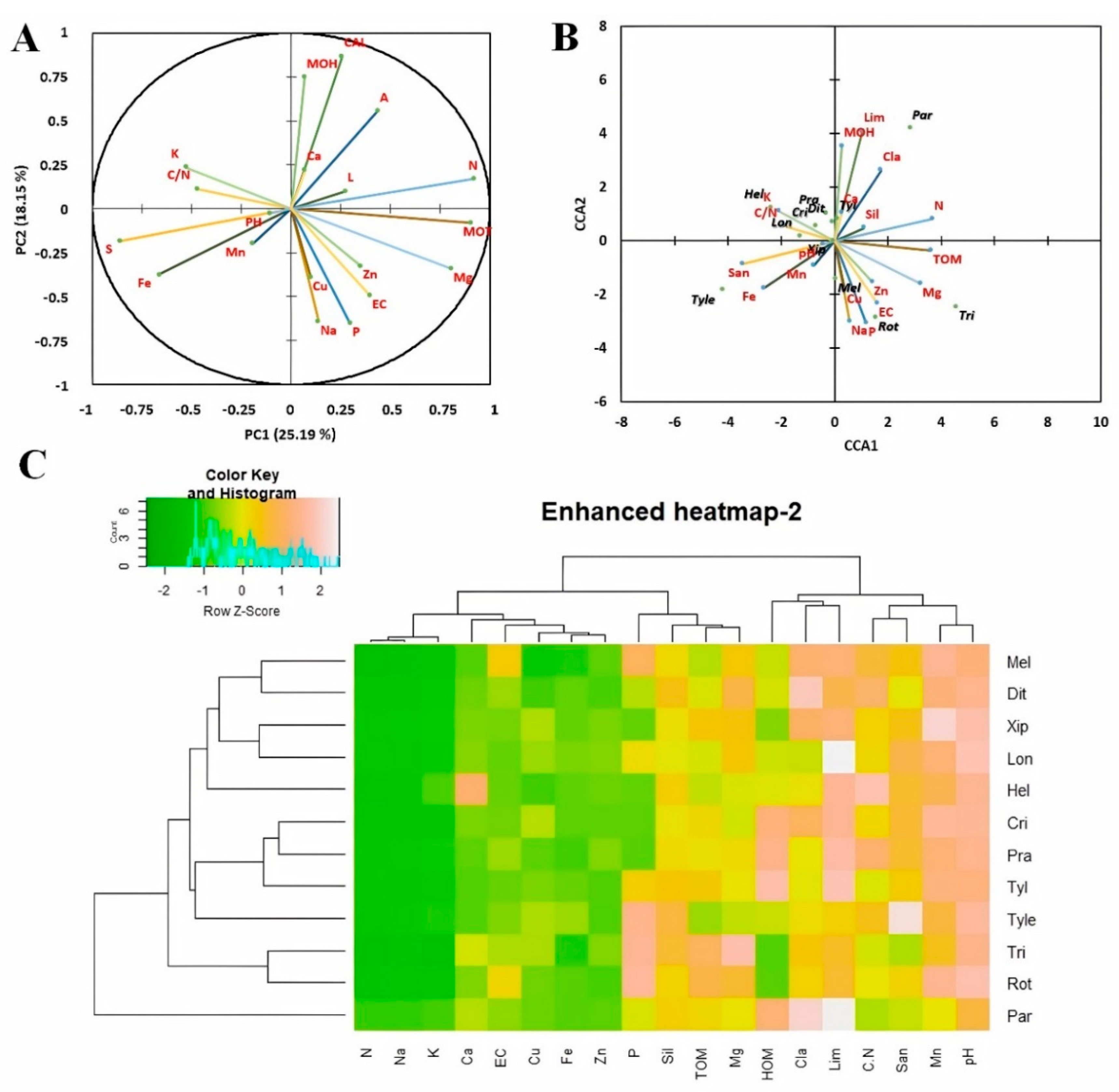
3.2. Community Patterns of PPNs in Organic Farming Systems
3.3. Distribution and Diversity of PPNs Associated with Organic Crops
3.4. Physico-Chemical Soil Characteristics and Their Interaction with Organic Crops Nematode Communities
3.5. Control Measures of Plant Parasitic Nematodes Adopted by Organic Farmers in Southern Morocco
4. Discussion
4.1. Distribution and Diversity of PPN Communities in Some Moroccan Organic Farming Systems
4.2. Effect of Soil Characteristics on PPN Abundance in Moroccan Organic Farming
4.3. PPN Management in Organic Farming Systems of Southern Morocco
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morgera, E.; Caro, C.B.; Durán, G.M. Organic Agriculture and the Law; FAO Legislative Study: Rome, Italy, 2012; Volume 107, pp. 6–10. [Google Scholar]
- Meerburg, B.G.; Borgsteede, F.H. Organic Agriculture and its Contribution to Zoonotic Pathogens. In Zoonotic Pathogens in the Food Chain; Krause, D.O., Hendrick, S., Eds.; CAB International: Wallingford, UK, 2010; pp. 167–181. [Google Scholar]
- Porciuncula, F.L.; Galang, L.M.; Parayno, R.S. Understanding the Organic Vegetables Production Environment in Central Luzon, Philippines. Int. J. Sci. Technol. Res. 2014, 3, 81–91. [Google Scholar]
- Willer, H.; Lernoud, J. The world of organic agriculture. Statistics and Emerging Trends 2019. In Bonn: Frick, and IFOAM, 20th ed.; (FiBL) RIoOA, Ed.; Organics International: Bonn, Germany, 2019. [Google Scholar]
- Willer, H.; Schlatter, B.; Trávníček, J.; Kemper, L.; Lernoud, J. The World of Organic Agriculture. Statistics and Emerging Trends 2020. In Bonn: Frick, and IFOAM; (FiBL) RIoOA, Ed.; Organics International: Bonn, Germany, 2020. [Google Scholar]
- Willer, H.; Lernoud, J. The World of Organic Agriculture. Statistics and Emerging Trends 2018. In Bonn: Frick, and IFOAM; (FiBL) RIoOA, Ed.; Organics International: Bonn, Germany, 2018. [Google Scholar]
- Ayoub, M. Study on the Introduction of Organic Tomato Greenhouse in Massa Plain, Agadir, South Morocco; Mediterranean Agronomic Institute of Bari: Bari, Italy, 2002. [Google Scholar]
- Alaoui, S. Organic Farming in the World, and Case Study of Morocco: Achievements, Drawbacks and Future Perspectives; Symposium (International) on Agriculture Durable en Region Méditerranéenne (AGDUMED): Rabat, Morocco, 2009; pp. 305–317. [Google Scholar]
- Kenny, L. Report on Organic Agriculture in the Mediterranean Area; Options Méditerranéennes: Agadir, Morocco, 2004. [Google Scholar]
- Kenny, L.; Hanafi, A. The Moroccan Experience in Organic Agriculture, Mediteranean Organic Agriculture; Symposium (International) on Arganic Agriculture: Agadir, Morocco, 2001; pp. 188–197. [Google Scholar]
- Chen, S.Y.; Sheaffer, C.C.; Wyse, D.L.; Nickel, P.; Kandel, H. Plant-parasitic nematode communities and their associations with soil factors in organically farmed fields in Minnesota. J. Nematol. 2012, 44, 361–369. [Google Scholar] [PubMed]
- Singh, S.K.; Hodda, M.; Ash, G.J. Plant-parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. Bull OEPP 2013, 43, 334–374. [Google Scholar] [CrossRef]
- Hallmann, J.; Frankenberg, A.; Paffrath, A.; Schmidt, H. Occurrence and importance of plant-parasitic nematodes in organic farming in German. Nematology 2007, 9, 869–879. [Google Scholar]
- Adam, M.; Heuer, H.; Ramadan, E.M.; Hussein, M.A.; Hallmann, J. Occurrence of plant-parasitic nematodes in organic farming in Egypt. Int. J. Nematol. 2013, 23, 82–90. [Google Scholar]
- Pascual, M.L.D.; Lim, A.K.R.; Cumagun, C.J.R. Plant-parasitic nematodes associated with organic and conventional vegetable farms in Laguna Province, Philippines. Arch. Phytopathol. Pflanzenschutz 2017, 50, 776–788. [Google Scholar] [CrossRef]
- Mokrini, F. Les nématodes de la tomate dans le Souss-Massa. Agric. Maghreb 2016, 93, 54–57. [Google Scholar]
- Janati, S.; Houari, A.; Wifaya, A.; Essarioui, A.; Mimouni, A.; Hormatallah, A.; Sbaghi, M.; Dababat, A.A.; Mokrini, F. Occurrence of the root-knot nematode species in vegetable crops in Souss Region of Morocco. Plant Pathol. J. 2018, 34, 308–315. [Google Scholar] [CrossRef]
- Mokrini, F.; El Aimani, A.; Houari, A.; Bouharroud, R.; Wifaya, A.; Dababat, A.A. Meloidogyne arenaria attacking eggplant in Souss region, Morocco. Australas. Plant Dis. Notes 2019, 14, 30. [Google Scholar] [CrossRef]
- Mokrini, F.; Dababat, A.A. First report of the dagger nematode Xiphinema pachtaicum on onion in Morocco. J. Nematol. 2019, 51, 1–2. [Google Scholar] [CrossRef]
- Janati, A.; Aouragh, E.; Meskine, M. (Eds.) The root-knot nematode. Meloidogyne spp. In Proceedings of the 3th Research and Planning Conference on Root-knot Nematode, Meloidogyne spp., Coimbria, Portugal, 14–17 September 1982. [Google Scholar]
- Van Capelle, C.; Schrader, S.; Brunotte, J. Tillage-induced changes in the functional diversity of soil biota–A review with a focus on German data. Eur. J. Soil Biol. 2012, 50, 165–181. [Google Scholar] [CrossRef]
- Briar, S.S.; Wichman, D.; Reddy, G.V. Plant-parasitic nematode problems in organic agriculture. In Organic Farming for Sustainable Agriculture; Nandwani, D., Ed.; Springer: Cham, Switzerland, 2016; pp. 107–122. [Google Scholar]
- Berkelmans, R.; Ferris, H.; Tenuta, M.; van Bruggen, A.H.C. Effects of long-term crop management on nematode trophic levels other than plant feeders disappear after 1 year of disruptive soil management. Appl. Soil Ecol. 2003, 23, 223–235. [Google Scholar] [CrossRef]
- Oka, Y.; Shapira, N.; Fine, P. Control of root-knot nematodes in organic farming systems by organic amendments and soil solarization. Crop Prot. 2007, 26, 1556–1565. [Google Scholar] [CrossRef]
- Hooper, D.J. Extraction of free-living nematode stages from soil. In Laboratory Methods for Work with Plant and Soil Nematodes; Southey, J.F., Ed.; Her Majesty’s Stationery Office: London, UK, 1986; pp. 5–22. [Google Scholar]
- Mai, W.F.; Lyon, H.H. Pictorial Key to the Genera of Plant-Parasitic Nematodes, 4th ed.; Cornell University Press: Ithaca, NY, USA, 1975. [Google Scholar]
- Mai, W.F.; Mullin, P.G. Plant-Parasitic Nematodes: A Pictorial Key to Genera; Cornell University Press: Ithaca, NY, USA, 1996. [Google Scholar]
- De Grisse, A.T. Redescription ou modification de quelques techniques utilisées dans l’étude des nématodes phytoparasitaires. Meded Rijksfakulteit Landbowwetenschappen Gent 1969, 34, 351–369. [Google Scholar]
- Ryss, A.Y. World Fauna of the Root Parasitic Nematodes of the Family Pratylenchidae (Tylenchida); Nauka: St Petersburg, Russia, 1988. [Google Scholar]
- Siddiqi, M.R. Tylenchida, Parasites of Plants and Insects, 2nd ed.; CAB International: Wallingford, UK, 2000. [Google Scholar]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management. Nematology Monographs and Perspectives 6; Brill: Leiden, The Netherlands, 2007. [Google Scholar]
- Brzeski, M.W. Review of the genus Ditylenchus Filipjev, 1936 (Nematoda: Anguinidae). Rev. Nématologie 1991, 14, 9–59. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary (Nematoda Errantia), Vol. I; Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2005. [Google Scholar]
- Geraert, E. The Tylenchidae of the World: Identification of the Family Tylenchidae (Nematoda); Academia Press: Ghent, Belgium, 2008. [Google Scholar]
- Geraert, E. The Dolichodoridae of the World: Identification of the Family Dolichodoridae (Nematoda); Academia Press: Ghent, Belgium, 2011. [Google Scholar]
- Geraert, E. The Criconematidae of the world: Identification of the family Criconematidae (Nematoda); Academia Press: Ghent, Belgium, 2010. [Google Scholar]
- Taylor, D.P.; Netscher, C. An improved technique for preparing perineal patterns of Meloidogyne spp. Nematology 1974, 20, 268–269. [Google Scholar]
- Boag, B. Standardization of ecological terms in nematology. Fundam. Appl. Nematol. 1993, 16, 190–191. [Google Scholar]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement Saline and Alkaline Soils; US Department of Agriculture: Washington, DC, USA, 1954.
- Sims, J.; Johnson, G.; Luxmoore, R. Micronutrient Soil Tests. Micronutrients. In R Package “corrplot”: Visualization of a Correlation Matrix; Version 0.84; Taiyun, W., Viliam, S., Eds.; Soil Science Society of America: Madison, WI, USA, 1991. [Google Scholar]
- Allison, L. Wet-combustion apparatus and procedure for organic and inorganic carbon in soil. Soil Sci. Soc. Am. J. 1960, 24, 36–40. [Google Scholar] [CrossRef]
- Nelson, P.W.; Sommers, C.E. Total C, organic C and organic matter. In Methods of Soil Analysis. Part 2. Chemical Methods; Page, A.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 539–579. [Google Scholar]
- Barbano, D.; Clark, J. Kjeldahl method for determination of total nitrogen content of milk: Collaborative study. J. AOAC Int. 1990, 73, 849–859. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance; Harper and Row: New York, NY, USA, 1985. [Google Scholar]
- Fortuner, R.; Merny, G. Les nématodes parasites des racines associés au riz en Basse-Casamance (Sénégal) et en Gambie. Cah. ORSTOM Série Biol. 1973, 21, 4–43. [Google Scholar]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Stephens, M.A. Statistics for goodness of fit and some comparisons. J. Am Stat. Assoc. 1974, 69, 730–737. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Mokbel, A.A.; Ibrahim, I.K.A.; El-Saedy, M.A.M.; Hammad, S.E. Plant-parasitic nematodes associated with some fruit trees and vegetable crops in northern Egypt. Egypt. J. Phytopathol. 2006, 34, 43–51. [Google Scholar]
- Ibrahim, I.K.A.; Handoo, Z.A.; El-Sherbiny, A.A. A survey of phytoparasitic nematodes on cultivated and non-cultivated plants in Northwestern Egypt. J. Nematol. 2000, 32, 478–485. [Google Scholar] [PubMed]
- Ibrahim, I.K.A.; Mokbel, A.A.; Handoo, Z.A. Current status of phytoparasitic nematodes and their host plants in Egypt. Nematropica 2010, 40, 239–262. [Google Scholar]
- Kranti, K.V.V.S.; Patil, J.A.; Yadav, S.A. Nematodes associated with organic farming systems and their management strategies: A review. J. Pharmacogn. Phytochem. 2019, 8, 713–720. [Google Scholar]
- Kranti, K.; Nishi, K. Nematode management in organic farming. Popular Kheti 2018, 6, 177–184. [Google Scholar]
- Di Vito, M.; Cianciotta, V.; Zaccheo, G. The effect of population densities of Meloidogyne incognita on yield of susceptible and resistant tomato. Nematol. Mediterr. 1991, 19, 265–268. [Google Scholar]
- Di Vito, M.; Zaccheo, G. Population density of root-knot nematode, Meloidogyne incognita and growth of artichoke (Cynara scolymus). Adv. Hortic. Sci. 1991, 5, 81–82. [Google Scholar]
- Avato, R.; Laquale, S.; Argentieri, M.P.; Radicci, V.; D’Addabbo, T. Nematicidal activity of essential oils from aromatic plants of Morocco. J. Pest Sci. 2017, 90, 711–722. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; Termorshuizen, A.J. Integrated approaches to root disease management in organic farming systems. Australas. Plant Pathol. 2003, 32, 141–156. [Google Scholar] [CrossRef]
- Mokrini, F.; Waeyenberge, L.; Viaene, N.; Abbad Andaloussi, F.; Moens, M. Diversity of root-lesion nematodes (Pratylenchus spp.) associated with wheat (Triticum aestivum and T. durum) in Morocco. Nematology 2016, 18, 781–801. [Google Scholar] [CrossRef]
- Mokrini, F.; Laasli, S.E.; Karra, Y.; El Aissami, A.; Dababat, A.A. Diversity and incidence of plant-parasitic nematodes associated with saffron (Crocus sativus L.) in Morocco and their relationship with soil physicochemical properties. Nematology 2019, 22, 87–102. [Google Scholar] [CrossRef]
- Sikora, R.A.; Fernandez, E. Nematode parasites of vegetables. In Plant-Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; Luc, M., Sikora, R.A., Bridge, J., Eds.; CAB International: Wallingford, UK, 2005; pp. 319–392. [Google Scholar]
- Nicol, J.M.; Davies, K.A.; Hancock, T.W.; Fisher, J.M. Yield loss caused by Pratylenchus thornei on wheat in south Australia. J. Nematol. 1991, 31, 367–376. [Google Scholar]
- Mai, W.F.; Bloom, J.R.; Chen, T.A. Biology and Ecology of the Plant-Parasitic Nematode, Pratylenchus Penetrans; The Pennsylvania State University College of Agriculture, Agriculture Experimental Station: University Park, PA, USA, 1977. [Google Scholar]
- Taylor, D.P.; Anderson, R.V.; Hlund, W.A. Nematodes associated with Minnesota crops, I. Preliminary survey of nematodes associated with alfalfa, flax, peas, and soybeans. Plant Dis. Rep. 1959, 43, 195–198. [Google Scholar]
- Blancard, D.A. Colour Handbook: Tomato Diseases: Identification, Biology and Control, 2nd ed.; CRC Press: London, UK, 2012. [Google Scholar]
- Orion, D.; Levy, Y.; Israeli, Y.; Fischer, E. Scanning electron microscope observations on spiral nematode (Helicotylenchus multicinctus)-infested banana roots. Nematropica 1999, 29, 179–183. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Yeates, G.W.; Wardle, D.A.; Watson, R.N. Responses of soil nematode populations, community structure, diversity and temporal variability to agricultural intensification over a seven-year period. Soil Biol. Biochem. 1999, 31, 1721–1733. [Google Scholar] [CrossRef]
- Koleva, L.; Tsolova, E. Population structure of free-living and plant-parasitic nematodes in strawberry plantations under organic farming conditions. In III Balkan Symposium on Fruit Growing; International Society for Horticultural Science: Belgrade, Serbia, 2015; pp. 745–752. [Google Scholar]
- Thoden, T.C.; Korthals, G.W.; Termorshuizen, A.J. Organic amendments and their influences on plant-parasitic and free-living nematodes: A promising method for nematode management? Nematology 2011, 13, 133–153. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.; Garey, J.R. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 2014, 23, 968–978. [Google Scholar] [CrossRef]
- Godefroid, M.; Delaville, L.; Marie-Luce, S.; Quénéhervé, P. Spatial stability of a plant-feeding nematode community in relation to macro-scale soil properties. Soil Biol. Biochem. 2013, 57, 173–181. [Google Scholar] [CrossRef]
- Van Diepeningen, A.D.; de Vos, O.J.; Korthals, G.W.; van Bruggen, A.H.C. Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Appl. Soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- Cadet, P.; Thioulouse, J. Identification of soil factors that relate to plant parasitic nematode communities on tomato and yam in the French West Indies. Appl. Soil Ecol. 1998, 8, 35–49. [Google Scholar] [CrossRef][Green Version]
- Melakeberhan, H.; Maung, Z.; Lee, C.L.; Poindexter, S.; Stewart, J. Soil type-driven variable effects on cover-and rotation-crops, nematodes and soil food web in sugar beet fields reveal a roadmap for developing healthy soils. Eur. J. Soil Biol. 2018, 85, 53–63. [Google Scholar] [CrossRef]
- Ardakani, A.S.; Mafi, Z.T.; Hesar, A.M.; Goltappeh, E.M. Relationship between soil properties and abundance of Tylenchulus semipenetrans in Citrus orchards, Kohgilouyeh va Boyerahmad Province. J. Agric. Sci. Technol. 2014, 16, 1699–1710. [Google Scholar]
- Aït-Hamza, M.; Ferji, Z.; Ali, N.; Tavoillot, J.; Chapuis, E.; El Oualkadi, A.; Moukhli, A.; Khadari, B.; Boubaker, H.; Lakhtar, H. Plant-parasitic nematodes associated with olive in southern Morocco. Int. J. Agric. Biol. 2015, 17, 719–726. [Google Scholar] [CrossRef]
- Aït Hamza, M.; Moukhli, A.; Ferji, Z.; Fossati-Gaschignard, O.; Tavoillot, J.; Ali, N.; Boubaker, H.; El Mousadik, A.; Mateille, T. Diversity of plant-parasitic nematode communities associated with olive nurseries in Morocco: Origin and environmental impacts. Appl. Soil Ecol. 2018, 124, 7–16. [Google Scholar] [CrossRef]
- Mokrini, F.; Laasli, S.E.; Iraqui, D.; Wifaya, A.; Mimouni, A.; Erginbas-Orakci, G. Distribution and occurrence of plant-parasitic nematodes associated with raspberry (Rubus idaeus) in Souss-Massa region of Morocco: Relationship with soil physico-chemical factors. Russ. J. Nematol. 2019, 27, 107–121. [Google Scholar]
- Prot, J.C.; Van Gundy, S.D. Effect of soil texture and the clay component on migration of Meloidogyne incognita second-stage juveniles. J. Nematol. 1981, 13, 213–217. [Google Scholar]
- Prasad, J.S.; Rao, Y.S. Influence of edaphic factors on the build-up of the root lesion nematode, Pratylenchus indicus Das, 1960 in rice. 1. Effect of type, texture, porosity and moisture of soil. Eur. J. Soil Biol. 1980, 17, 173–179. [Google Scholar]
- Grandison, G.; Wallace, H. The distribution and abundance of Pratylenchus thornei in fields of strawberry clover (Trifolium fragiferum). Nematologica 1974, 20, 283–290. [Google Scholar] [CrossRef]
- Thompson, J.; Clewett, T.; Sheedy, J.; Reen, R.; O’reilly, M.; Bell, K. Occurrence of root-lesion nematodes (Pratylenchus thornei and P. neglectus) and stunt nematode (Merlinius brevidens) in the northern grain region of Australia. Australas. Plant Pathol. 2010, 39, 254–264. [Google Scholar] [CrossRef]
- Wallace, H. Observations on the behaviour of Ditylenchus dipsaci in soil. Nematologica 1962, 7, 91–101. [Google Scholar] [CrossRef]
- Ferji, Z.; Geraert, E. Some Tylenchida from Morocco. Biol. Jaarb. Dod. 1997, 64, 109–124. [Google Scholar]
- Wang, C.; Bruening, G.; Williamson, V.M. Determination of preferred pH for root-knot nematode aggregation using Pluronic F-127 gel. J. Chem. Ecol. 2009, 35, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Norton, D.C. Abiotic soil factors and plant–parasitic nematode communities. J. Nematol. 1989, 21, 299–307. [Google Scholar]
- Cadet, P.; Thioulouse, J.; Albrecht, A. Relationships between ferrisol properties and the structure of plant-parasitic nematode communities on sugarcane in Martinique (French West Indies). Acta OEcol. 1994, 15, 767–780. [Google Scholar]
- Korthals, G.W.; Bongers, T.; Kammenga, J.E.; Alexiev, A.D.; Lexmond, T.M. Long-term effects of copper and pH on the nematode community in an agroecosystem. Environ. Toxicol. Chem. 1996, 15, 979–985. [Google Scholar] [CrossRef]
- Neher, D.A. Nematode communities in organically and conventionally managed agricultural soils. J. Nematol. 1999, 31, 142–154. [Google Scholar]
- Mulder, C.; Zwart, D.D.; Van Wijnen, H.J.; Schouten, A.J.; Breure, A.M. Observational and simulated evidence of ecological shifts within the soil nematode community of agroecosystems under conventional and organic farming. Funct. Ecol. 2003, 17, 516–525. [Google Scholar] [CrossRef]
- Wang, K.H.; McSorley, R.; Gallaher, R.N. Relationship of soil management history and nutrient status to nematode community structure. Nematropica 2004, 34, 83–95. [Google Scholar]
- Fiscus, D.A.; Neher, D.A. Distinguishing sensitivity of free-living soil nematode genera to physical and chemical disturbances. Ecol. Appl. 2002, 12, 565–575. [Google Scholar] [CrossRef]
- Francl, L.J. Multivariate analysis of selected edaphic factors and their relationship to Heterodera glycines population density. J. Nematol. 1993, 25, 270–276. [Google Scholar] [PubMed]
- Kincaid, R.R.; Martin, F.G.; Gammon, N.; Breland, H.L.; Pritchett, W.L. Multiple regression of tobacco black shank, root-knot and coarse root indexes on soil pH, potassium, calcium and magnesium. Phytopathology 1970, 60, 1513–1516. [Google Scholar] [CrossRef]
- Dabiré, R.K.; Ndiaye, S.; Mounport, D.; Mateille, T. Relationships between abiotic soil factors and epidemiology of the biocontrol bacterium Pasteuria penetrans in a root-knot nematode Meloidogyne javanica-infested field. Biol. Control. 2007, 40, 22–29. [Google Scholar] [CrossRef]
- Kandji, S.T.; Ogol, C.K.P.O.; Albrecht, A. Diversity of plant-parasitic nematodes and their relationships with some soil physico-chemical characteristics in improved fallows in western Kenya. Appl. Soil Ecol. 2001, 18, 143–157. [Google Scholar] [CrossRef]
- Georgieva, S.S.; McGrath, S.P.; Hooper, D.J.; Chambers, B.S. Nematode communities under stress: The long-term effects of heavy metals in soil treated with sewage sludge. Appl. Soil Ecol. 2002, 20, 27–42. [Google Scholar] [CrossRef]
- Benjlil, H.; Elkassemi, K.; Hamza, M.A.; Mateille, T.; Furze, J.N.; Cherifi, K.; Ferji, Z. Plant-parasitic nematodes parasitizing saffron in Morocco: Structuring drivers and biological risk identification. Appl. Soil Ecol. 2020, 147, 103362. [Google Scholar] [CrossRef]
- Rodriguez-Kabana, R.; King, P.; Pope, M. Combinations of anhydrous ammonia and ethylene dibromide for control of nematodes parasitic of soybeans. Nematropica 1981, 11, 27–41. [Google Scholar]
- Rodriguez-Kabana, R. Organic and inorganic nitrogen amendments to soil as nematode suppressants. J. Nematol. 1986, 18, 129. [Google Scholar]
- Oteifa, B.A. Nitrogen source of the host nutrition in relation to infection by a root-knot nematode, Meloidogyne incognita. Plant Dis. Rep. 1955, 39, 902–903. [Google Scholar]
- Yeates, G.W. Effect of fertiliser treatment and stocking rate on pasture nematode populations in a yellow-grey earth. N. Z. J. Agric. Res. 1976, 19, 405–408. [Google Scholar] [CrossRef]
- Widmer, T.L.; Mitkowski, N.A.; Abawi, G.S. Soil organic matter and management of plant-parasitic nematodes. J. Nematol. 2002, 34, 289–295. [Google Scholar] [PubMed]
- Hominick, B. Nematodes. In Proceedings of the International Workshop Tropical Soil Biology: Opportunities and Challenges for African Agriculture, Nairobi, Kenya, 14–23 March 1999. [Google Scholar]
- Qi, Y.; Hu, C. Soil nematode abundance in relation to diversity in different farming management system. World J. Agric. Sci. 2007, 3, 587–592. [Google Scholar]
- Barros, P.A.; Pedrosa, E.M.R.; de Oliveira Cardoso, M.S.; Rolim, M.M. Relationship between soil organic matter and nematodes in sugarcane fields. Semin. Ciências Agrárias 2017, 38, 551–560. [Google Scholar] [CrossRef]
- Papatheodorou, E.M.; Argyropoulou, M.D.; Stamou, G.P. The effects of large and small-scale differences in soil temperature and moisture on bacterial functional diversity and the community of bacterivorous nematodes. Appl. Soil Ecol. 2004, 25, 37–49. [Google Scholar] [CrossRef]
- Berry, S.; Cadet, P.; Spaull, V.W. Effect of certain cultural practices on nematode management in a small scale farming system. Proc. S. Afr. Sugar Technol. 2005, 79, 149–164. [Google Scholar]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Halbrendt, J.M.; LaMondia, J.A. Crop rotation and other cultural practices. In Nematology Advances and Perspectives, 2nd ed.; Chen, Z.X., Chen, S.Y., Dickson, D.W., Eds.; CAB International: Wallingford, UK, 2004; pp. 909–930. [Google Scholar]
- Green, B.D.; Kaminski, D.; Rapp, B.; Celetti, M.; Derksen, D.; Juras, L. Principles and practices of crop rotations. In Saskatchewan Agriculture and Food; Saskatchewan Agriculture and Food: Regina, SK, Canada, 2005. [Google Scholar]
- Dong, L.Q.; Zhang, K.Q. Microbial control of plant-parasitic nematodes: A five-party interaction. Plant Soil 2006, 288, 31–45. [Google Scholar] [CrossRef]
- Collange, B.; Navarrete, M.; Peyre, G.; Mateille, T.; Tchamitchian, M. Root-knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Prot. 2011, 30, 1251–1262. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M.; Askary, T.H. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt. J. Biol. Pest Control 2018, 28, 74. [Google Scholar] [CrossRef]
- Ladurner, E.; Benuzzi, M.; Fiorentini, F.; Lucchi, A. Efficacy of NemGuard® Granules, a new nematicide based on garlic extract for the control of root-knot nematodes on horticultural crops. In Conference on Atti, Giornate Fitopatologiche, Chianciano Terme (Siena); Brunelli, A., Collina, M., Eds.; Alma Mater Studiorum, Universitá di Bologna: Bologna, Italy, 2014; pp. 301–308. [Google Scholar]
- Makunde, P.T.; Mahere, T.S.; Dimbi, S. Root-Knot Nematode Control on Tobacco: Alternatives to Fumigant Nematicides; CORESTA Information Bulletin, Abstract AP08; Agronomy/Phytopathology Groups: Quebec, QC, Canada, 2014. [Google Scholar]
- D’Addabbo, T.; Laquale, S.; Perniola, M.; Candido, V. Biostimulants for plant growth promotion and sustainable management of phytoparasitic nematodes in vegetable crops. Agronomy 2019, 9, 616. [Google Scholar] [CrossRef]
- Zhao, N.N.; Zhang, H.; Zhang, X.C.; Luan, X.B.; Zhou, C.; Liu, Q.Z. Evaluation of acute toxicity of essential oil of garlic (Allium sativum) and its selected major constituent compounds against overwintering Cacopsylla chinensis (Hemiptera: Psyllidae). J. Econ. Entomol. 2013, 106, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, M. Fumigant and contact toxicity of Allium sativum (Alliaceae) essential oil against Sitophilus oryzae L. (Coleoptera: Dryophthoridae). Entomol. Appl. Sci. Lett. 2016, 3, 43–48. [Google Scholar]
- Park, I.K.; Kim, K.H.; Choi, K.S.; Kim, C.S.; Choi, I.H.; Park, J.Y.; Shin, S.C. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus). Nematology 2005, 7, 767–774. [Google Scholar] [CrossRef]
- Jardim, I.N.; Oliveira, D.F.; Campos, V.P.; Silva, G.H.; Souza, P.E. Garlic essential oil reduces the population of Meloidogyne incognita in tomato plants. Eur. J. Plant Pathol. 2020, 157, 197–209. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.; Kabeil, S.S.; Abd-El-Wahab, A.E. Changes in protein content and enzymatic activity of tomato plants in response to nematode infection. Egypt. J. Agronematol. 2009, 7, 49–61. [Google Scholar]
- Wanga, K.H.; Hooks, C.R.; Ploegb, A. Protecting crops from nematode pests: Using marigold as an alternative to chemical nematicides. Plant Dis. 2007, 35, 1–6. [Google Scholar]
- Ferji, Z.; Mayad, E.H.; Laghdaf, T.; Cherif, E.M. Effect of organic amendments of Ricinus communis and Azadirachta indica on root-knot nematodes Meloidogyne javanica infecting tomatoes in Morocco. In Proceedings of the Meeting of the IOBC/WPRS Working Group, Integrated Pest Control in Protected Crops, Mediterranean Climate, Murcia, Spain, 27–29 April 2006. [Google Scholar]
- Mokrini, F.; Janati, S.; Houari, A.; Essarioui, A.; Bouharroud, R.; Mimouni, A. Management of Plant-parasitc Nematodes by means of organic amendment. Rev. Mar. Sci. Agron. Vét. 2018, 6, 337–344. [Google Scholar]
- Fourie, H.; Ahuja, P.; Lammers, J.; Daneel, M. Brassicacea-based management strategies as an alternative to combat nematode pests: A synopsis. Crop Prot. 2016, 80, 21–41. [Google Scholar] [CrossRef]
- Oduor-Owino, P.; Waudo, S.W.; Sikora, R.A. Biological control of Meloidogyne javanica in Kenya: Effect of plant residues, benomyl and decomposition products of mustard (Brassica campestris). Nematologica 1993, 39, 127–134. [Google Scholar]
- Sturz, A.V.; Kimpinski, J. Endoroot bacteria derived from marigolds (Tagetes spp.) can decrease soil population densities of root-lesion nematodes in the potato root zone. Plant Soil 2004, 262, 241–249. [Google Scholar] [CrossRef]
- Tsvetkov, I.; Atanassov, A.; Vlahova, M.; Carlier, L.; Christov, N.; Lefort, F.; Rusanov, K.; Badjakov, I.; Dincheva, I.; Tchamitchian, M.; et al. Plant organic farming research—Current status and opportunities for future development. Biotechnol. Biotechnol. Equip. 2018. [Google Scholar] [CrossRef]


| Phylum | Class | Order | Family | Genus | Common Name | Code |
|---|---|---|---|---|---|---|
| Nematoda | Secernentea | Tylenchida | Heteroderidae | Meloidogyne | Root-knot nematode | MEL |
| Hoplolaimidae | Helicotylenchus | Spiral nematode | HEL | |||
| Pratylenchidae | Pratylenchus | Lesion nematode | PRA | |||
| Tylenchidae | Tylenchus | Tylenchids | TYL | |||
| Tylenchulidae | Paratylenchus | Pin nematode | PAR | |||
| Dolichodoridae | Tylenchorhynchus | Stunt nematode | TYLE | |||
| Hoplolaimidae | Rotylenchulus | Reniform nematode | ROT | |||
| Criconematidae | Criconemoides | Ring nematode | CRI | |||
| Anguinidae | Ditylenchus | Stem Nematode | DIT | |||
| Enoplea | Dorylaimida | Longidoridae | Xiphinema | Dagger nematode | XIP | |
| Longidorus | Needle nematode | LON | ||||
| Trichodorus | Stubby-root nematode | TRI |
| Host Plant | Location | Number of Fields Sampled | Control | Number of PPN Genera |
|---|---|---|---|---|
| Bean (Phaseolus vulgaris L.) | Belfaa | 24 | Crop rotation (Alfalfa/Corn/Maize) | 9 |
| Onion (Allium cepa L.) | Belfaa | 30 | Crop rotation (Tagetes spp./Ricinus communis L.) | 11 |
| Tomato (Solanum lycopersicum L.) | Biogra | 6 | Cover crop (Tagetes spp./R. communis L.) | 8 |
| Chive (Allium schoenoprasum L.) | Oulad dahou | 26 | Seyland + Nemguard granules | 9 |
| Rosemary (Salvia rosmarinus L.) | Oulad dahou | 12 | None | 10 |
| Thyme (Thymus vulgaris L.) | Oulad dahou | 2 | None | 7 |
| Calendula (Calendula officinalis L.) | Oulad dahou | 6 | None | 5 |
| Nematode Taxa/Genus and Species | Bean | Onion | Tomato | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cp-Value a | Prevalence (%) | Mean Intensity | Maximum Density | Prevalence (%) | Mean Intensity | Maximum Density | Prevalence (%) | Mean Intensity | Maximum Density | |||||||
| Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | |||||
| Meloidogyne | 3 | 100 | 5 | 11 | 13 | 29 | 63 | 2 | 3 | 8 | 9 | 83 | 1 | 6 | 2 | 7 |
| Pratylenchus | 3 | 80 | 3 | 3 | 5 | 7 | 70 | 2 | 3 | 6 | 13 | 68 | 1 | 6 | 3 | 9 |
| Ditylenchus | 2 | 29 | 1 | 1 | 3 | 3 | 30 | 1 | 1 | 3 | 4 | 17 | - | 1 | - | 4 |
| Helicotylenchus | 3 | 37.5 | 1 | 2 | 3 | 7 | 40 | 1 | 2 | 2 | 7 | 50 | - | 3 | - | 6 |
| Paratylenchus | 2 | 54 | 1 | 2 | 5 | 5 | 30 | 1 | 1 | 5 | 6 | 34 | - | 2 | - | 4 |
| Tylenchus | 2 | 33 | - | 2 | - | 9 | 26.6 | - | 2 | - | 11 | 33.7 | - | 1 | - | 3 |
| Tylenchorhynchus | 3 | 12.5 | - | 1 | - | 5 | 10 | - | 1 | - | 4 | 17 | - | 1 | - | 4 |
| Longidorus | 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Xiphinema | 5 | 4 | - | 1 | - | 2 | 26.6 | - | 3 | - | 7 | 34 | - | 1 | - | 3 |
| Rotylenchus | 3 | - | - | 1 | - | 4 | 20 | - | 2 | - | 8 | - | - | - | - | - |
| Criconemoides | 3 | - | - | - | - | - | 10 | - | 1 | - | 3 | - | - | - | - | - |
| Trichororus | 4 | - | - | - | - | - | 6.6 | - | 1 | - | 2 | - | - | - | - | - |
| Nematode Taxa/Genus and Species | Calendula | Rosemary | Thyme | Chive | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cp-Value a | Prevalence (%) | Mean Intensity | Maximum Density | Prevalence (%) | Mean Intensity | Maximum Density | Prevalence (%) | Mean Intensity | Maximum Density | Prevalence (%) | Mean Intensity | Maximum Density | |||||||||
| Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | Root | Soil | ||||||
| Meloidogyne | 3 | 83 | 4 | 4 | 5 | 5 | 67 | 2 | 1 | 5 | 4 | 50 | 1 | - | 3 | - | 50 | 3 | 8 | 7 | 22 |
| Pratylenchus | 3 | 50 | 2 | 2 | 2 | 2 | 75 | 2 | 3 | 5 | 9 | 50 | 1 | - | 2 | - | 54 | 3 | 4 | 5 | 10 |
| Ditylenchus | 2 | - | - | - | - | - | 17 | 1 | - | 2 | - | - | - | - | - | - | 11.5 | 3 | 3 | 5 | 3 |
| Helicotylenchus | 3 | 33.5 | - | 1 | - | 1 | 67 | 1 | 2 | 3 | 5 | 100 | 1 | 3 | 1 | 6 | 50 | 3 | 4 | 5 | 13 |
| Paratylenchus | 2 | 16.7 | 2 | - | 2 | - | 16.7 | - | 1 | - | 4 | 50 | - | 2 | - | 4 | 31 | 2 | 3 | 2 | 5 |
| Tylenchus | 2 | 50 | - | 1 | - | 1 | 33 | - | 2 | - | 6 | 100 | - | 1 | - | 2 | 23 | - | 4 | - | 12 |
| Tylenchorhynchus | 3 | - | - | - | - | - | 25 | - | 2 | - | 7 | - | - | - | - | - | 15.3 | - | 4 | - | 7 |
| Longidorus | 5 | - | - | - | - | - | 16.7 | - | 1 | - | 2 | - | - | - | - | - | - | - | - | - | - |
| Xiphinema | 5 | 16 | - | - | - | - | 8 | - | 1 | - | 1 | - | - | - | - | - | - | - | - | - | - |
| Rotylenchus | 3 | - | - | - | - | - | 25 | - | 1 | - | 2 | 50 | - | 1 | - | 2 | 4 | - | 2 | - | 2 |
| Criconemoides | 3 | - | - | - | - | - | 17 | - | 1 | - | 1 | 50 | - | 1 | - | 1 | 4 | - | 2 | - | 2 |
| Trichodorus | 4 | - | - | - | - | - | - | - | 0 | - | - | - | - | - | - | - | - | - | - | - | - |
| Vegetable Crop | Diversity Parameters | ||
|---|---|---|---|
| Number of PPN Genera | Shannon Diversity | Evenness (J) | |
| Index (H′) | |||
| Bean | 23.6 a | 1.64 ab | 0.58 a |
| Onion | 22.16 a | 2.03 a | 0.72 a |
| Chive | 17.3 ab | 1.73 ab | 0.61 a |
| Rosemary | 6.83 b | 1.84 a | 0.65 a |
| Tomato | 6 b | 1.87 a | 0.66 a |
| Calendula | 1.58 c | 1.13 c | 0.4 a |
| Thyme | 1.25 c | 1.26 c | 0.44 a |
| P | <0.001 | 0.0129 | 0.488 |
| Soil Characteristics | Code |
|---|---|
| Granulometry | |
| Clay | Cla |
| Sand | San |
| Silt | Sil |
| Organic matter | |
| Organic matter (Total, Humic) | OM (TOM, HOM) |
| Nitrogen | N |
| pH H2O | pH |
| Limestone | Lim |
| Soil solution | |
| Calcium | Ca |
| Phosphorus | P |
| Iron | Fe |
| Magnesium | Mg |
| Manganese | Mn |
| Potassium | K |
| Sodium | Na |
| Zinc | Zn |
| Copper | Cu |
| Electrical Conductivity | EC |
| Carbon to Nitrogen Ratio | C/N |
| Product Name | Formulation | Active Ingredient | Product Category | Dose | Treatment Mode | Crops | Max. Appli. |
|---|---|---|---|---|---|---|---|
| SESAMIN EC | EC | Sesame Oil (70%) | Nematicide | 10l/ha | Drip irrigation systems | Bean, Eggplant, Lentil, Pea, Pepper, Tomato, Watermelon, Melon, Zucchini | 4 |
| SEYLAND | WP | Bacillus subtilis (strain IAB/BS03) 7.5 × 106 CFU | Nematicide | 5 kg/ha × 2 | Soil treatment | Bean | - |
| NEMGUARD GRANULES | GR | Garlic extract 450 g/kg | Nematicide | 25 kg/ha | Soil treatment | Tomato | 1 |
| OIKOS | EC | Azadirachtine (31.95 g/L) | Insecticide | 1L/ha | Drip irrigation systems | Tomato | - |
| BIOACT WG | WG | Paecilomyces lilacinus strain | Nematicide | 4 kg/ha | Soil treatment | Banana | - |
| 251 | Strawberry | ||||||
| (6% (1 × 1010 spores/g) | Tomato |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krif, G.; Mokrini, F.; Aissami, A.E.; Laasli, S.-E.; Imren, M.; Özer, G.; Paulitz, T.; Lahlali, R.; Dababat, A.A. Diversity and Management Strategies of Plant Parasitic Nematodes in Moroccan Organic Farming and Their Relationship with Soil Physico-Chemical Properties. Agriculture 2020, 10, 447. https://doi.org/10.3390/agriculture10100447
Krif G, Mokrini F, Aissami AE, Laasli S-E, Imren M, Özer G, Paulitz T, Lahlali R, Dababat AA. Diversity and Management Strategies of Plant Parasitic Nematodes in Moroccan Organic Farming and Their Relationship with Soil Physico-Chemical Properties. Agriculture. 2020; 10(10):447. https://doi.org/10.3390/agriculture10100447
Chicago/Turabian StyleKrif, Ghizlane, Fouad Mokrini, Aicha El Aissami, Salah-Eddine Laasli, Mustafa Imren, Göksel Özer, Timothy Paulitz, Rachid Lahlali, and Abdelfattah A. Dababat. 2020. "Diversity and Management Strategies of Plant Parasitic Nematodes in Moroccan Organic Farming and Their Relationship with Soil Physico-Chemical Properties" Agriculture 10, no. 10: 447. https://doi.org/10.3390/agriculture10100447
APA StyleKrif, G., Mokrini, F., Aissami, A. E., Laasli, S.-E., Imren, M., Özer, G., Paulitz, T., Lahlali, R., & Dababat, A. A. (2020). Diversity and Management Strategies of Plant Parasitic Nematodes in Moroccan Organic Farming and Their Relationship with Soil Physico-Chemical Properties. Agriculture, 10(10), 447. https://doi.org/10.3390/agriculture10100447








