Clinical Features and Chest Imaging as Predictors of Intensity of Care in Patients with COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Level of Care Definition
2.3. Ethics Statement
2.4. Data Collection
2.5. Radiological Evaluation
2.6. Ultrasound Evaluation
2.7. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics at Baseline
3.2. Radiological Features on Admission
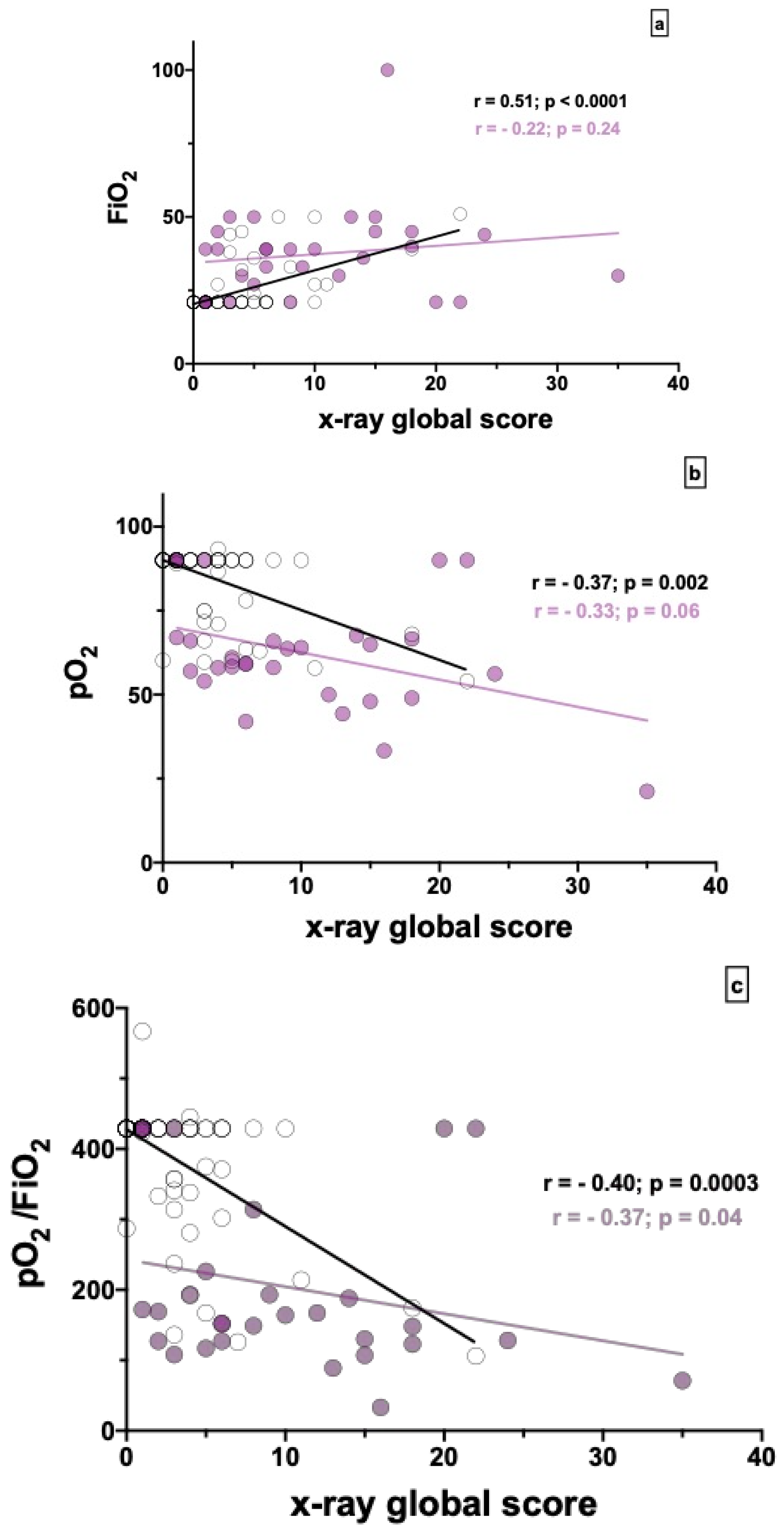
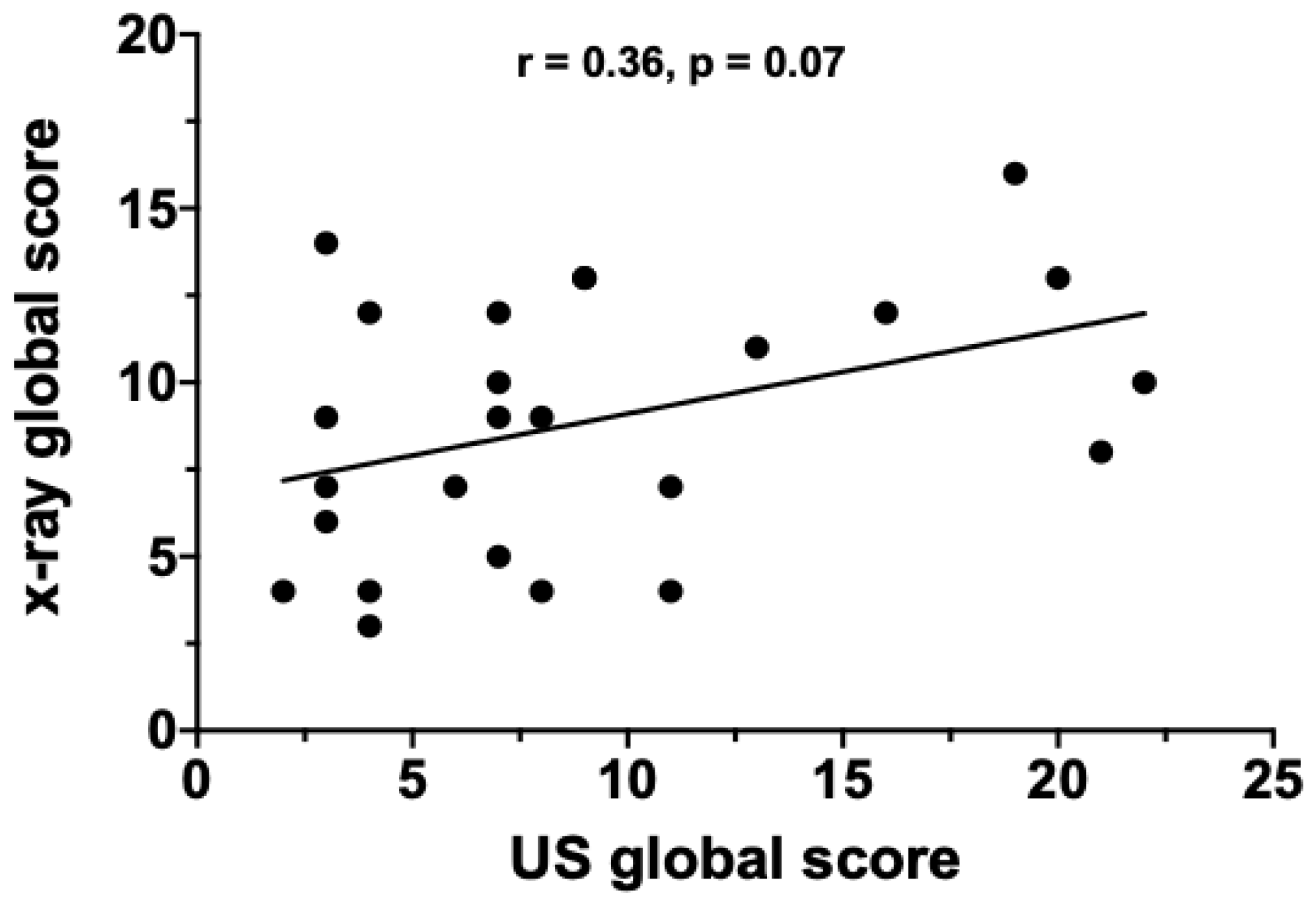
3.3. Radiological Correlations
3.4. Predictors of Level of Care Requirement
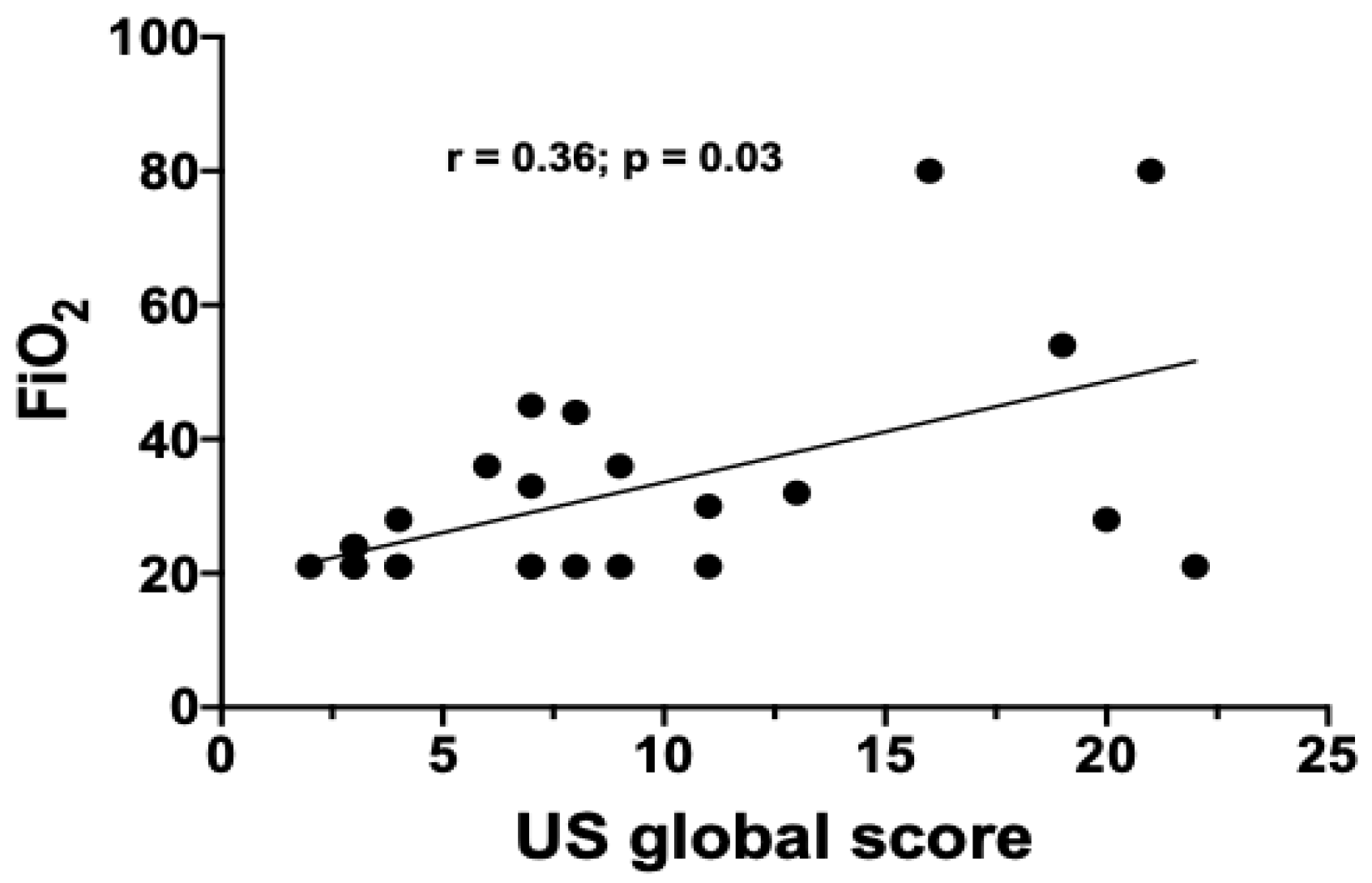
3.5. Ultrasound Evaluation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus type 2 |
| ICU | Intensive care unit |
| CT | computed tomography |
| CXR | chest X-ray |
| US | ultrasound |
| HFNC | high-flow nasal cannula |
| LIMC | low-intensity medical care |
| HIMC | high-intensity medical care |
| GGO— | ground glass opacities |
| CO | consolidations |
| CVD | cardiovascular diseases |
References
- Imperial College COVID-19 Response Team; Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020, 584, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Litmanovich, D.E.; Chung, M.; Kirkbride, R.R.; Kicska, G.; Kanne, J.P. Review of Chest Radiograph Findings of COVID-19 Pneumonia and Suggested Reporting Language. J. Thorac. Imaging 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Toussie, D.; Voutsinas, N.; Finkelstein, M.; Cedillo, M.A.; Manna, S.; Maron, S.Z.; Jacobi, A.; Chung, M.; Bernheim, A.; Eber, C.; et al. Clinical and Chest Radiography Features Determine Patient Outcomes In Young and Middle Age Adults with COVID-19. Radiology 2020, 201754. [Google Scholar] [CrossRef]
- Minns, F.C.; Mhuineachain, A.N.; Van Beek, E.J.R.; Ritchie, G.; Hill, A.; Murchison, J.T. Presenting CXR Phenotype of H1N1. Flu Compared with Contemporaneous Non-H1N1, Community Acquired Pneumonia, during Pandemic and Post-Pandemic Outbreaks’. Eur. J. Radiol. 2015, 84, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, D.; Albanesi, M.; Cavigli, E.; Moroni, C.; Bindi, A.; Luvarà, S.; Lucarini, S.; Busoni, S.; Mazzoni, L.N.; Miele, V. Chest X-ray in new Coronavirus Disease 2019 (COVID-19) infection: Findings and correlation with clinical outcome. Radiol. Med. 2020, 125, 730–737. [Google Scholar] [CrossRef]
- Wong, H.Y.F.; Lam, H.Y.S.; Fong, A.H.T.; Leung, S.T.; Chin, T.W.Y.; Lo, C.S.Y.; Lee, E.Y.P. Frequency and Distribution of Chest Radiographic Findings in COVID-19 Positive Patients Authors. Radiology 2020, 296, 2. [Google Scholar] [CrossRef] [Green Version]
- Rubin, G.D.; Ryerson, C.J.; Haramati, L.B.; Sverzellati, N.; Kanne, J.P.; Raoof, S.; Schluger, N.W.; Volpi, A.; Yim, J.-J.; Martin, I.B.; et al. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic. Chest 2020, 158, 106–116. [Google Scholar] [CrossRef]
- Orso, D.; Guglielmo, N.; Copetti, R. Lung ultrasound in diagnosing pneumonia in the emergency department: A Systematic Review and Meta-Analysis. Eur. J. Emerg. Med. 2018, 25, 312–321. [Google Scholar] [CrossRef]
- Gargani, L.; Soliman-Aboumarie, H.; Volpicelli, G.; Corradi, F.; Pastore, M.C.; Cameli, M. Why, when, and how to use lung ultrasound during the COVID-19 pandemic: Enthusiasm and caution. Eur. Hear. J. Cardiovasc. Imaging 2020, 21, 941–948. [Google Scholar] [CrossRef]
- Pierce, C.W. Clarifying the role of lung ultrasonography in COVID-19 respiratory disease. Can. Med. Assoc. J. 2020, 192, E436. [Google Scholar] [CrossRef] [Green Version]
- See, K.C.; Ong, V.; Tan, Y.L.; Sahagun, J.; Taculod, J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: A retrospective observational study. Crit. Care 2018, 22, 203. [Google Scholar] [CrossRef] [Green Version]
- Borghesi, A.; Maroldi, R. COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol. Med. 2020, 125, 509–513. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrera, L.; Rao, F.; Senarega, R.; Ferrari-Bravo, M. Chest radiological findings of influenza A H1N1 pneumonia. Rev. Port. Pneumol. 2012, 18, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Giraudo, C.; Cavaliere, A.; Fichera, G. Validation of a composed COVID-19 chest x-rAy scoRE: The CARE project. Eur. Respir. J. Open Res. 2020, in press. [Google Scholar]
- Smith, M.J.; Hayward, S.A.; Innes, S.; Miller, A.S.C. Point-of-Care Lung Ultrasound in Patients with COVID-19 –a Narrative Review. Anaesthesia 2020. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019-Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. SSRN Electron. J. 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Vardavas, C.I.; Nikitara, K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020, 18, 20. [Google Scholar] [CrossRef]
- Smith, J.C.; Sausville, E.L.; Girish, V.; Lou Yuan, M.; Vasudevan, A.; John, M.K.; Sheltzer, J.M. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev. Cell 2020, 53, 514–529.e3. [Google Scholar] [CrossRef]
- Huiart, L.; Ernst, P.; Suissa, S. Cardiovascular Morbidity and Mortality in COPD. Chest 2005, 128, 2640–2646. [Google Scholar] [CrossRef] [Green Version]
- Curkendall, S.M.; Lanes, S.; De Luise, C.; Stang, M.R.; Jones, J.; She, D.; Goehring, E. Chronic obstructive pulmonary disease severity and cardiovascular outcomes. Eur. J. Epidemiol. 2006, 21, 803–813. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of Comorbidities and Its Effects in Coronavirus Disease 2019 Patients: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Polverino, F.; Stern, D.A.; Ruocco, G. Comorbidities, cardiovascular therapies and COVID-19 Mortality: A Nationwide, Italian Observational Study (ItaliCO). Front. Cardiovasc. Med. 2020, 7, 170. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J. Ultrasound 2017, 20, 91–96. [Google Scholar] [CrossRef]
- Brogi, E.; Bignami, E.; Sidoti, A.; Shawar, M.; Gargani, L.; Vetrugno, L.; Volpicielli, G.; Forfori, F. Could the Use of Bedside Lung Ultrasound Reduce the Number of Chest X-Rays in the Intensive Care Unit? Cardiovasc. Ultrasound 2017, 15, 1–5. [Google Scholar] [CrossRef]
- Møller-Sørensen, H.; Gjedsted, J.; Jørgensen, V.L.; Hansen, K.L. COVID-19 Assessment with Bedside Lung Ultrasound in a Population of Intensive Care Patients Treated with Mechanical Ventilation and ECMO. Diagnostics 2020, 10, 447. [Google Scholar] [CrossRef]
- Soldati, G.; Smargiassi, A.; Inchingolo, R.; Buonsenso, D.; Perrone, T.; Briganti, D.F.; Perlini, S.; Torri, E.; Mariani, A.; Mossolani, E.E.; et al. Is There a Role for Lung Ultrasound During the COVID -19 Pandemic? J. Ultrasound Med. 2020, 39, 1459–1462. [Google Scholar] [CrossRef] [Green Version]
- Volpicelli, G.; Gargani, L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J. 2020, 12, 1–3. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Liu, Y.; Zhang, Y.; Zheng, C.; Zheng, Y.; Zhang, C.; Min, W.; Zhou, H.; Yu, M.; et al. A Preliminary Study on the Ultrasonic Manifestations of Peripulmonary Lesions of Non-Critical Novel Coronavirus Pneumonia (COVID-19). SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Nazerian, P.; Cerini, G.; Vanni, S.; Gigli, C.; Zanobetti, M.; Bartolucci, M.; Grifoni, S.; Volpicelli, G. Diagnostic accuracy of lung ultrasonography combined with procalcitonin for the diagnosis of pneumonia: A pilot study. Crit. Ultrasound J. 2016, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggiali, E.; Dacrema, A.; Bastoni, D.; Tinelli, V.; Demichele, E.; Ramos, P.; Marciano, T.; Silva, M.; Vercelli, A.; Magnacavallo, A. Can Lung US Help Critical Care Clinicians in the Early Diagnosis of Novel Coronavirus (COVID-19) Pneumonia? Radiology 2020, 295, E6. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.Y.; Wang, X.T.; Zhang, L.N. Findings of Lung Ultrasonography of Novel Corona Virus Pneumonia during the 2019–2020 Epidemic. Intensive Care Med. 2020, 46, 849–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Overall Population | Low-Intensity Medical Care (LIMC) | High-Intensity Medical Care (HIMC) | p Value | |
|---|---|---|---|---|
| (n = 102) | (n = 71) | (n = 31) | ||
| Male—n (%) | 75 (73) | 48 (67) | 27 (87) | 0.05 |
| Age at admission—years | 68 (22–94) | 63 (22–94) | 74 (28–85) | 0.03 |
| Smoking history—pack years | 0 (0–60) | 0 (0–60) | 10 (0–60) | 0.01 |
| 9 (9) | 8 (11) | 1 (3) | 0.18 |
| 43 (42) | 24 (34) | 19 (61) | 0.009 |
| 50 (49) | 41 (57) | 9 (29) | 0.007 |
| BMI (kg/m2) | 25 (16–43) | 24 (16–31) | 31 (21–43) | 0.02 |
| Lag time symptoms—diagnosis—days | 4 (−4–23) | 3 (−4–23) | 6 (−2–22) | 0.07 |
| FiO2 at admission (room air)—% | 21 (21–100) | 21 (21–51) | 39 (21–100) | <0.0001 |
| pO2 at admission (room air)—mmHg | 90 (21.2–119) | 90 (54–119) | 60 (21–90) | <0.0001 |
| P/F at admission—value | 429 (33–567) | 429 (106–567) | 158 (33–429) | <0.0001 |
| Hospitalization—days | 10.5 (2–119) | 8 (2–50) | 26 (7–119) | <0.0001 |
| Bacterial co-infections—n (%) | 24 (23) | 11 (15) | 13 (42) | 0.002 |
| Comorbidities | ||||
| 60 (59) | 35 (49) | 25 (80) | 0.002 |
| 18 (18) | 11 (15) | 7 (22) | 0.39 |
| 12 (12) | 10 (14) | 2 (6) | 0.34 |
| 45 (44) | 26 (37) | 19 (61) | 0.002 |
| 13 (13) | 6 (8) | 7 (22) | 0.05 |
| Death—n (%) | 6 (6) | 1 (1) | 4 (13) | 0.01 |
| Overall Population | Low-Intensity Medical Care (LIMC) | High-Intensity Medical Care (HIMC) | p Value | |
|---|---|---|---|---|
| (n = 102) | (n = 71) | (n = 31) | ||
| X-ray global score (GGO + consolidations) | 3 (0–35) | 3 (0–22) | 8 (0–35) | <0.0001 |
| GGO—score | 2 (0–18) | 1 (0–18) | 5 (0–15) | <0.0001 |
| Consolidation—score | 0 (0–35) | 0 (0–10) | 0 (0–35) | 0.02 |
| Normal—n (%) | 15 (15) | 14 (20) | 1 (3) | 0.003 |
| GGO prevalent—n (%) | 66 (65) | 44 (62) | 22 (71) | 0.38 |
| Consolidation prevalent—n (%) | 15 (15) | 11 (16) | 4 (13) | 0.73 |
| Mixed—n (%) | 6 (6) | 2 (3) | 4 (13) | 0.04 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% IC) | p | OR (95% IC) | p | |
| Sex (male vs. female) | 3.23 (1.01–11.89) | 0.04 | 0.54 (0.06–4.22) | 0.55 |
| Age (yr, ≥ 68 vs. < 68) | 3.34 (1.38–8.61) | 0.009 | 0.51 (0.06–3.03) | 0.49 |
| Smoking history (p/y, > 0 vs. ≤ 0) | 2.72 (1.08–7.27) | 0.03 | 6.55 (1.15–52.09) | 0.04 |
| FiO2 at admission (%, > 21 vs. ≤ 21) | 13.1 (4.92–39.2) | <0.0001 | 4.17 (0.60–29.89) | 0.14 |
| pO2 at admission (room air) (mmHg, < 90, ≥ 90) | 13 (4.78–40.4) | <0.0001 | 36.7 (3.64–681.4) | 0.005 |
| Lag time symptoms—diagnosis—(days, ≥ 4 vs. < 4) | 2.18 (0.90–5.50) | 0.08 | – | – |
| P/F at admission (≥ 429 vs. < 429) | 9.60 (3.59–29.26) | <0.0001 | 16.61 (3.34–128.3) | 0.002 |
| Bacterial co-infections (yes vs. no) | 4.64 (1.75–12.72) | 0.002 | 2.48 (0.38–17.78) | 0.34 |
| CVDs—(yes vs. no) | 5.14 (1.89–16.6) | 0.002 | 10.89 (1.44–112.0) | 0.02 |
| Respiratory diseases—(yes vs. no) | 5.14 (1.89–16.6) | 0.34 | – | – |
| Autoimmune diseases—(yes vs. no) | 0.43 (0.06–1.79) | 0.30 | – | – |
| Metabolic diseases—(yes vs. no) | 2.99 (1.25–7.44) | 0.01 | 2.63 (0.54–14.76) | 0.24 |
| Oncologic diseases—(yes vs. no) | 3.29 (1.00–11.25) | 0.04 | 17.13 (1.76–242.6) | 0.02 |
| X-ray global score (> 3 vs. < 3) | 3.33 (1.32–9.29) | 0.01 | 0.40 (0.02–3.63) | 0.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocconcelli, E.; Biondini, D.; Giraudo, C.; Lococo, S.; Bernardinello, N.; Fichera, G.; Barbiero, G.; Castelli, G.; Cavinato, S.; Ferrari, A.; et al. Clinical Features and Chest Imaging as Predictors of Intensity of Care in Patients with COVID-19. J. Clin. Med. 2020, 9, 2990. https://doi.org/10.3390/jcm9092990
Cocconcelli E, Biondini D, Giraudo C, Lococo S, Bernardinello N, Fichera G, Barbiero G, Castelli G, Cavinato S, Ferrari A, et al. Clinical Features and Chest Imaging as Predictors of Intensity of Care in Patients with COVID-19. Journal of Clinical Medicine. 2020; 9(9):2990. https://doi.org/10.3390/jcm9092990
Chicago/Turabian StyleCocconcelli, Elisabetta, Davide Biondini, Chiara Giraudo, Sara Lococo, Nicol Bernardinello, Giulia Fichera, Giulio Barbiero, Gioele Castelli, Silvia Cavinato, Anna Ferrari, and et al. 2020. "Clinical Features and Chest Imaging as Predictors of Intensity of Care in Patients with COVID-19" Journal of Clinical Medicine 9, no. 9: 2990. https://doi.org/10.3390/jcm9092990
APA StyleCocconcelli, E., Biondini, D., Giraudo, C., Lococo, S., Bernardinello, N., Fichera, G., Barbiero, G., Castelli, G., Cavinato, S., Ferrari, A., Saetta, M., Cattelan, A., Spagnolo, P., & Balestro, E. (2020). Clinical Features and Chest Imaging as Predictors of Intensity of Care in Patients with COVID-19. Journal of Clinical Medicine, 9(9), 2990. https://doi.org/10.3390/jcm9092990





