Surgical Management of Neuroendocrine Tumours of the Pancreas
Abstract
Highlights
- Surgical management of pNETs should be planned in a multidisciplinary staff meeting.
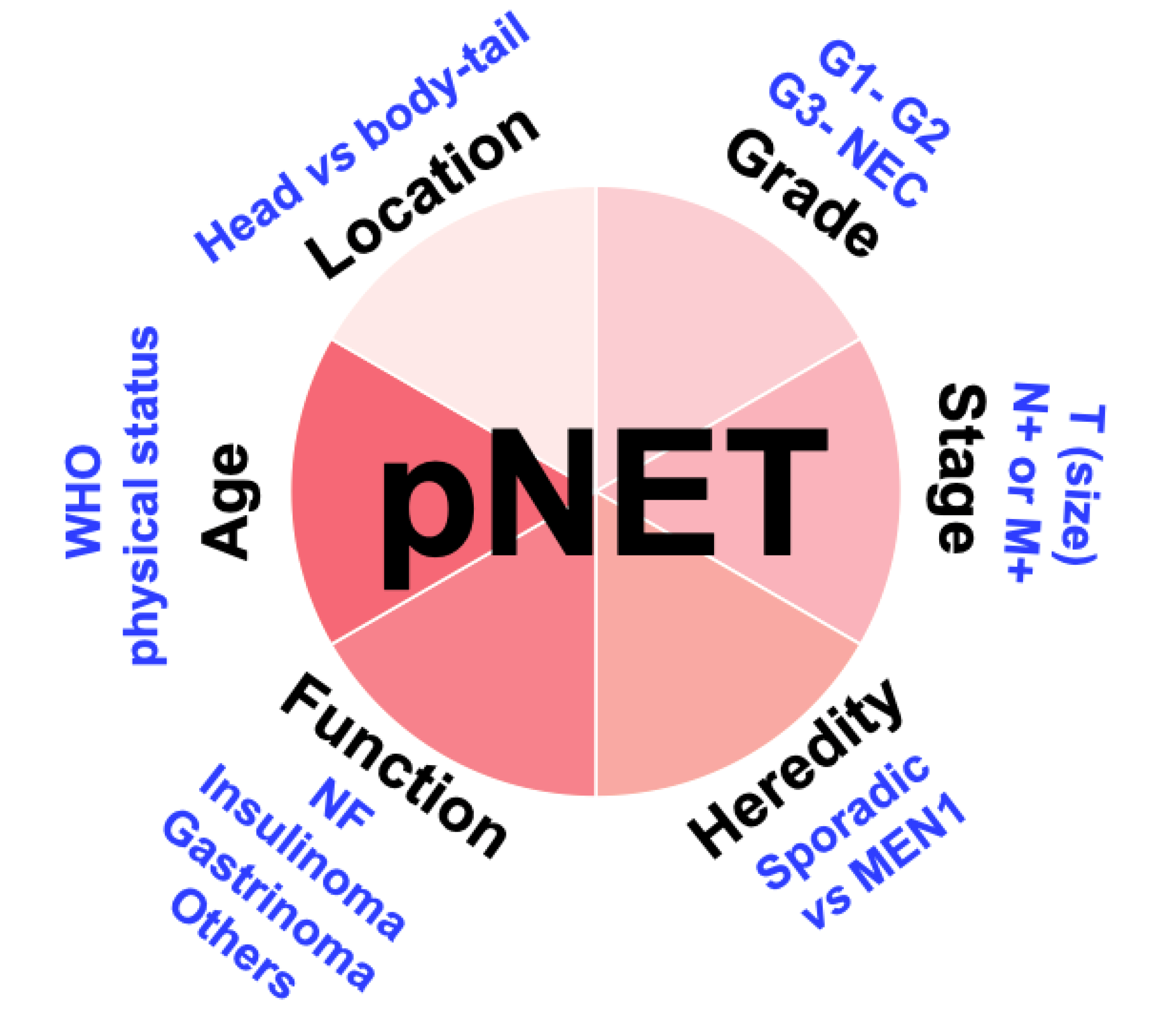
- The initial accurate assessment is the cornerstone in pNETs management and should include accurate localisation, grading, and staging.
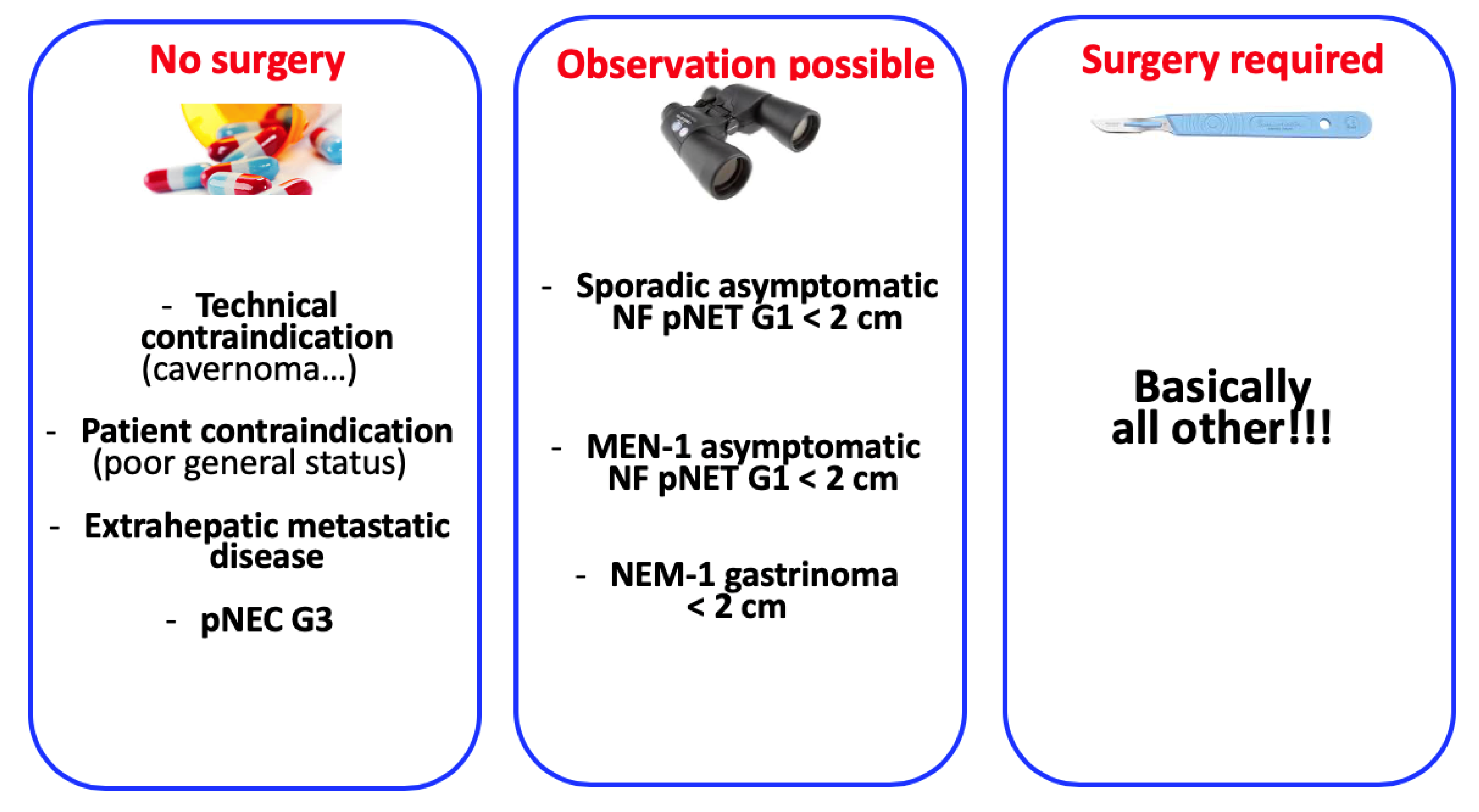
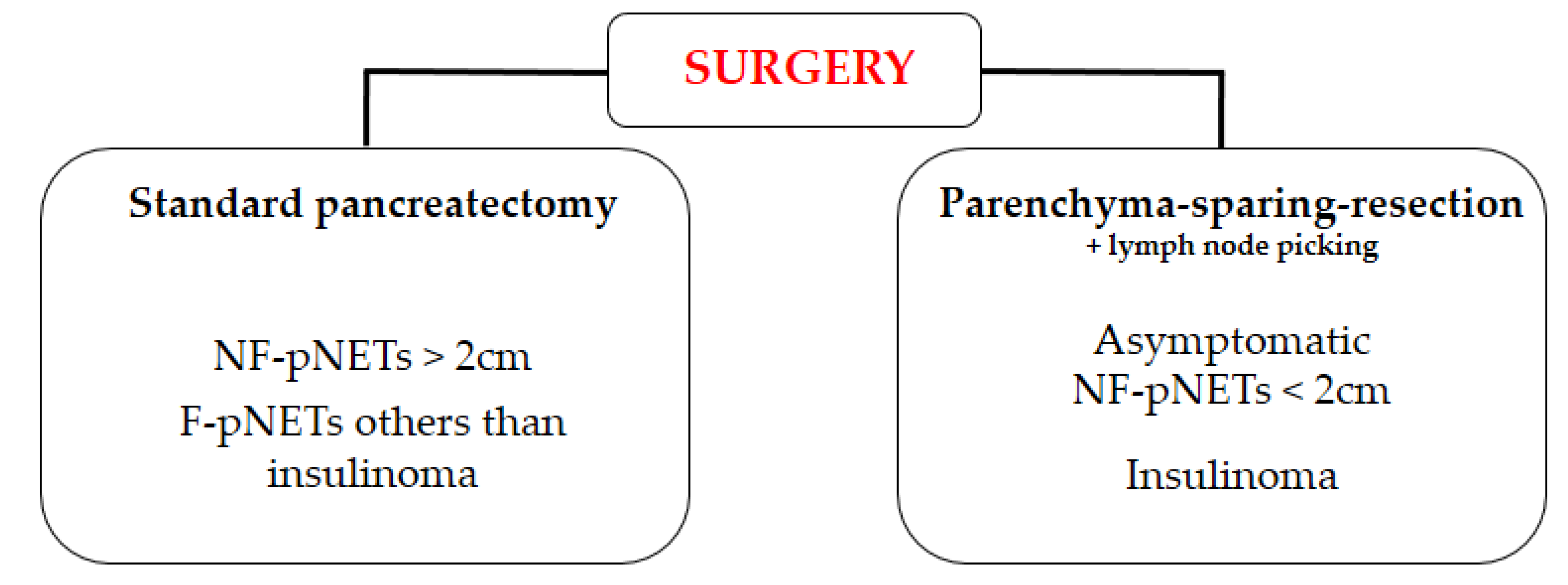
- Surgery should be performed for asymptomatic non-functional pNETs > 2 cm or non-functional symptomatic pNETs regardless of tumour size.
- Surgery should be performed for all functional sporadic pNETs, except those with unresectable distant metastasis.
- Parenchyma-sparing surgery is recommended for insulinoma and can be considered for non-functional pNETs < 2 cm if associated with lymph node picking.
1. Introduction
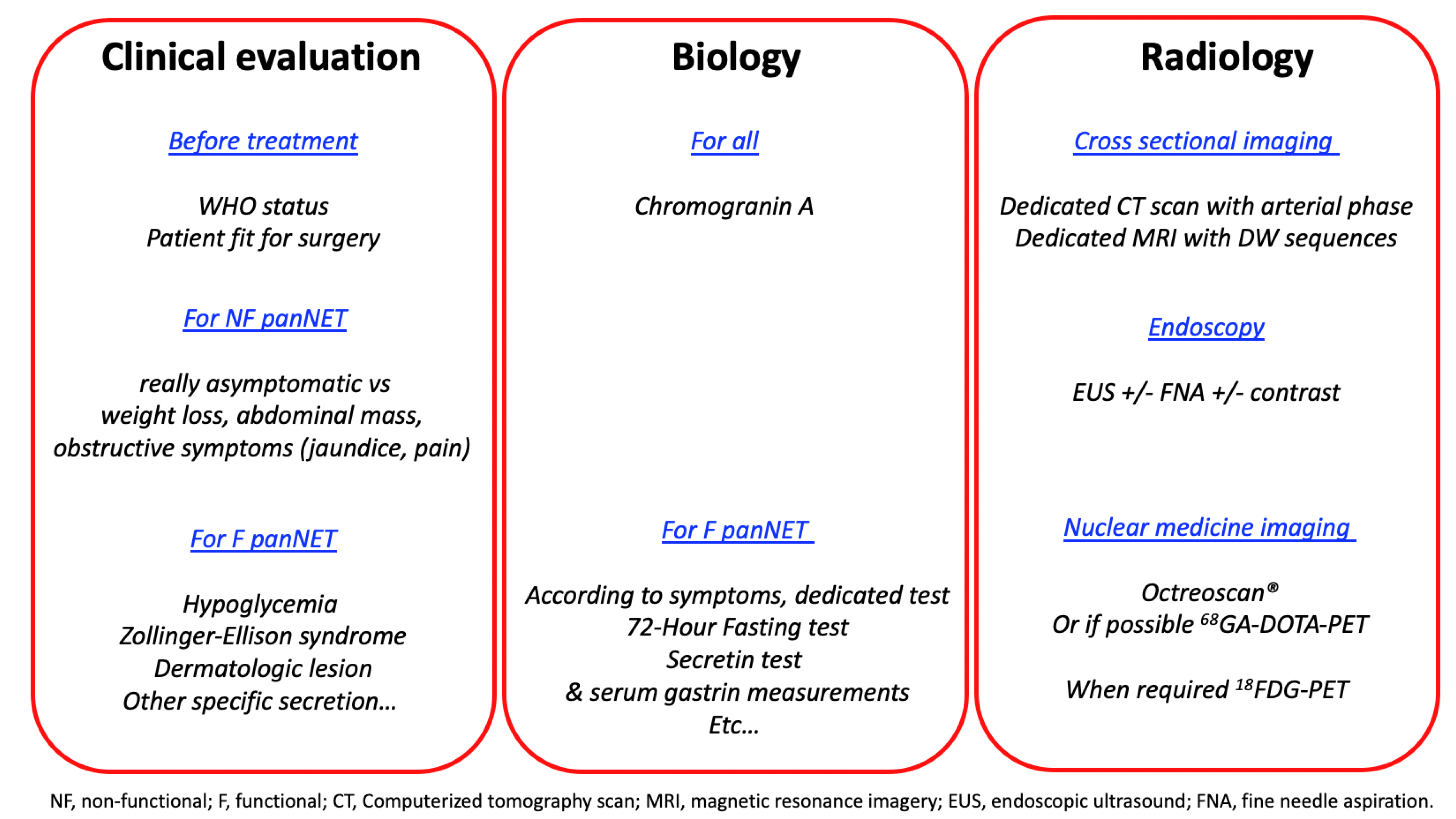
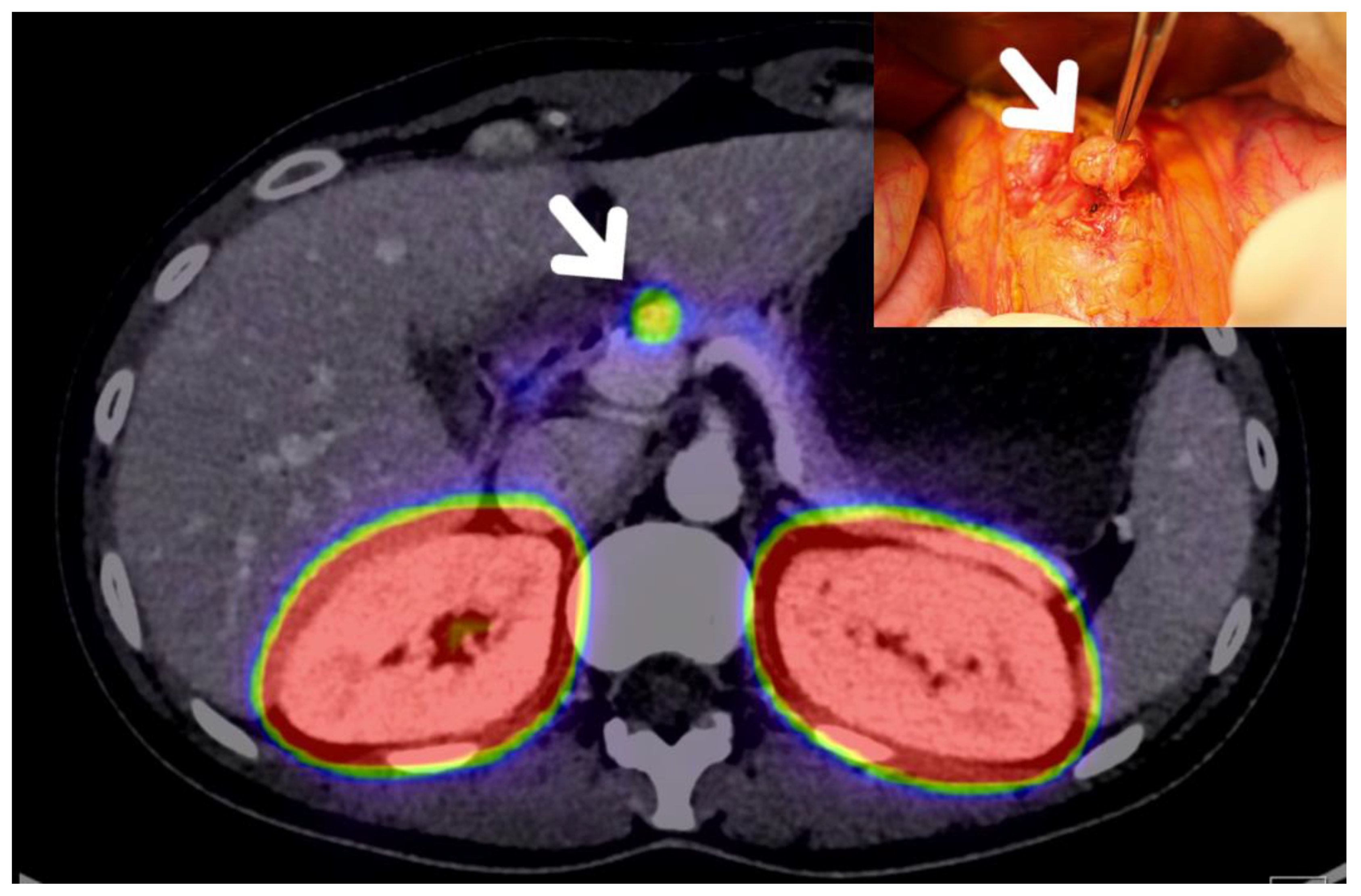
2. Preoperative Evaluation of pNET Patients
3. Indications of Surgical Resection of pNET Patients
3.1. Sporadic pNETs
3.1.1. Non-Functional pNETs
3.1.2. Insulinomas
3.1.3. Gastrinomas
3.1.4. VIPoma
3.2. pNETs Occurring in MEN1 Patients
3.2.1. MEN1 Related NF-pNETs
3.2.2. MEN1 Related Insulinoma
3.2.3. MEN1-Related Gastrinoma
3.3. G3 pNETs
3.4. Metastatic pNETs
4. Modalities of Pancreatic Resection of pNETs
4.1. Standard Surgery Versus Pancreatic Sparing Surgery
4.2. Surgical Approach: Minimally Invasive Versus Open Pancreatectomy
4.3. Short- and Long-Term Results of Pancreatic Resection in pNETs Patients
4.4. Pancreatic Resection of Functional pNETs
4.4.1. Gastrinoma
4.4.2. Insulinoma
4.5. The Value of Lymphadenectomy in pNETs Surgery
5. Recurrence Rate and Follow-Up after Resection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [PubMed]
- Kuo, E.J.; Salem, R.R. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann. Surg. Oncol. 2013, 20, 2815–2821. [Google Scholar]
- Kaltsas, G.A.; Besser, G.M.; Grossman, A.B. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr. Rev. 2004, 25, 458–511. [Google Scholar] [PubMed]
- Crona, J.; Norlen, O.; Antonodimitrakis, P.; Welin, S.; Stalberg, P.; Eriksson, B. Multiple and Secondary Hormone Secretion in Patients with Metastatic Pancreatic Neuroendocrine Tumours. J. Clin. Endocrinol. Metab. 2016, 101, 445–452. [Google Scholar]
- Halfdanarson, T.R.; Rabe, K.G.; Rubin, J.; Petersen, G.M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008, 19, 1727–1733. [Google Scholar] [PubMed]
- Ocuin, L.M.; Sarmiento, J.M.; Staley, C.A.; Galloway, J.R.; Johnson, C.D.; Wood, W.C.; Kooby, D.A. Comparison of central and extended left pancreatectomy for lesions of the pancreatic neck. Ann. Surg. Oncol. 2008, 15, 2096–2103. [Google Scholar]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar]
- Cheema, A.; Weber, J.; Strosberg, J.R. Incidental detection of pancreatic neuroendocrine tumors: An analysis of incidence and outcomes. Ann. Surg. Oncol. 2012, 19, 2932–2936. [Google Scholar]
- Birnbaum, D.J.; Gaujoux, S.; Cherif, R.; Dokmak, S.; Fuks, D.; Couvelard, A.; Vullierme, M.-P.; Ronot, M.; Ruszniewski, P.; Belghiti, J.; et al. Sporadic nonfunctioning pancreatic neuroendocrine tumors: Prognostic significance of incidental diagnosis. Surgery 2014, 155, 13–21. [Google Scholar]
- Crippa, S.; Partelli, S.; Zamboni, G.; Scarpa, A.; Tamburrino, D.; Bassi, C.; Pederzoli, P.; Falconi, M. Incidental diagnosis as prognostic factor in different tumor stages of nonfunctioning pancreatic endocrine tumors. Surgery 2014, 155, 145–153. [Google Scholar]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.; Capdevila, J.; Caplin, M.; Kos-Kudła, B.; Kwekkeboom, D.; Rindi, G.; Kloppel, G.; et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef]
- Schmid-Tannwald, C.; Schmid-Tannwald, C.M.; Morelli, J.N.; Neumann, R.; Haug, A.; Jansen, N.; Nikolaou, K.; Schramm, N.; Reiser, M.F.; Rist, C. Comparison of abdominal MRI with diffusion-weighted imaging to 68Ga-DOTATATE PET/CT in detection of neuroendocrine tumors of the pancreas. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Lestra, T.; Kanagaratnam, L.; Mule, S.; Janvier, A.; Brixi, H.; Cadiot, G.; Dohan, A.; Hoeffel, C. Measurement variability of liver metastases from neuroendocrine tumors on different magnetic resonance imaging sequences. Diagn. Interv. Imaging 2018, 99, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.; Taouli, B.; Guiu, B.; Koh, D.-M.; Luciani, A.; Manfredi, R.; Vilgrain, V.; Hoeffel, C.; Kanematsu, M.; Soyer, P. Diffusion-weighted MR imaging of the pancreas: Current status and recommendations. Radiology 2015, 274, 45–63. [Google Scholar] [PubMed]
- Sundin, A.; Garske, U.; Orlefors, H. Nuclear imaging of neuroendocrine tumours. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 69–85. [Google Scholar] [PubMed]
- Garin, E.; Le Jeune, F.; Devillers, A.; Cuggia, M.; De Lajarte-Thirouard, A.-S.; Bouriel, C.; Boucher, E.; Raoul, J.-L. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J. Nucl. Med. 2009, 50, 858–864. [Google Scholar] [PubMed]
- Abgral, R.; Leboulleux, S.; Deandreis, D.; Auperin, A.; Lumbroso, J.; Dromain, C.; Duvillard, P.; Elias, D.; de Baere, T.; Ducreux, M.; et al. Performance of (18)fluorodeoxyglucose-positron emission tomography and somatostatin receptor scintigraphy for high Ki67 (>/=10%) well-differentiated endocrine carcinoma staging. J. Clin. Endocrinol. Metab. 2011, 96, 665–671. [Google Scholar]
- Wild, D.; Macke, H.; Christ, E.; Gloor, B.; Reubi, J.C. Glucagon-like peptide 1-receptor scans to localize occult insulinomas. N. Engl. J. Med. 2008, 359, 766–768. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793. [Google Scholar] [CrossRef]
- Doppman, J.L.; Miller, D.L.; Chang, R.; Shawker, T.H.; Gorden, P.; Norton, J.A. Insulinomas: Localization with selective intraarterial injection of calcium. Radiology 1991, 178, 237–241. [Google Scholar]
- Guettier, J.-M.; Kam, A.; Chang, R.; Skarulis, M.C.; Cochran, C.; Alexander, H.R.; Libutti, S.K.; Pingpank, J.F.; Gorden, P. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: The NIH experience. J. Clin. Endocrinol. Metab. 2009, 94, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.-M.; Chen, J.-Y.; Won, J.G.-S.; Tseng, H.-S.; Yang, A.-H.; Wang, S.-E.; Lee, C.-H. The role of intra-arterial calcium stimulation test with hepatic venous sampling (IACS) in the management of occult insulinomas. Ann. Surg. Oncol. 2007, 14, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Hatoko, T.; Murakami, T.; Sone, M.; Yabe, D.; Masui, T.; Nakamoto, Y.; Furuta, A.; Uza, N.; Kodama, Y.; Harada, N.; et al. Low-dose Selective Arterial Calcium Stimulation Test for Localizing Insulinoma: A Single-center Experience of Five Consecutive Cases. Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Braatvedt, G.; Jennison, E.; Holdaway, I.M. Comparison of two low-dose calcium infusion schedules for localization of insulinomas by selective pancreatic arterial injection with hepatic venous sampling for insulin. Clin. Endocrinol. 2014, 80, 80–84. [Google Scholar] [CrossRef]
- Wong, M.; Isa, S.H.; Zahiah, M.; Azmi, K.N. Intraoperative ultrasound with palpation is still superior to intra-arterial calcium stimulation test in localising insulinoma. World J. Surg. 2007, 31, 586–592. [Google Scholar] [CrossRef]
- Gorman, B.; Charboneau, J.; James, E.; Reading, C.; Galiber, A.; Grant, C.; Van Heerden, J.; Telander, R.; Service, F. Benign pancreatic insulinoma: Preoperative and intraoperative sonographic localization. AJR Am. J. Roentgenol. 1986, 147, 929–934. [Google Scholar] [CrossRef]
- Bettini, R.; Partelli, S.; Boninsegna, L.; Capelli, P.; Crippa, S.; Pederzoli, P.; Scarpa, A.; Falconi, M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011, 150, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.; Grant, C.S.; Salomao, D.R.; Fletcher, J.G.; Takahashi, N.; Fidler, J.L.; Levy, M.J.; Huebner, M. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): Role for nonoperative management. Surgery 2012, 152, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Partelli, S.; Maire, F.; D’Onofrio, M.; Larroque, B.; Tamburrino, D.; Sauvanet, A.; Falconi, M.; Ruszniewski, P. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2013, 98, 4784–4789. [Google Scholar] [CrossRef]
- Norton, J.A.; Jensen, R.T. Role of surgery in Zollinger-Ellison syndrome. J. Am. Coll. Surg. 2007, 205, S34–S37. [Google Scholar]
- Cadiot, G.; Vuagnat, A.; Doukhan, I.; Murat, A.; Bonnaud, G.; Delemer, B.; Thiefin, G.; Beckers, A.; Veyrac, M.; Proye, C.; et al. Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Groupe d’Etude des Neoplasies Endocriniennes Multiples [GENEM and groupe de Recherche et d’Etude du Syndrome de Zollinger-Ellison (GRESZE). Gastroenterology 1999, 116, 286–293. [Google Scholar] [PubMed]
- Norton, J.A.; Alexander, H.R.; Fraker, D.L.; Venzon, D.J.; Gibril, F.; Jensen, R.T. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann. Surg. 2001, 234, 495–505. [Google Scholar] [PubMed]
- Tsoli, M.; Chatzellis, E.; Koumarianou, A.; Kolomodi, D.; Kaltsas, G. Current best practice in the management of neuroendocrine tumors. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018818804698. [Google Scholar] [PubMed]
- Sallinen, V.; Le Large, T.Y.; Galeev, S.; Kovalenko, Z.; Tieftrunk, E.; Araujo, R.L.C.; Ceyhan, G.O.; Gaujoux, S. Surveillance strategy for small asymptomatic non-functional pancreatic neuroendocrine tumors—A systematic review and meta-analysis. HPB 2017, 19, 310–320. [Google Scholar] [PubMed]
- Sallinen, V.J.; Le Large, T.Y.S.; Tieftrunk, E.; Galeev, S.; Kovalenko, Z.; Haugvik, S.P.; Antila, A.; Franklin, O.; Martinez-Moneo, E.; Panzuto, F.; et al. Prognosis of sporadic resected small (</=2 cm) nonfunctional pancreatic neuroendocrine tumors—A multi-institutional study. HPB 2018, 20, 251–259. [Google Scholar]
- Mansour, J.C.; Chen, H. Pancreatic endocrine tumors. J. Surg. Res. 2004, 120, 139–161. [Google Scholar]
- Jensen, R.T.; Cadiot, G.; Brandi, M.L.; De Herder, W.W.; Kaltsas, G.; Komminoth, P.; Scoazec, J.-Y.; Salazar, R.; Sauvanet, A.; Kianmanesh, R.; et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: Functional pancreatic endocrine tumor syndromes. Neuroendocrinology 2012, 95, 98–119. [Google Scholar]
- Ito, T.; Igarashi, H.; Jensen, R.T. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): Recent insights and advances. J. Gastroenterol. 2012, 47, 941–960. [Google Scholar]
- Tabarin, A.; Goichot, B.; French Endocrine, S. Treatment: Symptomatic treatment of hypoglycaemia. Ann. Endocrinol. 2013, 74, 196–199. [Google Scholar]
- Baudin, E.; Caron, P.; Lombard-Bohas, C.; Tabarin, A.; Mitry, E.; Reznick, Y.; Taïeb, D.; Pattou, F.; Goudet, P.; Vezzosi, D.; et al. Malignant insulinoma: Recommendations for characterisation and treatment. Ann. Endocrinol. 2013, 74, 523–533. [Google Scholar]
- Ferrer-García, J.C.; González-Cruz, V.I.; Navas-DeSolís, S.; Civera-Andrés, M.; Morillas-Ariño, C.; Merchante-Alfaro, Á.; Caballero-Díaz, C.; Sánchez-Juan, C.; Herrero, C.C. Management of malignant insulinoma. Clin. Transl. Oncol. 2013, 15, 725–731. [Google Scholar] [PubMed]
- Jawiarczyk, A.; Bolanowski, M.; Syrycka, J.; Bednarek-Tupikowska, G.; Kałużny, M.; Kołodziejczyk, A.; Domosławski, P. Effective therapy of insulinoma by using long-acting somatostatin analogue. A case report and literature review. Exp. Clin. Endocrinol. Diabetes 2012, 120, 68–72. [Google Scholar] [PubMed]
- Oberg, K.E.; Reubi, J.C.; Kwekkeboom, D.J.; Krenning, E.P. Role of somatostatins in gastroenteropancreatic neuroendocrine tumor development and therapy. Gastroenterology 2010, 139, 742–753. [Google Scholar] [PubMed]
- Ito, T.; Igarashi, H.; Jensen, R.T. Pancreatic neuroendocrine tumors: Clinical features, diagnosis and medical treatment: Advances. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 737–753. [Google Scholar] [PubMed]
- Crippa, S.; Zerbi, A.; Boninsegna, L.; Capitanio, V.; Partelli, S.; Balzano, G.; Pederzoli, P.; Di Carlo, V.; Falconi, M. Surgical management of insulinomas: Short- and long-term outcomes after enucleations and pancreatic resections. Arch. Surg. 2012, 147, 261–266. [Google Scholar]
- Silva, F.A.O.B.; Colaiacovo, R.; Araki, O.; Domene, A.F.; Junior, J.V.L.; De Moricz, A.; Rossini, L. Endoscopic ultrasound-guided fine needle injection of alcohol for ablation of an insulinoma: A well documented successful procedure. Endoscopy 2019, 51, E57–E58. [Google Scholar]
- Park, D.H.; Choi, J.-H.; Oh, D.; Lee, S.S.; Seo, N.-W.; Lee, S.K.; Kim, M.-H. Endoscopic ultrasonography-guided ethanol ablation for small pancreatic neuroendocrine tumors: Results of a pilot study. Clin. Endosc. 2015, 48, 158–164. [Google Scholar]
- Barthet, M.; Giovannini, M.; Lesavre, N.; Boustiere, C.; Napoleon, B.; Koch, S.; Gasmi, M.; Vanbiervliet, G.; Gonzalez, J.-M. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: A prospective multicenter study. Endoscopy 2019, 51, 836–842. [Google Scholar]
- Bartsch, D.K.; Waldmann, J.; Fendrich, V.; Boninsegna, L.; López, C.L.; Partelli, S.; Falconi, M. Impact of lymphadenectomy on survival after surgery for sporadic gastrinoma. Br. J. Surg. 2012, 99, 1234–1240. [Google Scholar]
- Norton, J.A.; Fraker, D.L.; Alexander, H.R.; Gibril, F.; Liewehr, D.J.; Venzon, D.J.; Jensen, R.T. Surgery increases survival in patients with gastrinoma. Ann. Surg. 2006, 244, 410–419. [Google Scholar]
- Angelousi, A.; Koffas, A.; Grozinsky-Glasberg, S.; Gertner, J.; Kassi, E.; Alexandraki, K.; Caplin, M.E.; Kaltsas, G.; Toumpanakis, C. Diagnostic and Management Challenges in Vasoactive Intestinal Peptide Secreting Tumors: A Series of 15 Patients. Pancreas 2019, 48, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Branton, S.A.; Avino, A.J.; Martin, J.; Klingler, P.J.; Thompson, G.B.; Grant, C.S.; Van Heerden, J.A. Vasoactive intestinal polypeptide secreting islet cell tumors: A 15-year experience and review of the literature. Surgery 1998, 124, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.M. Rare endocrine tumours of the GI tract. Best Pract. Res. Clin. Gastroenterol. 2005, 19, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Brandi, M.L.; Gagel, R.F.; Angeli, A. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J. Clin. Endocrinol. Metab. 2001, 86, 5658–5671. [Google Scholar] [CrossRef]
- Newey, P.J.; Thakker, R.V. Role of multiple endocrine neoplasia type 1 mutational analysis in clinical practice. Endocr. Pract. 2011, 17 (Suppl. 3), 8–17. [Google Scholar] [CrossRef]
- Yates, C.J.; Newey, P.J.; Thakker, R.V. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol. 2015, 3, 895–905. [Google Scholar] [CrossRef]
- Triponez, F.; Sadowski, S.M.; Pattou, F.; Cardot-Bauters, C.; Mirallié, E.; Le Bras, M.; Sebag, F.; Niccoli, P.; Deguelte, S.; Cadiot, G.; et al. Long-term Follow-up of MEN1 Patients Who Do Not Have Initial Surgery for Small </=2 cm Nonfunctioning Pancreatic Neuroendocrine Tumors, an AFCE and GTE Study: Association Francophone de Chirurgie Endocrinienne & Groupe d’Etude des Tumeurs Endocrines. Ann. Surg. 2018, 268, 158–164. [Google Scholar]
- Pieterman, C.R.; De Laat, J.M.; Twisk, J.W.; Van Leeuwaarde, R.S.; de Herder, W.W.; Dreijerink, K.M.; Hermus, A.R.; Dekkers, O.M.; van der Horst-Schrivers, A.N.; Drent, M.L.; et al. Long-Term Natural Course of Small Nonfunctional Pancreatic Neuroendocrine Tumors in MEN1-Results from the Dutch MEN1 Study Group. J. Clin. Endocrinol. Metab. 2017, 102, 3795–3805. [Google Scholar]
- van Hilst, J.; de Rooij, T.; Klompmaker, S.; Rawashdeh, M.; Aleottil, F.; Al-Sarireh, B.; Alseidi, A.; Ateeb, Z.; Balzano, G.; Berrevoet, F.; et al. Minimally Invasive versus Open Distal Pancreatectomy for Ductal Adenocarcinoma (DIPLOMA): A Pan-European Propensity Score Matched Study. Ann. Surg. 2019, 269, 10–17. [Google Scholar] [CrossRef]
- Nikfarjam, M.; Warshaw, A.L.; Axelrod, L.; Deshpande, V.; Thayer, S.P.; Ferrone, C.R.; Castillo, C.F.-D. Improved contemporary surgical management of insulinomas: A 25-year experience at the Massachusetts General Hospital. Ann. Surg. 2008, 247, 165–172. [Google Scholar] [CrossRef]
- O’Riordain, D.S.; O’Brien, T.; van Heerden, J.A.; Service, F.J.; Grant, C.S. Surgical management of insulinoma associated with multiple endocrine neoplasia type I. World J. Surg. 1994, 18, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Service, F.J.; McMahon, M.M.; O’Brien, P.C.; Ballard, D.J. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: A 60-year study. Mayo Clin. Proc. 1991, 66, 711–719. [Google Scholar] [CrossRef]
- Tonelli, F.; Giudici, F.; Nesi, G.; Batignani, G.; Brandi, M.L. Operation for insulinomas in multiple endocrine neoplasia type 1: When pancreatoduodenectomy is appropriate. Surgery 2017, 161, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.A.; Fang, T.D.; Jensen, R.T. Surgery for gastrinoma and insulinoma in multiple endocrine neoplasia type 1. J. Natl. Compr. Cancer Netw. 2006, 4, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Mignon, M.; Ruszniewski, P.; Podevin, P.; Sabbagh, L.; Cadiot, G.; Rigaud, D.; Bonfils, S. Current approach to the management of gastrinoma and insulinoma in adults with multiple endocrine neoplasia type I. World J. Surg. 1993, 17, 489–497. [Google Scholar] [CrossRef]
- Gibril, F.; Schumann, M.; Pace, A.; Jensen, R.T. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: A prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine 2004, 83, 43–83. [Google Scholar] [CrossRef]
- Vinault, S.; Mariet, A.-S.; Le Bras, M.; Mirallié, E.; Cardot-Bauters, C.; Pattou, F.; Ruszniewski, P.; Sauvanet, A.; Chanson, P.; Baudin, E.; et al. Metastatic Potential and Survival of Duodenal and Pancreatic Tumors in Multiple Endocrine Neoplasia Type 1: A GTE and AFCE Cohort Study (Groupe d’etude des Tumeurs Endocrines and Association Francophone de Chirurgie Endocrinienne). Ann. Surg. 2018. [Google Scholar] [CrossRef]
- Gibril, F.; Venzon, D.J.; Ojeaburu, J.V.; Bashir, S.; Jensen, R.T. Prospective study of the natural history of gastrinoma in patients with MEN1: Definition of an aggressive and a nonaggressive form. J. Clin. Endocrinol. Metab. 2001, 86, 5282–5293. [Google Scholar] [CrossRef]
- Inzani, F.; Petrone, G.; Rindi, G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol. Metab. Clin. N. Am. 2018, 47, 463–470. [Google Scholar] [CrossRef]
- Merola, E.; Rinke, A.; Partelli, S.; Gress, T.M.; Andreasi, V.; Kollár, A.; Perren, A.; Christ, E.; Panzuto, F.; Pascher, A.; et al. Surgery with Radical Intent: Is There an Indication for G3 Neuroendocrine Neoplasms? Ann. Surg. Oncol. 2020, 27, 1348–1355. [Google Scholar] [CrossRef]
- Yoshida, T.; Hijioka, S.; Hosoda, W.; Ueno, M.; Furukawa, M.; Kobayashi, N.; Ikeda, M.; Ito, T.; Kodama, Y.; Morizane, C.; et al. Surgery for Pancreatic Neuroendocrine Tumor G3 and Carcinoma G3 Should be Considered Separately. Ann. Surg. Oncol. 2019, 26, 1385–1393. [Google Scholar] [PubMed]
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.-Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21. [Google Scholar] [CrossRef]
- Howe, J.R.; Merchant, N.B.; Conrad, C.; Keutgen, X.M.; Hallet, J.; Drebin, J.A.; Minter, R.M.; Lairmore, T.C.; Tseng, J.F.; Zeh, H.J.; et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Bartsch, D.K.; Capdevila, J.; Chen, J.; Knigge, U.; Niederle, B.; Van Dijkum, E.J.N.; Pape, U.-F.; Pascher, A.; Ramage, J.; et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017, 105, 255–265. [Google Scholar] [PubMed]
- Pavel, M.; O’’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar]
- Sabater, L.; Ausania, F.; Bakker, O.J.; Boadas, J.; Domínguez-Muńoz, J.E.; Falconi, M.; Fernández-Cruz, L.; Frulloni, L.; González-Sánchez, V.; Larińo-Noia, J.; et al. Evidence-based Guidelines for the Management of Exocrine Pancreatic Insufficiency After Pancreatic Surgery. Ann. Surg. 2016, 264, 949–958. [Google Scholar] [CrossRef]
- Beger, H.G.; Poch, B.; Mayer, B.; Siech, M. New Onset of Diabetes and Pancreatic Exocrine Insufficiency After Pancreaticoduodenectomy for Benign and Malignant Tumors: A Systematic Review and Meta-analysis of Long-term Results. Ann. Surg. 2018, 267, 259–270. [Google Scholar]
- Crippa, S.; Bassi, C.; Salvia, R.; Falconi, M.; Butturini, G.; Pederzoli, P. Enucleation of pancreatic neoplasms. Br. J. Surg. 2007, 94, 1254–1259. [Google Scholar]
- Casadei, R.; Ricci, C.; Rega, D.; D’Ambra, M.; Pezzilli, R.; Tomassetti, P.; Campana, D.; Nori, F.; Minni, F. Pancreatic endocrine tumors less than 4 cm in diameter: Resect or enucleate? a single-center experience. Pancreas 2010, 39, 825–828. [Google Scholar] [CrossRef]
- Pitt, S.C.; Pitt, H.A.; Baker, M.S.; Christians, K.; Touzios, J.G.; Kiely, J.M.; Weber, S.M.; Wilson, S.D.; Howard, T.J.; Talamonti, M.S.; et al. Small pancreatic and periampullary neuroendocrine tumors: Resect or enucleate? J. Gastrointest. Surg. 2009, 13, 1692–1698. [Google Scholar]
- Cherif, R.; Gaujoux, S.; Couvelard, A.; Dokmak, S.; Vuillerme, M.-P.; Ruszniewski, P.; Belghiti, J.; Sauvanet, A. Parenchyma-sparing resections for pancreatic neuroendocrine tumors. J. Gastrointest. Surg. 2012, 16, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Toste, P.A.; Kadera, B.; Tatishchev, S.F.; Dawson, D.W.; Clerkin, B.M.; Muthusamy, R.; Watson, R.; Tomlinson, J.S.; Hines, O.J.; Reber, H.A.; et al. Nonfunctional pancreatic neuroendocrine tumors <2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J. Gastrointest. Surg. 2013, 17, 2105–2113. [Google Scholar]
- Clinical Outcomes of Surgical Therapy Study Group; Nelson, H.; Sargent, D.J.; Wieand, H.S.; Fleshman, J.; Anvari, M. A comparison of laparoscopically assisted and open colectomy for colon cancer. N. Engl. J. Med. 2004, 350, 2050–2059. [Google Scholar] [CrossRef] [PubMed]
- Bonjer, H.J.; Deijen, C.L.; Haglind, E.; Group, C.I.S. A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N. Engl. J. Med. 2015, 373, 194. [Google Scholar] [CrossRef]
- De Rooij, T.; Van Hilst, J.; Vogel, J.A.; Van Santvoort, H.C.; De Boer, M.T.; Boerma, D.; Boezem, P.B.V.D.; Bonsing, B.A.; Bosscha, K.; Coene, P.P.; et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): Study protocol for a randomized controlled trial. Trials 2017, 18, 166. [Google Scholar] [CrossRef]
- Van Der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Palanivelu, C.; Senthilnathan, P.; Sabnis, S.C.; Babu, N.S.; Gurumurthy, S.S.; Vijai, N.A.; Nalankilli, V.P.; Raj, P.; Parthasarathy, R.; Rajapandian, S. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br. J. Surg. 2017, 104, 1443–1450. [Google Scholar] [CrossRef]
- Edwin, B.; EAES Consensus Conference Study Group; Sahakyan, M.А.; Abu Hilal, M.; Besselink, M.G.; Braga, M.; Fabre, J.-M.; Fernández-Cruz, L.; Gayet, B.; Kim, S.C.; et al. Laparoscopic surgery for pancreatic neoplasms: The European association for endoscopic surgery clinical consensus conference. Surg. Endosc. 2017, 31, 2023–2041. [Google Scholar] [CrossRef]
- Asbun, H.J.; Moekotte, A.L.; Vissers, F.L.; Kunzler, F.; Cipriani, F.; Alseidi, A.; D’Angelica, M.I.; Balduzzi, A.; Bassi, C.; Björnsson, B.; et al. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann. Surg. 2020, 271, 1–14. [Google Scholar] [CrossRef]
- Nickel, F.; Haney, C.M.; Kowalewski, K.-F.; Probst, P.; Limen, E.F.; Kalkum, E.; Diener, M.K.; Strobel, O.; Müller-Stich, B.P.; Hackert, T. Laparoscopic Versus Open Pancreaticoduodenectomy: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2020, 271, 54–66. [Google Scholar] [CrossRef]
- Van Hilst, J.; De Rooij, T.; Bosscha, K.; Brinkman, D.J.; Van Dieren, S.; Dijkgraaf, M.G.; Gerhards, M.F.; De Hingh, I.H.; Karsten, T.M.; Lips, D.J.; et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): A multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 199–207. [Google Scholar] [PubMed]
- Butturini, G.; Damoli, I.; Crepaz, L.; Malleo, G.; Marchegiani, G.; Daskalaki, D.; Esposito, A.; Cingarlini, S.; Salvia, R.; Bassi, C. A prospective non-randomised single-center study comparing laparoscopic and robotic distal pancreatectomy. Surg. Endosc. 2015, 29, 3163–3170. [Google Scholar] [PubMed]
- Souche, R.; Herrero, A.; Bourel, G.; Chauvat, J.; Pirlet, I.; Guillon, F.; Nocca, D.; Borie, F.; Mercier, G.; Fabre, J.-M. Robotic versus laparoscopic distal pancreatectomy: A French prospective single-center experience and cost-effectiveness analysis. Surg. Endosc. 2018, 32, 3562–3569. [Google Scholar] [PubMed]
- Guerra, F.; Checcacci, P.; Vegni, A.; Di Marino, M.; Annecchiarico, M.; Farsi, M.; Coratti, A. Surgical and oncological outcomes of our first 59 cases of robotic pancreaticoduodenectomy. J. Visc. Surg. 2019, 156, 185–190. [Google Scholar]
- Kim, H.S.; Han, Y.; Kang, J.S.; Kim, H.; Kim, J.R.; Koon, W.; Kim, S.-W.; Jang, J.-Y. Comparison of surgical outcomes between open and robot-assisted minimally invasive pancreaticoduodenectomy. J. Hepato-Biliary Pancreat. Sci. 2018, 25, 142–149. [Google Scholar]
- Liu, R.; Zhang, T.; Zhao, Z.-M.; Tan, X.-L.; Zhao, G.-D.; Zhang, X.; Xu, Y. The surgical outcomes of robot-assisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: A comparative study of a single center. Surg. Endosc. 2017, 31, 2380–2386. [Google Scholar]
- Casadei, R.; Ricci, C.; D’Ambra, M.; Marrano, N.; Alagna, V.; Rega, D.; Monari, F.; Minni, F. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: A case-control study. Updat. Surg. 2010, 62, 171–174. [Google Scholar]
- Zhang, J.; Jin, J.; Chen, S.; Gu, J.; Zhu, Y.; Qin, K.; Zhan, Q.; Cheng, D.; Chen, H.; Deng, X.; et al. Minimally invasive distal pancreatectomy for PNETs: Laparoscopic or robotic approach? Oncotarget 2017, 8, 33872–33883. [Google Scholar]
- Sciuto, A.; Abete, R.; Reggio, S.; Pirozzi, F.; Settembre, A.; Corcione, F. Laparoscopic spleen-preserving distal pancreatectomy for insulinoma: Experience of a single center. Int. J. Surg. 2014, 12 (Suppl. 1), S152–S155. [Google Scholar]
- Fernandezcruz, L.; Martinez, I.; Cesarborges, G.; Astudillo, E.; Orduna, D.; Halperin, I.; Sesmilo, G.; Puig, M. Laparoscopic surgery in patients with sporadic and multiple insulinomas associated with multiple endocrine neoplasia type 1. J. Gastrointest. Surg. 2005, 9, 381–388. [Google Scholar]
- Al-Kurd, A.; Chapchay, K.; Grozinsky-Glasberg, S.; Mazeh, H. Laparoscopic resection of pancreatic neuroendocrine tumors. World J. Gastroenterol. 2014, 20, 4908–4916. [Google Scholar] [PubMed]
- Brown, K.M.; Shoup, M.; Abodeely, A.; Hodul, P.; Brems, J.J.; Aranha, G.V. Central pancreatectomy for benign pancreatic lesions. HPB 2006, 8, 142–147. [Google Scholar] [PubMed]
- Goldstein, M.J.; Toman, J.; Chabot, J.A. Pancreaticogastrostomy: A novel application after central pancreatectomy. J. Am. Coll. Surg. 2004, 198, 871–876. [Google Scholar] [CrossRef]
- Reber, H.A. Middle pancreatectomy: Why I rarely do it. J. Gastrointest. Surg. 2007, 11, 730–732. [Google Scholar] [PubMed]
- Norton, J.A.; Cromack, D.T.; Shawker, T.H.; Doppman, J.L.; Comi, R.; Gorden, P.; Maton, P.N.; Gardner, J.D.; Jensen, R.T. Intraoperative ultrasonographic localization of islet cell tumors. A prospective comparison to palpation. Ann. Surg. 1988, 207, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.A.; Alexander, H.R.; Fraker, D.L.; Venzon, D.J.; Gibril, F.; Jensen, R.T. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases, or survival in patients with Zollinger-Ellison syndrome? Ann. Surg. 2004, 239, 617–625. [Google Scholar]
- Norton, J.A. Intraoperative methods to stage and localize pancreatic and duodenal tumors. Ann. Oncol. 1999, 10 (Suppl. 4), 182–184. [Google Scholar]
- Mehrabi, A.; Fischer, L.; Hafezi, M.; Dirlewanger, A.; Grenacher, L.; Diener, M.K.; Fonouni, H.; Golriz, M.; Garoussi, C.; Fard, N.; et al. A systematic review of localization, surgical treatment options, and outcome of insulinoma. Pancreas 2014, 43, 675–686. [Google Scholar]
- Guo, Q.; Wu, Y. Surgical treatment of pancreatic islet cell tumor: Report of 44 cases. Hepatogastroenterology 2013, 60, 2099–2102. [Google Scholar]
- Knigge, U.; Hansen, C.P. Surgery for GEP-NETs. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 819–831. [Google Scholar]
- Chiara, M.; Brunani, A.; Damascelli, B.; Tichà, V.; Castello, L.; Aimaretti, G.; Scacchi, M.; Paolo, M. Non-surgical ablative therapies for inoperable benign insulinoma. J. Endocrinol. Investig. 2018, 41, 153–162. [Google Scholar]
- Fischer, L.; Bergmann, F.; Schimmack, S.; Hinz, U.; Pries, S.; Müller-Stich, B.P.; Werner, J.; Hackert, T.; Buchler, M.W. Outcome of surgery for pancreatic neuroendocrine neoplasms. Br. J. Surg. 2014, 101, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Curran, T.; Pockaj, B.A.; Gray, R.J.; Halfdanarson, T.R.; Wasif, N. Importance of lymph node involvement in pancreatic neuroendocrine tumors: Impact on survival and implications for surgical resection. J. Gastrointest. Surg. 2015, 19, 152–160, discussion 60. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Gaujoux, S.; Boninsegna, L.; Cherif, R.; Crippa, S.; Couvelard, A.; Scarpa, A.; Ruszniewski, P.; Sauvanet, A.; Falconi, M. Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). JAMA Surg. 2013, 148, 932–939. [Google Scholar] [CrossRef]
- Guarneri, G.; de Mestier, L.; Landoni, L.; Partelli, S.; Gaujoux, S.; Andreasi, V.; Nessi, C.; Dokmak, S.; Fontana, M.; Dousset, B.; et al. Prognostic role of examined and positive lymph nodes after distal pancreatectomy for non-functioning neuroendocrine neoplasms. Neuroendocrinology 2020. [Google Scholar] [CrossRef]
- Tierney, J.F.; Kosche, C.; Schadde, E.; Ali, A.; Virmani, S.; Pappas, S.G.; Poirier, J.; Keutgen, X.M. (68)Gallium-DOTATATE positron emission tomography-computed tomography (PET CT) changes management in a majority of patients with neuroendocrine tumors. Surgery 2019, 165, 178–185. [Google Scholar] [CrossRef]
- Gao, H.; Liu, L.; Wang, W.; Xu, H.; Jin, K.; Wu, C.; Qi, Z.; Zhang, S.; Liu, C.; Xu, J.; et al. Novel recurrence risk stratification of resected pancreatic neuroendocrine tumor. Cancer Lett. 2018, 412, 188–193. [Google Scholar] [CrossRef]
- Sho, S.; Court, C.M.; Winograd, P.; Toste, P.A.; Pisegna, J.R.; Lewis, M.; Donahue, T.R.; Hines, O.J.; Reber, H.A.; Dawson, D.W.; et al. A Prognostic Scoring System for the Prediction of Metastatic Recurrence Following Curative Resection of Pancreatic Neuroendocrine Tumors. J. Gastrointest. Surg. 2019, 23, 1392–1400. [Google Scholar] [CrossRef]
- Genç, C.G.; Jilesen, A.P.; Partelli, S.; Falconi, M.; Muffatti, F.; Van Kemenade, F.J.; Van Eeden, S.; Verheij, J.; Van Dieren, S.; Van Eijck, C.H.J.; et al. A New Scoring System to Predict Recurrent Disease in Grade 1 and 2 Nonfunctional Pancreatic Neuroendocrine Tumors. Ann. Surg. 2018, 267, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Ausania, F.; Senra Del Rio, P.; Gomez-Bravo, M.A.; Martin-Perez, E.; Perez-Daga, J.A.; Dorcaratto, D.; González-Nicolás, T.; Sanchez-Cabus, S.; Tardio-Baiges, A. Can we predict recurrence in WHO G1-G2 pancreatic neuroendocrine neoplasms? Results from a multi-institutional Spanish study. Pancreatology 2019, 19, 367–371. [Google Scholar] [CrossRef]
- Marchegiani, G.; Landoni, L.; Andrianello, S.; Masini, G.; Cingarlini, S.; D’Onofrio, M.; De Robertis, R.; Davì, M.; Capelli, P.; Manfrin, E.; et al. Patterns of Recurrence after Resection for Pancreatic Neuroendocrine Tumors: Who, When, and Where? Neuroendocrinology 2019, 108, 161–171. [Google Scholar] [PubMed]
| Name | Symptoms | Secretion | Incidence New Case//Million/yr. | Location | Malignant | MEN-1 Context | Surgery | Procedure |
|---|---|---|---|---|---|---|---|---|
| Insulinoma | Whipple’s triad: Low blood sugar, presence of symptoms, and reversal of these symptoms when the glucose serum level is restored to normal Many other, like confusion, behavioral changes, visual troubles | insulin | 1–32 | Variable | <10% | 4–5% | Always | Sparing parenchymal pancreatectomy |
| Gastrinoma | Zollinger-Ellison syndrome: Gastric acid hypersecretion, severe peptic ulceration, profuse diarrhea | gastrin | 0.5–21.5 | Stabile & Passaro triangle | 60% | 20–25% | yes (unless MEN-1 gastrinoma < 2 cm) | Sparing parenchymal or standard pancreatectomy |
| Glucagonoma | Hyperglycemia, necrotic migratory erythema | glucagon | 0.01–0.1 | Variable | 50–80% | 1–20% | yes | Sparing parenchymal or standard pancreatectomy |
| Vipoma | WDHA syndrome Watery diarrhea, hypokalemia, acidosis | VIP | 0.05–0.2 | Variable | 60–80% | 6% | yes | Standard pancreatectomy |
| Somatostinoma | Pain, diabetes, diarrhea, gallstones | somatostatin | <0.02 | Variable | 70–92% | 45% | yes | Standard pancreatectomy |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souche, R.; Hobeika, C.; Hain, E.; Gaujoux, S. Surgical Management of Neuroendocrine Tumours of the Pancreas. J. Clin. Med. 2020, 9, 2993. https://doi.org/10.3390/jcm9092993
Souche R, Hobeika C, Hain E, Gaujoux S. Surgical Management of Neuroendocrine Tumours of the Pancreas. Journal of Clinical Medicine. 2020; 9(9):2993. https://doi.org/10.3390/jcm9092993
Chicago/Turabian StyleSouche, Regis, Christian Hobeika, Elisabeth Hain, and Sebastien Gaujoux. 2020. "Surgical Management of Neuroendocrine Tumours of the Pancreas" Journal of Clinical Medicine 9, no. 9: 2993. https://doi.org/10.3390/jcm9092993
APA StyleSouche, R., Hobeika, C., Hain, E., & Gaujoux, S. (2020). Surgical Management of Neuroendocrine Tumours of the Pancreas. Journal of Clinical Medicine, 9(9), 2993. https://doi.org/10.3390/jcm9092993






