Distinct Expression Patterns of VEGFR 1-3 in Gastroenteropancreatic Neuroendocrine Neoplasms: Supporting Clinical Relevance, but not a Prognostic Factor
Abstract
1. Introduction
2. Material and Methods
2.1. Patient Cohort
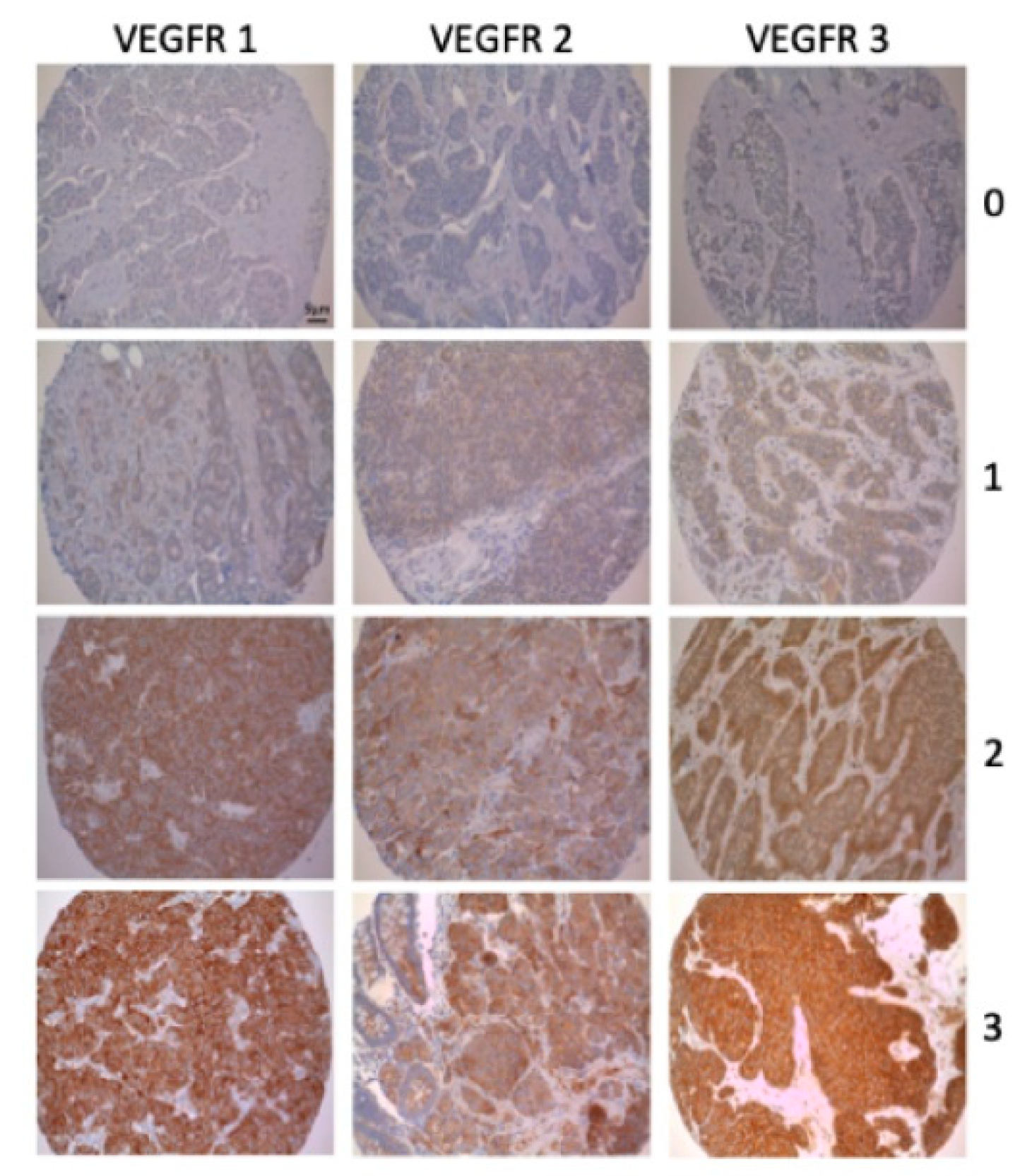
2.2. Tissue Microarrays and Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. General Clinicopathological Findings
3.2. Correlations
3.3. Survival Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GEP-NEN | Gastroenteropancreatic neuroendocrine neoplasm |
| NEC | Neuroendocrine carcinoma |
| PD-1 | Programmed death-1 |
| PDGFR | Platelet-derived growth factor receptor |
| PD-L1 | Programmed death ligand-1 |
| pNEN | Neuroendocrine neoplasm of the pancreas |
| si-NEN | Neuroendocrine neoplasm of the small intestine |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag. Res. 2018, 10, 5629–5638. [Google Scholar] [CrossRef] [PubMed]
- Modlin, I.M.; Oberg, K.; Chung, D.C.; Jensen, R.T.; de Herder, W.W.; Thakker, R.V.; Caplin, M.; Delle Fave, G.; Kaltsas, G.A.; Krenning, E.P.; et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008, 9, 61–72. [Google Scholar] [CrossRef]
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Oberg, K.; Steinmuller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R.; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Schurr, P.G.; Strate, T.; Rese, K.; Kaifi, J.T.; Reichelt, U.; Petri, S.; Kleinhans, H.; Yekebas, E.F.; Izbicki, J.R. Aggressive surgery improves long-term survival in neuroendocrine pancreatic tumors: An institutional experience. Ann. Surg. 2007, 245, 273–281. [Google Scholar] [CrossRef]
- Frilling, A.; Li, J.; Malamutmann, E.; Schmid, K.W.; Bockisch, A.; Broelsch, C.E. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. Br. J. Surg. 2009, 96, 175–184. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Cwikla, J.B.; Phan, A.T.; Raderer, M.; Sedlackova, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Rinke, A.; Muller, H.H.; Schade-Brittinger, C.; Klose, K.J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.F.; Blaker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.; Bazhin, A.V.; Heublein, S.; Bruwer, K.; Knosel, T.; Reiter, F.P.; Auernhammer, C.J.; Guba, M.O.; Spitzweg, C.; Werner, J.; et al. Treatment with somatostatin analogs induces differentially expressed let-7c-5p and mir-3137 in small intestine neuroendocrine tumors. BMC Cancer 2019, 19, 575. [Google Scholar] [CrossRef] [PubMed]
- Auernhammer, C.J.; Spitzweg, C.; Angele, M.K.; Boeck, S.; Grossman, A.; Nolting, S.; Ilhan, H.; Knosel, T.; Mayerle, J.; Reincke, M.; et al. Advanced neuroendocrine tumours of the small intestine and pancreas: Clinical developments, controversies, and future strategies. Lancet Diabetes Endocrinol. 2018, 6, 404–415. [Google Scholar] [CrossRef]
- Capdevila, J.; Hernando, J.; Perez-Hoyos, S.; Roman-Gonzalez, A.; Grande, E. Meta-Analysis of Randomized Clinical Trials Comparing Active Treatment with Placebo in Metastatic Neuroendocrine Tumors. Oncologist 2019, 24, e1315–e1320. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, B.M.; Tan, P.K.; Niederleithner, H.; Ferrara, N.; Petzelbauer, P.; Sibilia, M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 2010, 140, 268–279. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.; Uccella, S.; Finzi, G.; Albarello, L.; Sessa, F.; Capella, C. Localization of vascular endothelial growth factor and its receptors in digestive endocrine tumors: Correlation with microvessel density and clinicopathologic features. Hum. Pathol. 2003, 34, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Raymond, E.; Dahan, L.; Raoul, J.L.; Bang, Y.J.; Borbath, I.; Lombard-Bohas, C.; Valle, J.; Metrakos, P.; Smith, D.; Vinik, A.; et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N. Engl. J. Med. 2011, 364, 501–513. [Google Scholar] [CrossRef]
- Grillo, F.; Florio, T.; Ferrau, F.; Kara, E.; Fanciulli, G.; Faggiano, A.; Colao, A.; Group, N. Emerging multitarget tyrosine kinase inhibitors in the treatment of neuroendocrine neoplasms. Endocr. Relat. Cancer 2018, 25, R453–R466. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.H.L.C.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients With Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Finn Richard, Q.S.; Masafumi, I.; Peter, G.; Michel, D.; Tae-You, K.; Masatoshi, K.; Valeriy, B.; Philippe, M.; Ahmed, K.; Daneng, L.; et al. IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Kuiper, P.; Hawinkels, L.J.; de Jonge-Muller, E.S.; Biemond, I.; Lamers, C.B.; Verspaget, H.W. Angiogenic markers endoglin and vascular endothelial growth factor in gastroenteropancreatic neuroendocrine tumors. World J. Gastroenterol. 2011, 17, 219–225. [Google Scholar] [CrossRef]
- Pavel, M.E.; Hassler, G.; Baum, U.; Hahn, E.G.; Lohmann, T.; Schuppan, D. Circulating levels of angiogenic cytokines can predict tumour progression and prognosis in neuroendocrine carcinomas. Clin. Endocrinol. 2005, 62, 434–443. [Google Scholar] [CrossRef]
- Board WCoTE. Digestive System Tumours, 5th ed.; WHO Classification of Tumours: Geneva, Switzerland, 2019. [Google Scholar]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Knosel, T.; Emde, A.; Schluns, K.; Chen, Y.; Jurchott, K.; Krause, M.; Dietel, M.; Petersen, I. Immunoprofiles of 11 biomarkers using tissue microarrays identify prognostic subgroups in colorectal cancer. Neoplasia 2005, 7, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.; Bruwer, K.; Altendorf-Hofmann, A.; Auernhammer, C.J.; Spitzweg, C.; Westphalen, C.B.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Heinemann, V.; et al. Immune checkpoint markers in gastroenteropancreatic neuroendocrine neoplasia. Endocr. Relat. Cancer 2019, 26, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, E.; Altendorf-Hofmann, A.; Gibis, S.; Lindner, L.H.; Issels, R.; Kirchner, T.; Knosel, T. VEGFR2 predicts decreased patients survival in soft tissue sarcomas. Pathol. Res. Pract. 2015, 211, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Scharf, M.; Petry, V.; Daniel, H.; Rinke, A.; Gress, T.M. Bone Metastases in Patients with Neuroendocrine Neoplasm: Frequency and Clinical, Therapeutic, and Prognostic Relevance. Neuroendocrinology 2018, 160, 30–37. [Google Scholar] [CrossRef]
- Panzuto, F.; Puscedddu, S.; Faggiano, A.; Rinzivillo, M.; Brighi, N.; Prinzi, N.; Riccardi, F.; Iannicelli, E.; Maggio, I.; Femia, D.; et al. Prognostic impact of tumour burden in stage IV neuroendocrine neoplasia: A comparison between pancreatic and gastrointestinal localizations. Pancreatology 2019, 19, 1067–1073. [Google Scholar] [CrossRef]
- Terris, B.; Scoazec, J.Y.; Rubbia, L.; Bregeaud, L.; Pepper, M.S.; Ruszniewski, P.; Belghiti, J.; Flejou, J.; Degott, C. Expression of vascular endothelial growth factor in digestive neuroendocrine tumours. Histopathology 1998, 32, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Sartelet, H.; Decaussin, M.; Devouassoux, G.; Nawrocki-Raby, B.; Brichon, P.Y.; Brambilla, C.; Brambilla, E. Expression of vascular endothelial growth factor (VEGF) and its receptors (VEGF-R1 [Flt-1] and VEGF-R2 [KDR/Flk-1]) in tumorlets and in neuroendocrine cell hyperplasia of the lung. Hum. Pathol. 2004, 35, 1210–1217. [Google Scholar] [CrossRef]
- Petrova, T.V.; Makinen, T.; Alitalo, K. Signaling via vascular endothelial growth factor receptors. Exp. Cell Res. 1999, 253, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Berruti, A.; Fazio, N.; Ferrero, A.; Brizzi, M.P.; Volante, M.; Nobili, E.; Tozzi, L.; Bodei, L.; Torta, M.; D’Avolio, A.; et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: The XELBEVOCT study. BMC Cancer 2014, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.A.; Amoyal, N.; Nisotel, L.; Fishbein, J.N.; MacDonald, J.; Stagl, J.; Lennes, I.; Temel, J.S.; Safren, S.A.; Pirl, W.F. A Systematic Review of Adherence to Oral Antineoplastic Therapies. Oncologist 2016, 21, 354–376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, J. Cardiovascular Toxicities with Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer Patients: A Meta-Analysis of 77 Randomized Controlled Trials. Clin. Drug Investig. 2018, 38, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.T.; Halperin, D.M.; Chan, J.A.; Fogelman, D.R.; Hess, K.R.; Malinowski, P.; Regan, E.; Ng, C.S.; Yao, J.C.; Kulke, M.H. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: A multicentre, single-group, phase 2 study. Lancet Oncol. 2015, 16, 695–703. [Google Scholar] [CrossRef]
- Ng, C.S.; Wei, W.; Duran, C.; Ghosh, P.; Anderson, E.F.; Chandler, A.G.; Yao, J.C. CT perfusion in normal liver and liver metastases from neuroendocrine tumors treated with targeted antivascular agents. Abdom. Radiol. 2018, 43, 1661–1669. [Google Scholar] [CrossRef]
- D’Haene, N.; Koopmansch, C.; Van Eycke, Y.R.; Hulet, F.; Allard, J.; Bouri, S.; Rorive, S.; Remmelink, M.; Decaestecker, C.; Maris, C.; et al. The Prognostic Value of the Combination of Low VEGFR-1 and High VEGFR-2 Expression in Endothelial Cells of Colorectal Cancer. Int. J. Mol. Sci. 2018, 19, 3536. [Google Scholar] [CrossRef]
- Berardi, R.; Torniai, M.; Partelli, S.; Rubini, C.; Pagliaretta, S.; Savini, A.; Polenta, V.; Santoni, M.; Giampieri, R.; Onorati, S.; et al. Impact of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) single nucleotide polymorphisms on outcome in gastroenteropancreatic neuroendocrine neoplasms. PLoS ONE 2018, 13, e0197035. [Google Scholar] [CrossRef] [PubMed]
- Bowen, K.A.; Silva, S.R.; Johnson, J.N.; Doan, H.Q.; Jackson, L.N.; Gulhati, P.; Qiu, S.; Riall, T.S.; Evers, B.M. An Analysis of Trends and Growth Factor Receptor Expression of GI Carcinoid Tumors. J. Gastrointest. Surg. 2009, 13, 1773–1780. [Google Scholar] [CrossRef]
- Nakayama, T.; Cho, Y.C.; Mine, Y.; Yoshizaki, A.; Naito, S.; Wen, C.Y.; Sekine, I. Expression of vascular endothelial growth factor and its receptors VEGFR-1 and 2 in gastrointestinal stromal tumors, leiomyomas and schwannomas. World J. Gastroenterol. 2006, 12, 6182–6187. [Google Scholar] [CrossRef]
- Besig, S.; Voland, P.; Baur, D.M.; Perren, A.; Prinz, C. Vascular Endothelial Growth Factors, Angiogenesis, and Survival in Human Ileal Enterochromaffin Cell Carcinoids. Neuroendocrinology 2008, 90, 402–415. [Google Scholar] [CrossRef]
- Casanovas, O.; Hicklin, D.J.; Bergers, G.; Hanahan, D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 2005, 8, 299–309. [Google Scholar] [CrossRef]
- Kulke, M.H.; Lenz, H.J.; Meropol, N.J.; Posey, J.; Ryan, D.P.; Picus, J.; Bergsland, E.; Stuart, K.; Tye, L.; Huang, X.; et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2008, 26, 3403–3410. [Google Scholar] [CrossRef]
- Kasuya, K.; Nagakawa, Y.; Suzuki, M.; Suzuki, Y.; Kyo, B.; Suzuki, S.; Matsudo, T.; Itoi, T.; Tsuchida, A.; Aoki, T. Combination therapy of gemcitabine or oral S-1 with the anti-VEGF monoclonal antibody bevacizumab for pancreatic neuroendocrine carcinoma. Exp. Ther. Med. 2012, 3, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Stuart, K.; Earle, C.C.; Clark, J.W.; Bhargava, P.; Miksad, R.; Blaszkowsky, L.; Enzinger, P.C.; Meyerhardt, J.A.; Zheng, H.; et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J. Clin. Oncol. 2012, 30, 2963–2968. [Google Scholar] [CrossRef] [PubMed]
- Hadoux, J.; Ducreux, M. Bevacizumab and subtype-adapted chemotherapy backbone in neuroendocrine tumors. J. Clin. Oncol. 2013, 31, 976–977. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.; Todorova, R.; Link, H.; Westphalen, C.B.; Boeck, S.; Heinemann, V.; Werner, J.; Kirchner, T.; Angele, M.K.; Neumann, J. Molecular subtyping of gastric cancer with respect to the growth pattern of lymph-node metastases. J. Cancer Res. Clin. Oncol. 2019, 145, 2689–2697. [Google Scholar] [CrossRef]
- Auernhammer, C.J.; Spitzweg, C.; Bock, S.; Knosel, T.; Bartenstein, P. Current standards and novel developments in the treatment of neuroendocrine tumors of the gastroenteropancreatic system. Dtsch. Med. Wochenschr. 2019, 144, 1390–1395. [Google Scholar] [CrossRef]
- Fazio, N.; Cella, C.A.; Del Re, M.; Laffi, A.; Rubino, M.; Zagami, P.; Spada, F. Pharmacodynamics, clinical findings and approval status of current and emerging tyrosine-kinase inhibitors for pancreatic neuroendocrine tumors. Expert Opin. Drug Metab. Toxicol. 2019, 15, 993–1004. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Cives, M.; Hwang, J.; Weber, T.; Nickerson, M.; Atreya, C.E.; Venook, A.; Kelley, R.K.; Valone, T.; Morse, B.; et al. A phase II study of axitinib in advanced neuroendocrine tumors. Endocr. Relat. Cancer 2016, 23, 411–418. [Google Scholar] [CrossRef]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Weber, M.M.; Fottner, C. Immune Checkpoint Inhibitors in the Treatment of Patients with Neuroendocrine Neoplasia. Oncol. Res. Treat. 2018, 41, 306–312. [Google Scholar] [CrossRef]
- Chauhan, A.; Arnold, S.N.; Wu, J.; Nair, R.; Slone, S.; Dressler, E.; Flynn, H.; Adams, V.; Weiss, H.; Evers, M.; et al. Clinical Efficacy and Toxicity Data on Phase 1 Study of Fosbretabulin in Combination with Everolimus in Neuroendocrine Tumors. NANETS 2019 Symp. Abstr. 2019, C16, 4114. [Google Scholar] [CrossRef]

| Patient Characteristics | n | % |
|---|---|---|
| Gender | ||
| Male | 137 | 55 |
| Female | 112 | 45 |
| Localization | ||
| Esophagus/stomach | 16 | 6.4 |
| Small intestine | 126 | 50.6 |
| Colon | 18 | 7.2 |
| Rectum | 8 | 3.2 |
| Papilla vateri | 1 | 0.4 |
| Pancreas | 64 | 25.7 |
| Appendix | 16 | 6.4 |
| VEGFR 1 | ||
| No/low expression | 102 | 41 |
| High expression | 147 | 59 |
| VEGFR 2 | ||
| No/low expression | 233 | 93.6 |
| High expression | 16 | 6.4 |
| VEGFR 3 | ||
| No/low expression | 95 | 38.2 |
| High expression | 154 | 61.8 |
| PD-L1 (1) | ||
| Low expression | 218 | 91.2 |
| High expression | 21 | 8.8 |
| PD-1 (2) | ||
| Low expression | 188 | 83.2 |
| High expression | 38 | 16.8 |
| Grading | ||
| G1 | 152 | 61 |
| G2 | 81 | 32.5 |
| G3 | 16 | 6.4 |
| Surgical resection (3) | ||
| Yes | 229 | 92 |
| No | 20 | 8 |
| Localization (n) | VEGFR 1 High n (%) | VEGFR 2 High n (%) | VEGFR 3 High n (%) |
|---|---|---|---|
| Esophagus/stomach (16) | 6 (37.5) | 4 (20) | 11 (68.8) |
| Small intestine (126) | 74 (58.7) | 3 (2.4) | 64 (50.8) |
| Colon (18) | 5 (28.7) | 3 (16.7) | 11 (61.1) |
| Rectum (8) | 6 (75) | 1 (12.5) | 7 (87.5) |
| Papilla vateri (1) | 0 (0) | 0 (0) | 1 (100) |
| Pancreas (64) | 52 (81.3) | 5 (7.8) | 52 (81.3) |
| Appendix (16) | 4 (25) | 0 (0) | 8 (50) |
| VEGFR 1 high | n.a. | 9 (6.1) | 121 (82.3) |
| VEGFR 2 high | 9 (6.1) | n.a. | 15 (93.8) |
| PD-1 Low Expression n (%) | PD-1 High Expression n (%) | p-Value | |
|---|---|---|---|
| VEGFR 1 | 74 | 17 | 0.588 |
| no/low expression | 81.3% | 18.7% | |
| VEGFR 1 | 114 | 21 | |
| high expression | 84.4% | 15.6% | |
| VEGFR 2 | 183 | 32 | 0.004 |
| no/low expression | 85.1% | 14.9% | |
| VEGFR 2 | 5 | 6 | |
| high expression | 45.5% | 54.5% | |
| VEGFR 3 | 79 | 13 | 0.47 |
| no/low expression | 85.9% | 14.1% | |
| VEGFR 3 | 109 | 25 | |
| high expression | 81.3% | 18.7% |
| PD-L1 Low Expression n (%) | PD-L1 High Expression n (%) | p-Value | |
|---|---|---|---|
| VEGFR 1 | 89 | 7 | 0.643 |
| no/low expression | 92.7% | 7.3% | |
| VEGFR 1 | 129 | 14 | |
| high expression | 90.2% | 9.8% | |
| VEGFR 2 | 209 | 18 | 0.077 |
| no/low expression | 92.1% | 7.9% | |
| VEGFR 2 | 9 | 3 | |
| high expression | 75.0% | 25.0% | |
| VEGFR 3 | 91 | 1 | 0.001 |
| no/low expression | 98.9% | 1.1% | |
| VEGFR 3 | 127 | 20 | |
| high expression | 86.4% | 13.6% |
| p-Value | Exp (B) | CI 95% | |
|---|---|---|---|
| Distant metastases | 0.042 | 2.09 | 1.029–4.246 |
| Grading | 0.000 | 1.036 | 1.024–1.048 |
| Bone metastases | 0.006 | 3.242 | 1.398–7.516 |
| Liver metastases | 0.542 | 1.185 | 0.687–2.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bösch, F.; Altendorf-Hofmann, A.; Jacob, S.; Auernhammer, C.J.; Spitzweg, C.; Boeck, S.; Schubert-Fritschle, G.; Werner, J.; Kirchner, T.; Angele, M.K.; et al. Distinct Expression Patterns of VEGFR 1-3 in Gastroenteropancreatic Neuroendocrine Neoplasms: Supporting Clinical Relevance, but not a Prognostic Factor. J. Clin. Med. 2020, 9, 3368. https://doi.org/10.3390/jcm9103368
Bösch F, Altendorf-Hofmann A, Jacob S, Auernhammer CJ, Spitzweg C, Boeck S, Schubert-Fritschle G, Werner J, Kirchner T, Angele MK, et al. Distinct Expression Patterns of VEGFR 1-3 in Gastroenteropancreatic Neuroendocrine Neoplasms: Supporting Clinical Relevance, but not a Prognostic Factor. Journal of Clinical Medicine. 2020; 9(10):3368. https://doi.org/10.3390/jcm9103368
Chicago/Turabian StyleBösch, Florian, Annelore Altendorf-Hofmann, Sven Jacob, Christoph J. Auernhammer, Christine Spitzweg, Stefan Boeck, Gabriele Schubert-Fritschle, Jens Werner, Thomas Kirchner, Martin K. Angele, and et al. 2020. "Distinct Expression Patterns of VEGFR 1-3 in Gastroenteropancreatic Neuroendocrine Neoplasms: Supporting Clinical Relevance, but not a Prognostic Factor" Journal of Clinical Medicine 9, no. 10: 3368. https://doi.org/10.3390/jcm9103368
APA StyleBösch, F., Altendorf-Hofmann, A., Jacob, S., Auernhammer, C. J., Spitzweg, C., Boeck, S., Schubert-Fritschle, G., Werner, J., Kirchner, T., Angele, M. K., & Knösel, T. (2020). Distinct Expression Patterns of VEGFR 1-3 in Gastroenteropancreatic Neuroendocrine Neoplasms: Supporting Clinical Relevance, but not a Prognostic Factor. Journal of Clinical Medicine, 9(10), 3368. https://doi.org/10.3390/jcm9103368





