Computational Analysis of Clinical and Molecular Markers and New Theranostic Possibilities in Primary Open-Angle Glaucoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Proceedings
2.2.1. Study Population
2.2.2. Demographics and Participant Characteristics
2.2.3. Clinical Variables
2.2.4. Sample Handling
2.2.5. Biochemical and Molecular Variables
Oxidative/Nitrosative Stress
Inflammation and Immune Response
Apoptosis Assays
Neuroprotection Status
Neurotransmitter Determinations
2.2.6. Bioinformatics
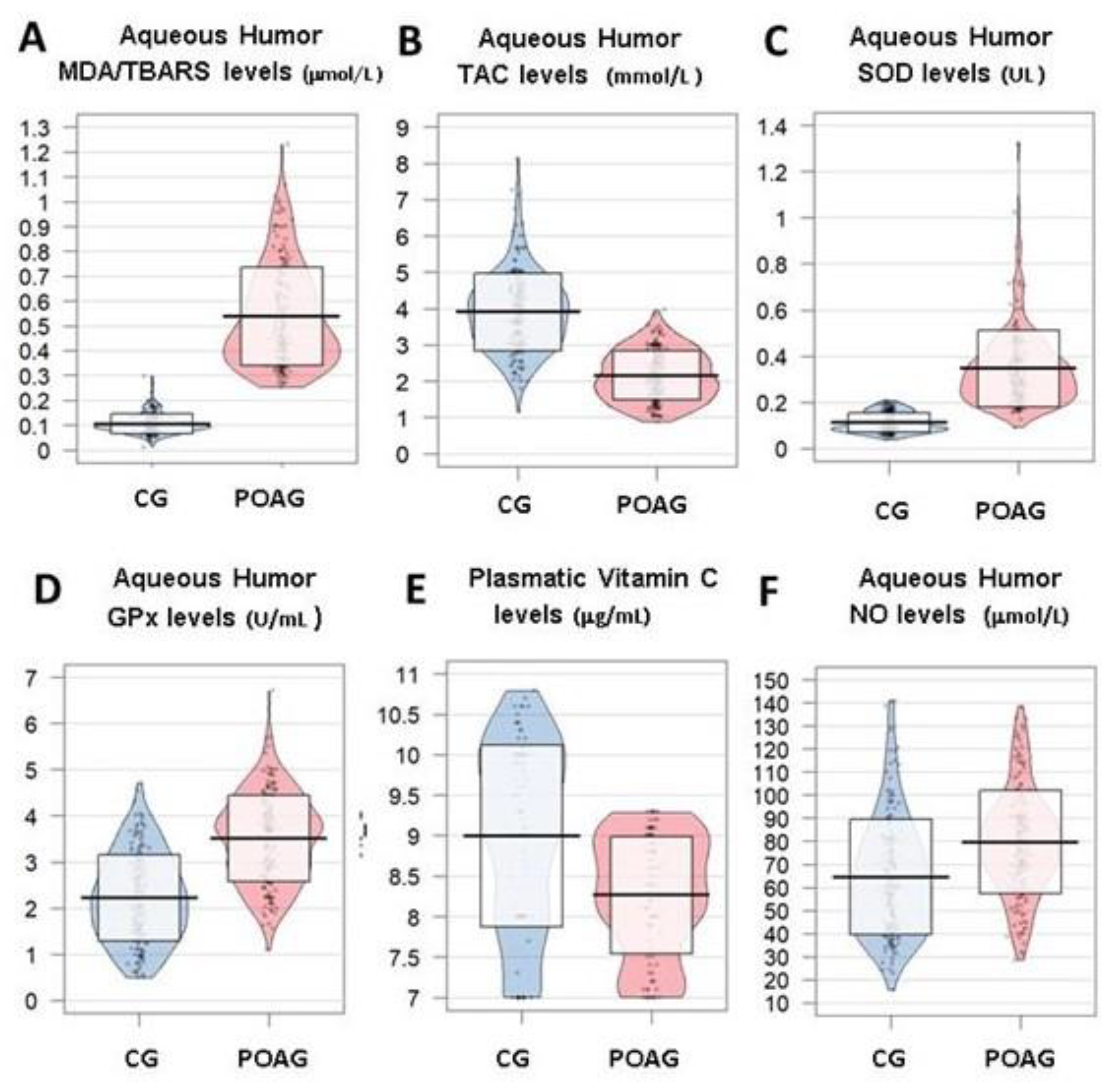
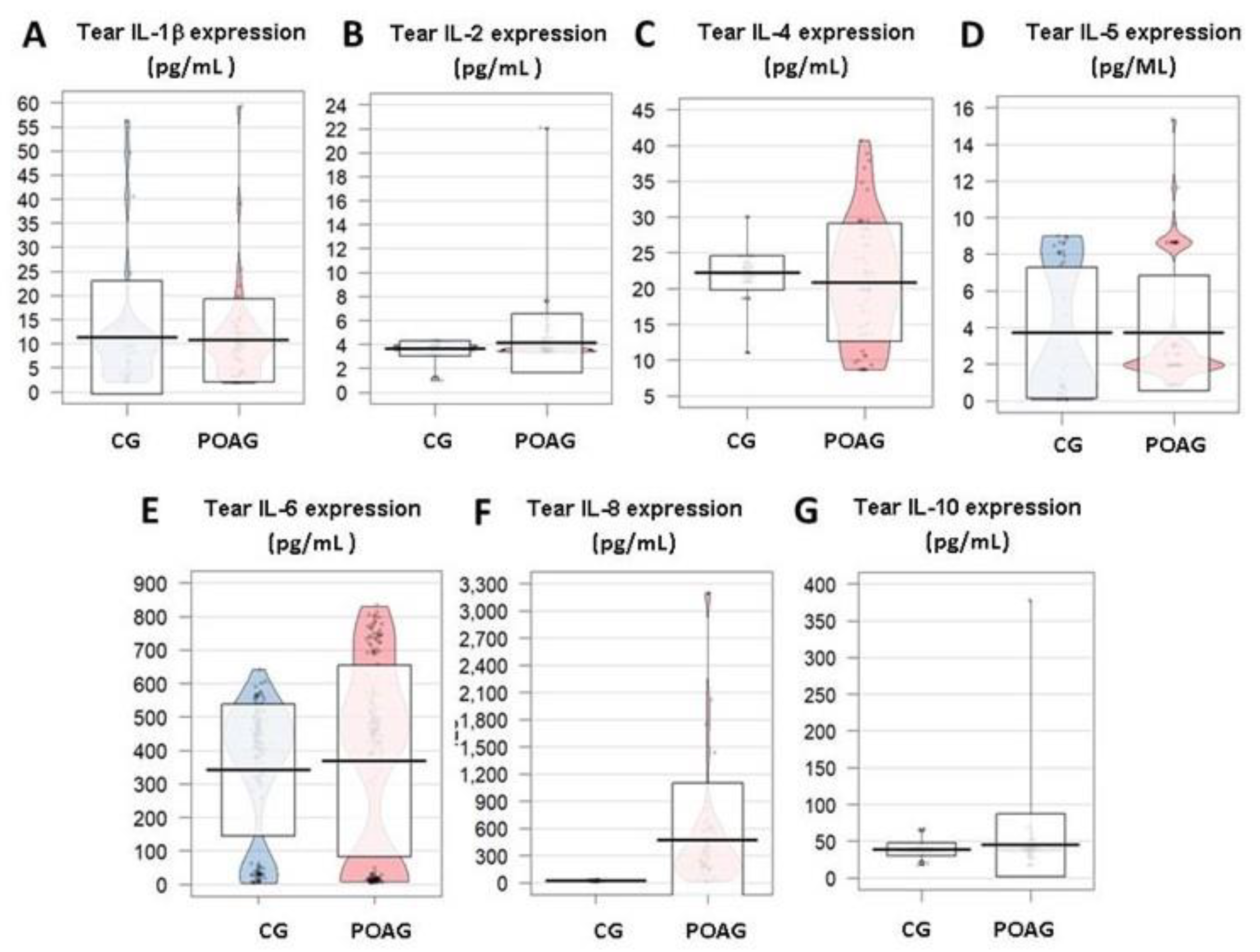
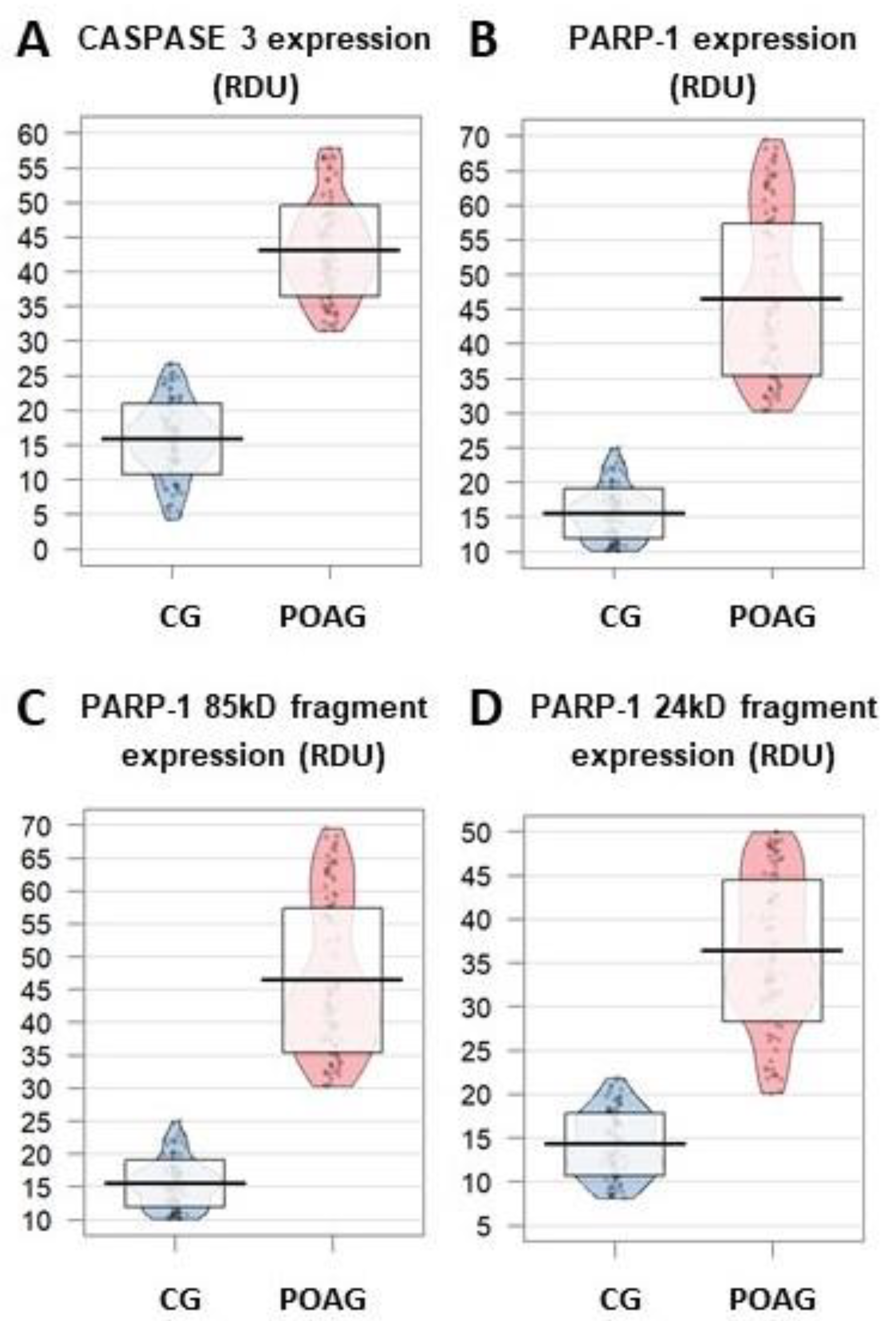
3. Results
3.1. Demographics and Participant Characteristics
3.2. Ophthalmologic Evaluation
3.3. Molecular Biomarkers
3.3.1. Oxidative Stress
3.3.2. Inflammation and Immune Response
3.3.3. Apoptosis
3.3.4. Neurodegeneration/Neuroprotection
3.3.5. Neurotransmitters
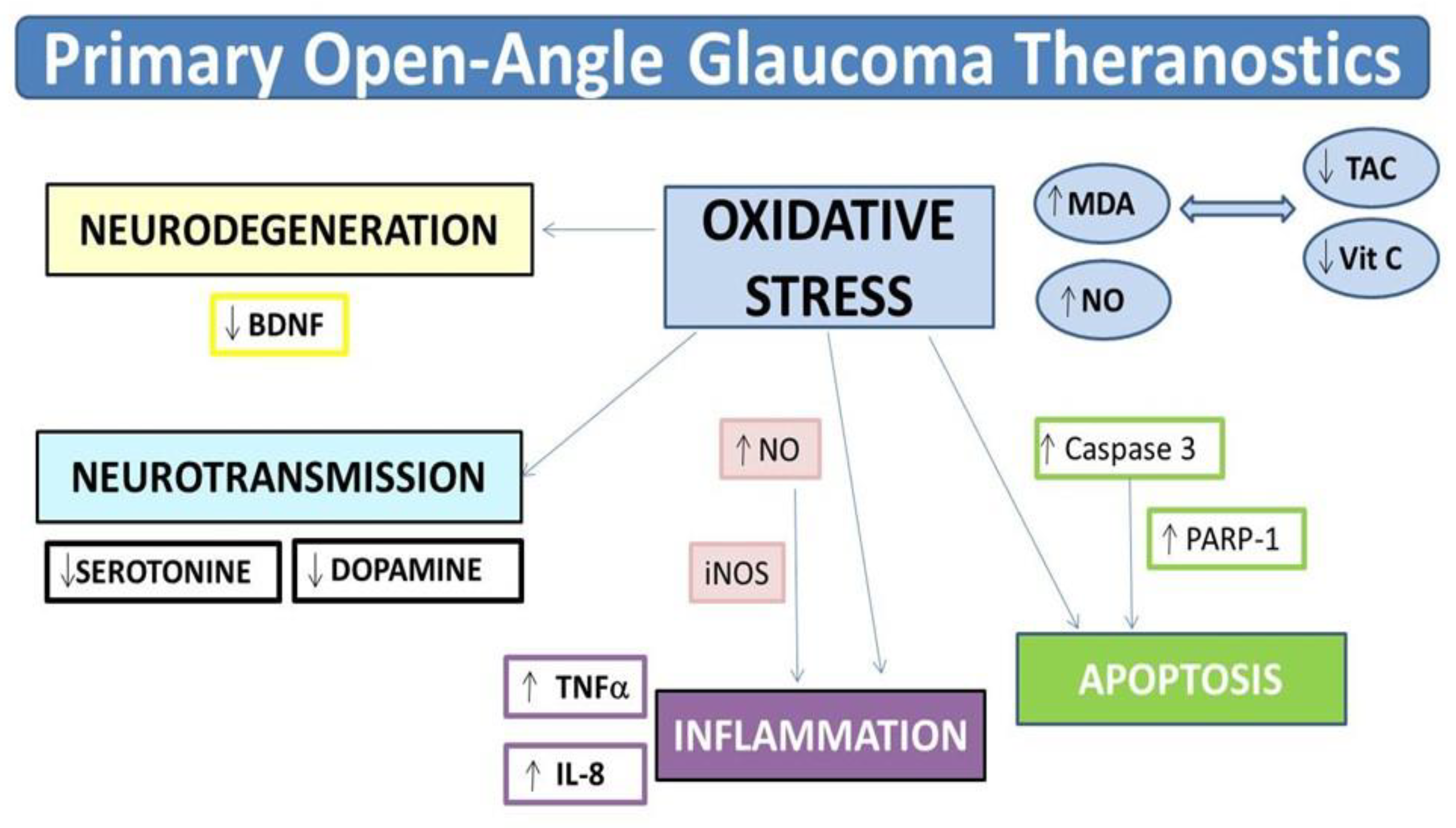
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| 5HT: | 5-hydroxytriptamine |
| AA: | alpha-agonists |
| ADP | adenosine diphosphate |
| AP: | apoptosis |
| BB: | beta-blockers |
| BCVA: | best corrected visual acuity |
| BDNF: | brain-derived neurotrophic factor |
| BMB: | biochemical and molecular biomarkers |
| BMC: | biomicroscopy |
| CAI: | carbonic anhydrase inhibitors |
| CAS3: | caspase-3 |
| CCT: | central corneal thickness |
| CG: | control group |
| DARC: | detection of apoptosing retail cells |
| ELISA: | enzyme-linked immunosorbent assays |
| FLIO: | fluorescence lifetime imaging ophthalmoscope |
| GABA: | y-aminobutyric acid |
| GM-CSF: | colony-stimulating factor |
| GPx: | glutathione peroxidase |
| GTP: | guanosine triphosphatase |
| H2O2: | hydrogen peroxide |
| HPLC: | high performance liquid chromatography |
| IFγ: | interferon gamma |
| IL: | interleukin |
| IOP: | intraocular pressure |
| LE: | left eye |
| MD: | mean derivation |
| MDA: | malondialdehyde |
| MD: | mediterranean diet |
| MIGS: | minimally invasive glaucoma surgery |
| NF-κB: | nuclear factor kappa B |
| NO: | nitric oxide |
| NPDS: | non-perforating deep sclerectomy |
| OCT: | optical coherence tomography |
| OCTA: | optical coherence tomography angiography |
| OF: | ocular fundus |
| OH: | hydroxyl radical |
| ORAC: | oxygen-radical absorbance capacity method |
| OS: | oxidative stress |
| PA: | prostaglandin analogues |
| PARP1: | poly ADP-ribose polymerase-1 |
| POAG: | primary open-angle glaucoma |
| RE: | right eye |
| RGC: | retinal ganglion cells |
| RNFL: | retinal nerve fiber layer |
| ROS: | reactive oxygen species |
| SD: | standard derivation |
| SOD: | superoxide dismutase |
| TAC: | total antioxidant capacity |
| TBARS: | thiobarbituric acid reactive substances |
| TM: | trabecular meshwork |
| TNFα: | tumour necrosis factor alpha |
| TrkB: | tyrosine kinase B |
| VEGF: | vascular endothelial growth factor |
| VF: | visual field |
References
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. On behalf of the Vision Loss Expert Group of the Global Burden of Disease Study† et al., Vision loss expert group of the global burden of disease study. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar]
- Bourne, R.R.A. Vision 2020: Where are we? Curr. Opin. Ophthalmol. 2020, 31, 81–84. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Muñoz-Negrete, F.J.; Sanz-González, S.M.; Benítez-del-Castillo, J.; Giménez-Gómez, R.; Serrano, M.; Zanón-Moreno, V.; García-Medina, J.J. The role of neuroinflammation in the pathogenesis of glaucoma neurodegeneration. Prog. Brain Res. 2020, in press. [Google Scholar]
- Lee, R.M.H.; Bouremel, Y.; Eames, I.; Brocchini, S.; Khaw, P.T. Translating Minimally Invasive Glaucoma Surgery Devices. Clin. Transl. Sci. 2020, 13, 14–25. [Google Scholar] [CrossRef]
- Occhiutto, M.L.; Maranhão, R.C.; Costa, V.P.; Konstas, A.G. Nanotechnology for Medical and Surgical Glaucoma Therapy-A Review. Adv. Ther. 2020, 37, 155–199. [Google Scholar]
- Sheybani, A.; Scott, R.; Samuelson, T.W.; Kahook, M.Y.; Bettis, D.I.; Ahmed, I.I.K.; Stehpehs, J.D.; Kent, D.; Ferguson, T.J.; Herndon, L.W. Open-Angle Glaucoma: Burden of Illness, Current Therapies, and the Management of Nocturnal IOP Variation. Ophthalmol. Ther. 2020, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A. Biomarkers and Surrogate Endpoints: Lessons Learned From Glaucoma. Invest. Ophthalmol. Vis. Sci. 2017, 58, BIO20–BIO26. [Google Scholar] [CrossRef] [PubMed]
- Yohannan, J.; Boland, M.V. The Evolving Role of the Relationship between Optic Nerve Structure and Function in Glaucoma. Ophthalmology 2017, 124, S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Bagnis, A.; Saccá, S. The role of oxidative stress in glaucoma. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Erdurmuş, M.; Yağcı, R.; Atış, Ö.; Karadağ, R.; Akbaş, A.; Hepşen, I.F. Antioxidant status and oxidative stress in primary open angle glaucoma and pseudoexfoliative glaucoma. Curr. Eye Res. 2011, 36, 713–718. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martínez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative stress and its downstream signaling in aging eyes. Clin. Interv. Aging. 2014, 9, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Gandolfi, S.; Bagnis, A.; Manni, G.; Damonte, G.; Traverso, C.E.; Izzotti, A. From DNA damage to functional changes of the trabecular meshwork in aging and glaucoma. Ageing Res. Rev. 2016, 29, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Sathiyanathan, P.; Tay, C.Y.; Stanton, L.W. Transcriptome analysis for the identification of cellular markers related to trabecular meshwork differentiation. BMC Genom. 2017, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Benitez-Del-Castillo Sánchez, J.; Morillo-Rojas, M.D.; Galbis-Estrada, C.; Pinazo-Duran, M.D. Determination of inmune response and inflammation mediators in tears: Changes in dry eye and glaucoma as compared to healthy controls. Arch. Soc. Esp. Oftalmol. 2017, 92, 210–217. [Google Scholar] [CrossRef]
- Mayordomo-Febrer, A.; Lopez-Murcia, M.; Morales-Tatay, J.M.; Monleón-Salvadó, D.; Pinazo-Durán, M.D. Metabolomics of the aqueous humor in the rat glaucoma model induced by a series of intracamerular sodium hyaluronate injection. Exp. Eye Res. 2015, 131, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lanza, M.; Benincasa, G.; Costa, D.; Napoli, C. Clinical role of epigenetics and network analysis in eye diseases. A translational science review. J. Ophthalmol. 2019, 2019, 2424956. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Cicalini, I.; Cufaro, M.C.; Agnifili, L.; Mastropasqua, L.; Marchisio, M.; de Laurenzi, V.; del Boccio, P.; Pieragostino, D. Multiomics approach for studying tears in treatment naïve glaucoma patients. Int. J. Mol. Sci. 2019, 20, 4029. [Google Scholar] [CrossRef]
- Izzotti, A.; Cecaroli, C.; Longobardi, G.M.; Micale, T.R.; Pulliero, A.; La Maestra, S.; Saccá, S. Molecular damage in glaucoma: From anterior to posterior eye segment. The microRNA role. Microma 2015, 4, 3–17. [Google Scholar] [CrossRef]
- Altman, R.B. Translational bioinformatics: Linking the molecular world to the clinical world. Clin. Pharmacol. Ther. 2012, 91, 994–1000. [Google Scholar] [CrossRef]
- Kievit, F.M.; Zhang, M. Cancer nanotheranostics: Improving imaging and therapy by targeted delivery across biological barriers. Adv. Mater. 2011, 23, H217–H247. [Google Scholar] [CrossRef]
- Zinnhardt, B.; Belloy, M.; Fricke, I.B.; Orije, J.; Guglielmetti, C.; Hermann, S.; Wagner, S.; Schäfers, M.; van der Linden, A.; Jacobs, A.H. Molecular Imaging of Immune Cell Dynamics During De- and Remyelination in the Cuprizone Model of Multiple Sclerosis by [18F]DPA-714 PET and MRI. Theranostics 2019, 9, 1523–1537. [Google Scholar] [CrossRef]
- Vaz, S.C.; Oliveira, F.; Herrmann, K.; Veit-Haibach, P. Nuclear medicine and molecular imaging advances in the 21st century. Br. J. Radiol. 2020, 93, 20200095. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. Featuring advanced translational strategies: Principles, techniques, devices and applications. Cancer Lett. 2020, 489, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Doupe, M.B.; Poss, J.; Norton, P.G.; Garland, A.; Dik, N.; Zinnick, S.; Lix, L.M. How well does the minimum data set measure healthcare use? A validation study. BMC Health Serv. Res. 2018, 18, 279. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.P.; Budenz, D.L.; Lee, P.P.; Noecker, R.J.; Walt, J.G.; Siegartel, L.R.; Evans, S.J.; Doyle, J.J. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am. J. Ophthalmol. 2006, 141, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hodapp, E.; Parrish, R.K. II Anderson D.R. Clinical Decisions in Glaucoma; Mosby Year Book Medical Publishers: St. Louis, MO, USA, 1993; pp. 52–61. [Google Scholar]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, L.; et al. PREDIMED Study Investigators Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lima-Oliveira, G.; Volanski, W.; Lippi, G.; Picheth, G.; Guidi, G.C. Pre-analytical phase management: A review of the procedures from patient preparation to laboratory analysis. Scand. J. Clin. Lab. Investig. 2017, 77, 153–16329. [Google Scholar] [CrossRef] [PubMed]
- Zanon-Moreno, V.; Marco-Ventura, P.; Lleo-Perez, A.; Pons-Vazquez, S.; Garcia-Medina, J.J.; Vinuesa-Silva, I.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Oxidative stress in primary open-angle glaucoma. J. Glaucoma 2008, 17, 263–268. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Galbis-Estrada, C.; Pons-Vázquez, S.; Cantú-Dibildox, J.; Marco-Ramírez, C.; Benítez-del-Castillo, J. Effects of a nutraceutical formulation based on the combination of antioxidants and ω-3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clin. Interv. Aging 2013, 8, 139–148. [Google Scholar] [CrossRef][Green Version]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Pinazo-Duran, M.D.; Shoaie-Nia, K.; Zanon-Moreno, V.; Sanz-Gonzalez, S.M.; del Castillo, J.B.; Garcia-Medina, J.J. Strategies to Reduce Oxidative Stress in Glaucoma Patients. Curr. Neuropharmacol. 2018, 16, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Csallany, A.S.; der Guan, M.; Manwaring, J.D.; Addis, P.B. Free Malonaldehyde Determination in Tissues by High-Performance Liquid Chromatography. Anal. Biochem. 1984, 142, 277–283. [Google Scholar] [CrossRef]
- Nucci, C.; Di Pierro, D.; Varesi, C.; Ciuffoletti, E.; Russo, R.; Gentile, R.; Cedrone, C.; Pinazo-Duran, M.D.; Coletta, M.; Mancino, R. Increased malondialdehyde concentration and reduced total antioxidant capacity in aqueous humor and blood samples from patients with glaucoma. Mol. Vis. 2013, 19, 1841–1846. [Google Scholar] [PubMed]
- Zanon-Moreno, V.; Garcia-Medina, J.J.; Gallego-Pinazo, R.; Vinuesa-Silva, I.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Antioxidant status modifications by topical administration of dorzolamide in primary open-angle glaucoma. Eur. J. Ophthalmol. 2009, 19, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Zanon-Moreno, V.; Asensio-Marquez, E.M.; Ciancotti-Oliver, L.; García Medina, J.J.; Sanz, P.; Ortega-Azorin, C.; Pinazo-Duran, M.D.; Ordovás, M.; Corella, D. Effects of polymorphisms in vitamin E-, vitamin C-, and glutathione peroxidase-related genes on serum biomarkers and associations with glaucoma. Mol. Vis. 2013, 19, 231–242. [Google Scholar] [PubMed]
- Li, X.; Franke, A.A. Fast HPLC–ECD analysis of ascorbic acid, dehydroascorbic acid and uric acid. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 853–856. [Google Scholar] [CrossRef]
- Zanon-Moreno, V.; Ciancotti-Olivares, L.; Asencio, J.; Sanz, P.; Ortega-Azorin, C.; Pinazo-Duran, M.D.; Corella, D. Association between a SLC23A2 gene variation, plasma vitamin C levels, and risk of glaucoma in a Mediterranean population. Mol. Vis. 2011, 17, 2997–3004. [Google Scholar]
- Zanón-Moreno, V.; Pons, S.; Gallego-Pinazo, R.; García-Medina, J.J.; Vinuesa, I.; Vila-Bou, V.; Pinazo-Durán, M.D. Involvement of nitric oxide and other molecules with redox potential in primary open angle glaucoma. Arch. Soc. Esp. Oftalmol. 2008, 83, 365–372. [Google Scholar]
- Benitez-Del-Castillo, J.; Cantu-Dibildox, J.; Sanz-González, S.M.; Zanón-Moreno, V.; Pinazo-Duran, M.D. Cytokine expression in tears of patients with glaucoma or dry eye disease: A prospective, observational cohort study. Eur. J. Ophthalmol. 2019, 29, 437–443. [Google Scholar] [CrossRef]
- Zanon-Moreno, V.; Garcia-Medina, J.J.; Zanon-Viguer, V.; Moreno-Nadal, M.A.; Pinazo-Duran, M.D. Smoking, an additional risk factor in elder women with primary open-angle glaucoma. Mol. Vis. 2009, 15, 2953–2959. [Google Scholar]
- Chandra, D.; Tang, D.G. Detection of apoptosis in cell-free systems. Methods Mol. Biol. Clifton N. J. 2009, 559, 65–75. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Naegelin, Y.; Dingsdale, H.; Säuberli, K.; Schädelin, S.; Kappos, L.; Barde, Y.A. Measuring and Validating the Levels of Brain-Derived Neurotrophic Factor in Human Serum. eNeuro 2018, 5, ENEURO.0419-17.2018. [Google Scholar] [CrossRef]
- Ali, S.F.; Newport, G.D.; Scallet, A.C.; Binienda, Z.; Ferguson, S.A.; Bailey, J.R.; Paule, M.G.; Slikker, W., Jr. Oral administration of 3,4-methylenedioxymethamphetamine (MDMA) produces selective serotonergic depletion in the nonhuman primate. Neurotoxicol. Teratol. 1993, 15, 91–96. [Google Scholar] [CrossRef]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–830. [Google Scholar] [CrossRef] [PubMed]
- Hollands, H.; Johnson, D.; Hollands, S.; Simel, D.L.; Jinapriya, D.; Sharma, S. Do findings on routine examination identify patients at risk for primary open-angle glaucoma? The rational clinical examination systematic review. JAMA 2013, 309, 2035–2042. [Google Scholar] [CrossRef]
- Kreft, D.; Doblhammer, G.; Guthoff, R.F.; Frech, S. Prevalence, incidence, and risk factors of primary open-angle glaucoma—A cohort study based on longitudinal data from a German public health insurance. BMC Public Health 2019, 19, 851. [Google Scholar] [CrossRef]
- Vianna, J.R.; Danthurebandara, V.M.; Sharpe, G.P.; Hutchison, D.M.; Belliveau, A.C.; Shuba, L.M.; Nicolela, M.T.; Chauhan, B.C. Importance of Normal Aging in Estimating the Rate of Glaucomatous Neuroretinal Rim and Retinal Nerve Fiber Layer Loss. Ophthalmology 2015, 122, 2392–2398. [Google Scholar] [CrossRef]
- Liu, S.A.; Zhao, Z.N.; Sun, N.N.; Han, Y.; Chen, J.; Fan, Z.G. Transitions of the Understanding and Definition of Primary Glaucoma. Chin. Med. J. Engl. 2018, 131, 2852–2859. [Google Scholar]
- Kang, J.H.; Pasquale, L.R.; Willett, W.; Rosner, B.; Egan, K.M.; Faberowski, N.; Hankinson, S.E. Antioxidant intake and primary open-angle glaucoma: A prospective study. Am. J. Epidemiol. 2003, 158, 337–346. [Google Scholar] [CrossRef]
- Bussel, I.I.; Aref, A.A. Dietary factors and the risk of glaucoma: A review. Ther. Adv. Chronic Dis. 2014, 5, 188–194. [Google Scholar] [CrossRef]
- Abreu-Reyes, J.A.; Álvarez-Luis, D.; Arteaga-Hernández, V.; Sánchez-Mendez, M.; Abreu-González, R. Mediterranean diet adherence of patients with primary open-angle glaucoma. Arch. Soc. Esp. Oftalmol. 2017, 92, 353–358. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Tuulonen, A.; Forsman, E.; Hagman, J.; Harju, M.; Kari, O.; Lumme, P.; Luodonpää, M.; Määttä, M.; Saarela, V.; Vaajanen, A.; et al. [Update on Current Care Guideline: Glaucoma]. Duodecim 2015, 131, 356–358. [Google Scholar]
- Nucci, C.; Martucci, A.; Giannini, C.; Morrone, L.A.; Bagetta, G.; Mancino, R. Neuroprotective agents in the management of glaucoma. Eye 2018, 32, 938–945. [Google Scholar] [CrossRef]
- Konstantakopoulou, E.; Gazzard, G.; Vickerstaff, V.; Jiang, Y.; Nathwani, N.; Hunter, R.; Ambler, G.; Bunce, C.; LiGHT Trial Study Group. The Laser in Glaucoma and Ocular Hypertension (LiGHT) trial. A multicentre randomised controlled trial: Baseline patient characteristics. Br. J. Ophthalmol. 2018, 102, 599–603. [Google Scholar] [CrossRef]
- Pinazo-Durán, M.D.; Zanón-Moreno, V.; García-Medina, J.J.; Gallego-Pinazo, R. Evaluation of presumptive biomarkers of oxidative stress, immune response and apoptosis in primary open-angle glaucoma. Curr. Opin. Pharmacol. 2013, 13, 98–107. [Google Scholar] [CrossRef]
- Kambayashi, Y.; Binh, N.T.; Asakura, H.W. Efficient assay for total antioxidant capacity in human plasma using a 96-well microplate. J. Clin. Biochem. Nutr. 2009, 44, 46–51. [Google Scholar] [CrossRef]
- Lymperaki, E.; Tsikopoulos, A.; Makedou, K.; Paliogianni, E.; Kiriazi, L.M.; Charisi, C.; Vagdatli, E. Impact of iron and folic acid supplementation on oxidative stress during pregnancy. J. Obstet. Gynaecol. 2015, 35, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Reg. Res. 2013, 8, 2003–2014. [Google Scholar]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Durán, M.D.; Zanón-Moreno, V.; Gallego-Pinazo, R.; García-Medina, J.J. Oxidative stress and mitochondrial failure in the pathogenesis of glaucoma neurodegeneration. Prog. Brain Res. 2015, 220, 127–153. [Google Scholar] [PubMed]
- Saccà, S.C.; Pascotto, A.; Camicione, P.; Capris, P.; Izzotti, A. Oxidative DNA damage in the human trabecular meshwork. Clinical correlation in patients with primary open-angle glaucoma. Arch. Ophthalmol. 2005, 123, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Ishikawa, K.; Nakao, S.; Sonoda, K.H. Innate immune response in retinal homeostasis and inflammatory disorders. Prog. Retin. Eye Res. 2020, 74, 100778. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Yang, X.; Luo, C.; Peng, Y.; Sun, S.L.; Sun, D. Mechanisms of immune system activation in glaucoma: Oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Investig. Ophthalmol. Vis. Sci. 2007, 48, 705–714. [Google Scholar] [CrossRef]
- Yerramothu, P.; Vijay, A.K.; Willcox, M.D.P. Inflammasomes, the eye and anti-inflammasome therapy. Eye 2018, 32, 491–505. [Google Scholar] [CrossRef]
- Zanon-Moreno, V.; Raga-Cervera, J.; García-Medina, J.J.; Benitez-del-Castillo, J.; Vinuesa-Silva, I.; Torregrosa, S.; Pinazo-Durán, M.D. New horizons for glaucoma therapy. I: Neuroinflammation and inflammasomes. Arch. Soc. Esp. Oftalmol. 2018, 93, e7–e9. [Google Scholar] [CrossRef]
- Cordeiro, M.F.; Guo, L.; Luong, V.; Harding, G.; Wang, W.; Jones, H.E.; Moss, S.E.; Sillito, A.M.; Fitzke, F.W. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc. Natl. Acad. Sci. USA 2004, 10, 13352–13356. [Google Scholar] [CrossRef]
- Gauthier, A.C.; Liu, J. Epigenetics and Signaling Pathways in Glaucoma. Biomed. Res. Int. 2017, 2017, 5712341. [Google Scholar] [CrossRef]
- Vasudevan, S.K.; Gupta, V.; Crowston, J.G. Neuroprotection in glaucoma. Indian J. Ophthalmol. 2011, 59 (Suppl. S1), S102–S113. [Google Scholar] [CrossRef]
- Pietrucha-Dutczak, M.; Amadio, M.; Govoni, S.; Lewin-Kowalik, J.; Smedowski, A. The Role of Endogenous Neuroprotective Mechanisms in the Prevention of Retinal Ganglion Cells Degeneration. Front. Neurosci. 2018, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Durán, M.D.; Zanón-Moreno, V.; del-Rio-Vellosillo, M. Update on the effects of antioxidants on diabetic. Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Albarral, J.A.; de Hoz, R.; Ramirez, A.I.; López-Cuenca, I.; Salobrar-García, E.; Pinazo-Durá, M.D.; Ramírez, J.M.; Salazar, J.J. Beneficial effects of saffron (Crocus sativus L.) in ocular pathologies, particularly neurodegenerative retinal diseases. Neural Regen. Res. 2020, 15, 1408–1416. [Google Scholar] [PubMed]
- Fernández-Albarral, J.A.; Ramírez, A.I.; de Hoz, R.; López-Villarín, N.; Salobrar-García, E.; López-Cue, I.; Licastro, E.; Inarejos-García, A.M.; Almodóvar, P.; Pinazo-Durán, M.D.; et al. Neuroprotective and Anti-Inflammatory Effects of a Hydrophilic Saffron Extract in a Model of.Glaucoma. Int. J. Mol. Sci. 2019, 20, 4110. [Google Scholar] [CrossRef]
- Zhou, X.; Li, G.; Zhang, S.; Wu, J. 5-HT1A Receptor Agonist Promotes Retinal Ganglion Cell Function by Inhibiting OFF-Type Presynaptic Glutamatergic Activity in a Chronic Glaucoma Model. Front. Cell. Neurosci. 2019, 13, 167. [Google Scholar] [CrossRef]
- Pesscosolido, N.; Parisi, F.; Russo, P.; Buomprisco, G.; Nebbioso, M. Role of dopaminergic receptors in glaucomatous disease modulation. BioMed Res. Int. 2013, 2013, 193048. [Google Scholar] [CrossRef]
- Porter, L.F.; Black, G.C. Personalized ophthalmology. Clin. Genet. 2014, 86, 1–11. [Google Scholar] [CrossRef]
- Kubelick, K.P.; Snider, E.J.; Ethier, R.; Emelianov, S. Development of a stem cell tracking platform for ophthalmic applications using ultrasound and photoacoustic imaging. Theranostics 2019, 9, 3812–3824. [Google Scholar] [CrossRef]
- Shiri, I.; Abdollahi, H.; Atashzar, M.R.; Rahmim, A.; Zaidi, H. A theranostic approach based on.radiolabeled antiviral drugs, antibodies and CRISPR-associated proteins for early detection and treatment of SARS-CoV-2 disease. Nucl. Med. Commun. 2020, 41, 837–840. [Google Scholar] [CrossRef]



| Inclusion | Exclusion |
|---|---|
| Individuals aged 40 years or more | Individuals under 40 years of age |
| Accurate POAG diagnosis (initial stage) for the corresponding group of participants. | Other glaucoma type than the POAG or glaucoma suspects. |
| Healthy individuals for the CG of participants | Patients suffering other eye diseases than POAG, or systemic disorders. |
| Patients under treatment that may interfere with the results of the study. | |
| Laser and/or eye surgery in the previous 12 months. | |
| Complete and precise data at the medical history. | Incomplete and/or confounding data. Other diagnosis or procedures that do not fit with the study purpose. Impossibility to obtain a complete and thorough clinical history |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinazo-Durán, M.D.; García-Medina, J.J.; Bolarín, J.M.; Sanz-González, S.M.; Valero-Vello, M.; Abellán-Abenza, J.; Zanón-Moreno, V.; Moreno-Montañés, J. Computational Analysis of Clinical and Molecular Markers and New Theranostic Possibilities in Primary Open-Angle Glaucoma. J. Clin. Med. 2020, 9, 3032. https://doi.org/10.3390/jcm9093032
Pinazo-Durán MD, García-Medina JJ, Bolarín JM, Sanz-González SM, Valero-Vello M, Abellán-Abenza J, Zanón-Moreno V, Moreno-Montañés J. Computational Analysis of Clinical and Molecular Markers and New Theranostic Possibilities in Primary Open-Angle Glaucoma. Journal of Clinical Medicine. 2020; 9(9):3032. https://doi.org/10.3390/jcm9093032
Chicago/Turabian StylePinazo-Durán, María D., José J. García-Medina, José M. Bolarín, Silvia M. Sanz-González, Mar Valero-Vello, Javier Abellán-Abenza, Vicente Zanón-Moreno, and Javier Moreno-Montañés. 2020. "Computational Analysis of Clinical and Molecular Markers and New Theranostic Possibilities in Primary Open-Angle Glaucoma" Journal of Clinical Medicine 9, no. 9: 3032. https://doi.org/10.3390/jcm9093032
APA StylePinazo-Durán, M. D., García-Medina, J. J., Bolarín, J. M., Sanz-González, S. M., Valero-Vello, M., Abellán-Abenza, J., Zanón-Moreno, V., & Moreno-Montañés, J. (2020). Computational Analysis of Clinical and Molecular Markers and New Theranostic Possibilities in Primary Open-Angle Glaucoma. Journal of Clinical Medicine, 9(9), 3032. https://doi.org/10.3390/jcm9093032







