Outcomes of Inhaled Amikacin and Clofazimine-Containing Regimens for Treatment of Refractory Mycobacterium avium Complex Pulmonary Disease
Abstract
:1. Introduction
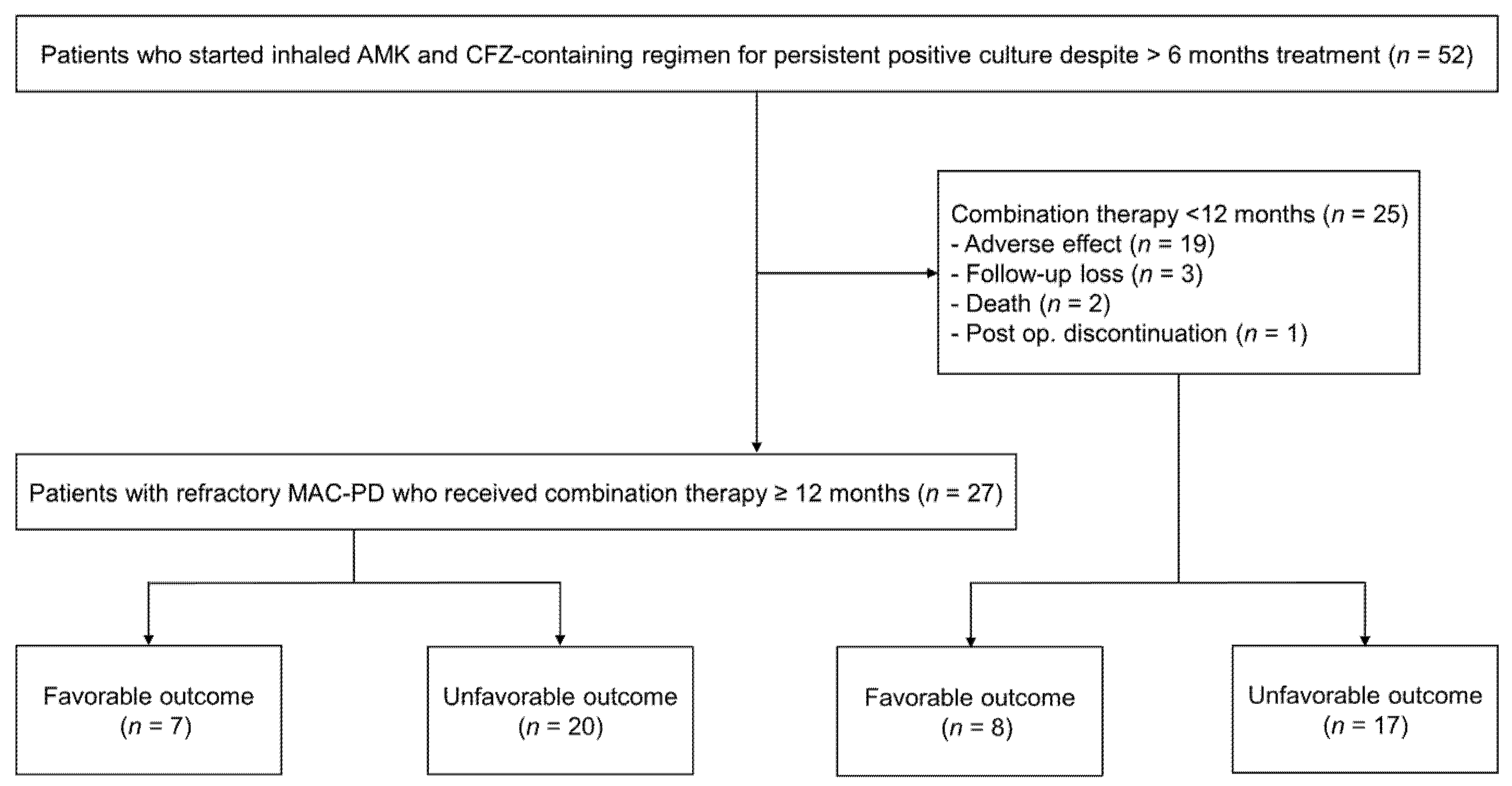
2. Materials and Methods
2.1. Study Patients
2.2. Inhaled AMK and CFZ Therapy
2.3. Assessment of Symptomatic, Radiological, and Microbiological Response
2.4. Evaluation of Treatment Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Antibiotic Treatment Regimens
3.3. Treatment Response of Study Patients
3.4. Adverse Effects Associated with Inhaled AMK and CFZ
3.5. Factors Associated with a Favorable Outcome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Prevots, D.R.; Marras, T.K. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clin. Chest Med. 2015, 36, 13–34. [Google Scholar]
- Lee, H.; Myung, W.; Koh, W.J.; Moon, S.M.; Jhun, B.W. Epidemiology of nontuberculous mycobacterial infection, South Korea, 2007–2016. Emerg. Infect. Dis. 2019, 25, 569–572. [Google Scholar]
- Prevots, D.R.; Shaw, P.A.; Strickland, D.; Jackson, L.A.; Raebel, M.A.; Blosky, M.A.; Montes de Oca, R.; Shea, Y.R.; Seitz, A.E.; Holland, S.M.; et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am. J. Respir. Crit. Care Med. 2010, 182, 970–976. [Google Scholar]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar]
- Daley, C.L.; Iaccarino, J.M.; Lange, C.; Cambau, E.; Wallace, R.J.J.; Andrejak, C.; Böttger, E.C.; Brozek, J.; Griffith, D.E.; Guglielmetti, L.; et al. Treatment of nontuberculous mycobacterial pulmonary disease: An official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur. Respir. J. 2020, 56, 2000535. [Google Scholar]
- Xu, H.B.; Jiang, R.H.; Li, L. Treatment outcomes for Mycobacterium avium complex: A systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 347–358. [Google Scholar]
- Kwak, N.; Park, J.; Kim, E.; Lee, C.H.; Han, S.K.; Yim, J.J. Treatment outcomes of Mycobacterium avium complex lung disease: A systematic review and meta-analysis. Clin. Infect. Dis. 2017, 65, 1077–1084. [Google Scholar]
- Pasipanodya, J.G.; Ogbonna, D.; Deshpande, D.; Srivastava, S.; Gumbo, T. Meta-analyses and the evidence base for microbial outcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J. Antimicrob. Chemother. 2017, 72 (Suppl. 2), i3–i19. [Google Scholar]
- Diel, R.; Nienhaus, A.; Ringshausen, F.C.; Richter, E.; Welte, T.; Rabe, K.F.; Loddenkemper, R. Microbiologic outcome of interventions against Mycobacterium avium complex pulmonary disease: A systematic review. Chest 2018, 153, 888–921. [Google Scholar]
- Haworth, C.S.; Banks, J.; Capstick, T.; Fisher, A.J.; Gorsuch, T.; Laurenson, I.F.; Leitch, A.; Loebinger, M.R.; Milburn, H.J.; Nightingale, M.; et al. British thoracic society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017, 72 (Suppl 2), ii1–ii64. [Google Scholar]
- Floto, R.A.; Olivier, K.N.; Saiman, L.; Daley, C.L.; Herrmann, J.L.; Nick, J.A.; Noone, P.G.; Bilton, D.; Corris, P.; Gibson, R.L.; et al. US cystic fibrosis foundation and european cystic fibrosis society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016, 71 (Suppl 1), i1–i22. [Google Scholar]
- Jhun, B.W.; Yang, B.; Moon, S.M.; Lee, H.; Park, H.Y.; Jeon, K.; Kwon, O.J.; Ahn, J.; Moon, I.J.; Shin, S.J.; et al. Amikacin inhalation as salvage therapy for refractory nontuberculous mycobacterial lung disease. Antimicrob. Agents Chemother. 2018, 62, e00011-18. [Google Scholar]
- Martiniano, S.L.; Wagner, B.D.; Levin, A.; Nick, J.A.; Sagel, S.D.; Daley, C.L. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial Infection. Chest 2017, 152, 800–809. [Google Scholar]
- Griffith, D.E.; Eagle, G.; Thomson, R.; Aksamit, T.R.; Hasegawa, N.; Morimoto, K.; Addrizzo-Harris, D.J.; O’Donnell, A.E.; Marras, T.K.; Flume, P.A.; et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium Complex (CONVERT). A prospective, open-label, randomized study. Am. J. Respir. Crit. Care Med. 2018, 198, 1559–1569. [Google Scholar]
- Van Ingen, J.; Totten, S.E.; Helstrom, N.K.; Heifets, L.B.; Boeree, M.J.; Daley, C.L. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob. Agents Chemother. 2012, 56, 6324–6327. [Google Scholar]
- Lee, H.; Sohn, Y.M.; Ko, J.Y.; Lee, S.Y.; Jhun, B.W.; Park, H.Y.; Jeon, K.; Kim, D.H.; Kim, S.Y.; Choi, J.E.; et al. Once-daily dosing of amikacin for treatment of Mycobacterium abscessus lung disease. Int. J. Tuberc. Lung Dis. 2017, 21, 818–824. [Google Scholar]
- Kon, S.S.; Canavan, J.L.; Jones, S.E.; Nolan, C.M.; Clark, A.L.; Dickson, M.J.; Haselden, B.M.; Polkey, M.I.; Man, W.D. Minimum clinically important difference for the COPD Assessment Test: A prospective analysis. Lancet Respir. Med. 2014, 2, 195–203. [Google Scholar]
- Koh, W.J.; Moon, S.M.; Kim, S.Y.; Woo, M.A.; Kim, S.; Jhun, B.W.; Park, H.Y.; Jeon, K.; Huh, H.J.; Ki, C.S.; et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur. Respir. J. 2017, 50, 1602503. [Google Scholar]
- American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 2000, 161, 1376–1395. [Google Scholar]
- Griffith, D.E.; Adjemian, J.; Brown-Elliott, B.A.; Philley, J.V.; Prevots, D.R.; Gaston, C.; Olivier, K.N.; Wallace, R.J., Jr. Semiquantitative culture analysis during therapy for Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2015, 192, 754–760. [Google Scholar]
- Yang, B.; Jhun, B.W.; Moon, S.M.; Lee, H.; Park, H.Y.; Jeon, K.; Kim, D.H.; Kim, S.Y.; Shin, S.J.; Daley, C.L.; et al. Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob. Agents Chemother. 2017, 61, e02052-16. [Google Scholar]
- Woods, G.L.; Brown-Elliott, B.A.; Conville, P.S.; Desmond, E.P.; Hall, G.S.; Lin, G.; Pfyffer, G.E.; Ridderhof, J.C.; Siddiqi, S.H.; Wallace, R.J.J.; et al. CLSI Standards: Guidelines for health care excellence. In Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- Van Ingen, J.; Aksamit, T.; Andrejak, C.; Bottger, E.C.; Cambau, E.; Daley, C.L.; Griffith, D.E.; Guglielmetti, L.; Holland, S.M.; Huitt, G.A.; et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: An NTM-NET consensus statement. Eur. Respir. J. 2018, 51, 1800170. [Google Scholar]
- Yagi, K.; Ishii, M.; Namkoong, H.; Asami, T.; Iketani, O.; Asakura, T.; Suzuki, S.; Sugiura, H.; Yamada, Y.; Nishimura, T.; et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect. Dis. 2017, 17, 558. [Google Scholar]
- Jhun, B.W.; Kim, S.Y.; Moon, S.M.; Jeon, K.; Kwon, O.J.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; Shin, S.J.; Daley, C.L.; et al. Development of macrolide resistance and reinfection in refractory Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2018, 198, 1322–1330. [Google Scholar]
- Griffith, D.E.; Brown-Elliott, B.A.; Langsjoen, B.; Zhang, Y.; Pan, X.; Girard, W.; Nelson, K.; Caccitolo, J.; Alvarez, J.; Shepherd, S.; et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am. J. Respir. Crit. Care Med. 2006, 174, 928–934. [Google Scholar]
- Kadota, T.; Matsui, H.; Hirose, T.; Suzuki, J.; Saito, M.; Akaba, T.; Kobayashi, K.; Akashi, S.; Kawashima, M.; Tamura, A.; et al. Analysis of drug treatment outcome in clarithromycin-resistant Mycobacterium avium complex lung disease. BMC Infect. Dis. 2016, 16, 31. [Google Scholar]
- Moon, S.M.; Park, H.Y.; Kim, S.Y.; Jhun, B.W.; Lee, H.; Jeon, K.; Kim, D.H.; Huh, H.J.; Ki, C.S.; Lee, N.Y.; et al. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant Mycobacterium avium complex lung disease. Antimicrob. Agents Chemother. 2016, 60, 6758–6765. [Google Scholar]
- Morimoto, K.; Namkoong, H.; Hasegawa, N.; Nakagawa, T.; Morino, E.; Shiraishi, Y.; Ogawa, K.; Izumi, K.; Takasaki, J.; Yoshiyama, T.; et al. Macrolide-resistant Mycobacterium avium complex lung disease: Analysis of 102 consecutive cases. Ann. Am. Thorac. Soc. 2016, 13, 1904–1911. [Google Scholar]
- Cho, E.H.; Huh, H.J.; Song, D.J.; Moon, S.M.; Lee, S.H.; Shin, S.Y.; Kim, C.K.; Ki, C.S.; Koh, W.J.; Lee, N.Y. Differences in drug susceptibility pattern between Mycobacterium avium and Mycobacterium intracellulare isolated in respiratory specimens. J. Infect. Chemother. 2018, 24, 315–318. [Google Scholar]
- Kim, O.H.; Kwon, B.S.; Han, M.; Koh, Y.; Kim, W.S.; Song, J.W.; Oh, Y.M.; Lee, S.D.; Lee, S.W.; Lee, J.S.; et al. Association between duration of aminoglycoside treatment and outcome of cavitary Mycobacterium avium complex lung disease. Clin. Infect. Dis. 2019, 68, 1870–1876. [Google Scholar]
- Davis, K.K.; Kao, P.N.; Jacobs, S.S.; Ruoss, S.J. Aerosolized amikacin for treatment of pulmonary Mycobacterium avium infections: An observational case series. BMC Pulm. Med. 2007, 7, 2. [Google Scholar]
- Olivier, K.N.; Shaw, P.A.; Glaser, T.S.; Bhattacharyya, D.; Fleshner, M.; Brewer, C.C.; Zalewski, C.K.; Folio, L.R.; Siegelman, J.R.; Shallom, S.; et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann. Am. Thorac. Soc. 2014, 11, 30–35. [Google Scholar]
- Jarand, J.; Davis, J.P.; Cowie, R.L.; Field, S.K.; Fisher, D.A. Long-term follow-up of Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest 2016, 149, 1285–1293. [Google Scholar]
- Field, S.K.; Cowie, R.L. Treatment of Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest 2003, 124, 1482–1486. [Google Scholar]
- Kwak, N.; Whang, J.; Yang, J.S.; Kim, T.S.; Kim, S.A.; Yim, J.J. Minimal Inhibitory concentration of clofazimine among clinical isolates of nontuberculous mycobacteria and its impact on treatment outcome. Chest 2020. [Google Scholar] [CrossRef]
| Characteristics | Value |
|---|---|
| Age, years | 59 (51–70) |
| Female | 25 (48%) |
| Body mass index, kg/m2 | 20.7 (18.3–22.2) |
| Weight, kg | 54 (48–62) |
| Never-smoker | 29 (56%) |
| Underlying condition | |
| Previous pulmonary tuberculosis | 27 (52%) |
| Chronic pulmonary aspergillosis | 17 (33%) |
| Chronic obstructive pulmonary disease | 14 (27%) |
| Previous lung cancer * | 3 (6%) |
| Diabetes mellitus | 4 (8%) |
| Chronic liver disease | 2 (4%) |
| Chronic kidney disease | 1 (2%) |
| Rheumatoid disease | 1 (2%) |
| Other malignancy † | 2 (4%) |
| Etiologic organism | |
| M. intracellulare | 35 (67%) |
| M. avium | 17 (33%) |
| Radiologic findings | |
| Nodular bronchiectatic form | 27 (52%) |
| With cavity | 10/27 (37%) |
| Without cavity | 17/27 (63%) |
| Fibrocavitary form | 25 (48%) |
| Laboratory findings | |
| Sputum AFB smear positivity | 34 (65%) |
| Serum albumin, g/dL | 4.1 (3.8–4.4) |
| C-reactive protein, mg/dL | 0.6 (0.1–1.7) |
| Erythrocyte sedimentation rate, mm/h | 30.0 (18.0–67.3) |
| FEV1, % | 68 (53–81) |
| FVC, % | 74 (56–90) |
| Macrolide resistance, ≥32 μ/mL | 25 (48%) |
| MIC level of AMK | 16 (16–32) |
| 4–16 μg/mL | 37 (71%) |
| 32 μg/mL | 15 (29%) |
| Variables | N = 52 | Treatment Duration (Months) |
|---|---|---|
| Drugs before starting inhaled AMK and CFZ therapy | ||
| Azithromycin | 52 (100%) | 27.0 (19.0–56.0) |
| Ethambutol | 52 (100%) | 27.0 (16.0–56.0) |
| Rifamycin | 50 (96%) | 28.0 (19.0–56.0) |
| Moxifloxacin | 13 (25%) | 12.0 (6.0–16.0) |
| Aminoglycoside injection | 30 (58%) | 7.0 (5.5–12.0) |
| AMK | 2 (4%) | 3.0, 8.0 |
| Streptomycin | 28 (54%) | 7.0 (6.0–12.0) |
| Total duration before starting AMK and CFZ therapy | 52 (100%) | 28.5 (20.3–55.5) |
| Inhaled AMK and CFZ therapy | ||
| Total duration of inhaled AMK and CFZ | 52 (100%) | 11.9 (4.7–18.8) |
| ≥12 months | 27/52 (52%) | 18.7 (12.6–26.4) |
| <12 months | 25/52 (48%) | 4.6 (1.2–6.6) |
| Total duration of inhaled AMK | 52 (100%) | 12.9 (7.2–20.6) |
| Total duration of CFZ | 52 (100%) | 12.5 (5.5–18.9) |
| Companion drugs used with inhaled AMK and CFZ | ||
| Azithromycin | 51 (98%) | 15.4 (12.4–20.9) |
| Ethambutol | 50 (96%) | 16.3 (12.7–22.2) |
| Rifamycin | 11 (21%) | 4.0 (3.0–13.0) |
| Moxifloxacin | 10 (19%) | 8.5 (3.0–14.2) |
| Linezolid | 1 (2%) | 12.4 |
| Total duration after starting AMK and CFZ therapy | 52 (100%) | 15.4 (12.5–21.2) |
| Variables | N = 52 |
|---|---|
| Symptomatic response by CAT score change | |
| Improved | 25 (48%) |
| Unchanged | 11 (21%) |
| Worsened | 16 (31%) |
| Radiological response | |
| Improved | 17 (33%) |
| Unchanged | 20 (39%) |
| Worsened | 15 (29%) |
| Microbiological response | |
| At least one sputum negative culture | 22 (42%) |
| Time to at least one culture negative, months | 5.1 (1.0–10.1) |
| Culture conversion | 17 (33%) |
| Time to culture conversion, months | 3.0 (1.0–9.2) |
| Microbiological cure | 12 (23%) |
| Cure | 6 (12%) |
| Clinical cure | 3 (6%) |
| Favorable outcome | 15 (29%) |
| Microbiological response in ≥12 months treatment group (n = 27) | |
| Culture conversion | 10/27 (37%) |
| Time to culture conversion, months | 3.0 (0.9–9.3) |
| Microbiological cure | 7/27 (26%) |
| Cure | 2/27 (7%) |
| Clinical cure | 0/27 (0%) |
| Microbiological response in <12 months treatment group (n = 25) | |
| Culture conversion | 7/25 (28%) |
| Time to culture conversion, months | 3.0 (1.0–9.7) |
| Microbiological cure | 5/25 (20%) |
| Cure | 4/25 (16%) |
| Clinical cure | 3/25 (12%) |
| Death * | 6 (12%) |
| Time from starting inhaled AMK and CFZ to death, months | 11.1 (5.8–20.5) |
| Discontinuation | Dose Change | Total | |
|---|---|---|---|
| Amikacin inhalation | |||
| Ototoxicity | 12 (23%) | 5 (10%) | 17 (33%) |
| Fatigue | 2 (4%) | 0 (0%) | 2 (4%) |
| Tinnitus | 1 (2%) | 0 (0%) | 1 (2%) |
| Dizziness | 1 (2%) | 0 (0%) | 1 (2%) |
| Nausea | 1 (2%) | 0 (0%) | 1 (2%) |
| Hoarseness | 0 (0%) | 1 (2%) | 1 (2%) |
| Nephrotoxicity | 0 (0%) | 1 (2%) | 1 (2%) |
| Total | 17 (33%) * | 7 (14%) † | 24 (46%) |
| Clofazimine | |||
| Skin color change | 1 (2%) | 9 (17%) | 10 (19%) |
| Loss of appetite | 1 (2%) | 0 (0%) | 2 (4%) |
| Diarrhea | 0 (0%) | 1 (2%) | 1 (2%) |
| Fatigue | 1 (2%) | 0 (0%) | 1 (2%) |
| Hepatotoxicity (>3 times the normal level) | 1 (2%) | 0 (0%) | 1 (2%) |
| Total | 4 (8%) ‡ | 10 (19%) | 14 (27%) |
| Variable | Favorable Outcome * (n = 15) | Univariable | Multivariable | ||
|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | ||
| Age ≤ 65 years | 2 (13%) | 4.432 (0.871–22.550) | 0.073 | ||
| Female | 9 (60%) | 1.969 (0.581–6.673) | 0.277 | ||
| Body mass index, kg/m2 | 1.058 (0.857–1.308) | 0.599 | |||
| Never-smoker | 10 (67%) | 1.895 (0.542–6.628) | 0.317 | ||
| No previous pulmonary tuberculosis | 8 (53%) | 1.345 (0.404–4.477) | 0.630 | ||
| No chronic obstructive pulmonary disease | 14 (93%) | 7.583 (0.894–64.331) | 0.063 | ||
| M. avium (reference: M. intracellulare) | 8 (53%) | 3.556 (1.006–12.562) | 0.049 | ||
| Negative sputum AFB smear | 6 (40%) | 1.389 (0.401–4.806) | 0.604 | ||
| No cavity | 7 (47%) | 2.362 (0.679–8.222) | 0.177 | ||
| No macrolide resistance | 8 (53%) | 1.083 (0.325–3.602) | 0.897 | ||
| Amikacin MIC < 32 μg/mL | 11 (73%) | 1.163 (0.303–4.461) | 0.825 | ||
| Treatment duration ≥12 months | 10 (67%) | 2.353 (0.672–8.239) | 0.181 | ||
| FEV1 > 60% | 14 (93%) | 9.545 (1.132–80.506) | 0.038 | ||
| ESR, mm/h † | 0.950 (0.914–0.988) | 0.010 | 0.950 (0.914–0.988) | 0.010 | |
| CRP, mg/dL † | 0.228 (0.057–0.904) | 0.035 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.-G.; Kim, H.; Kwon, O.J.; Huh, H.J.; Lee, N.Y.; Baek, S.-Y.; Sohn, I.; Jhun, B.W. Outcomes of Inhaled Amikacin and Clofazimine-Containing Regimens for Treatment of Refractory Mycobacterium avium Complex Pulmonary Disease. J. Clin. Med. 2020, 9, 2968. https://doi.org/10.3390/jcm9092968
Kim B-G, Kim H, Kwon OJ, Huh HJ, Lee NY, Baek S-Y, Sohn I, Jhun BW. Outcomes of Inhaled Amikacin and Clofazimine-Containing Regimens for Treatment of Refractory Mycobacterium avium Complex Pulmonary Disease. Journal of Clinical Medicine. 2020; 9(9):2968. https://doi.org/10.3390/jcm9092968
Chicago/Turabian StyleKim, Bo-Guen, Hojoong Kim, O. Jung Kwon, Hee Jae Huh, Nam Yong Lee, Sun-Young Baek, Insuk Sohn, and Byung Woo Jhun. 2020. "Outcomes of Inhaled Amikacin and Clofazimine-Containing Regimens for Treatment of Refractory Mycobacterium avium Complex Pulmonary Disease" Journal of Clinical Medicine 9, no. 9: 2968. https://doi.org/10.3390/jcm9092968
APA StyleKim, B.-G., Kim, H., Kwon, O. J., Huh, H. J., Lee, N. Y., Baek, S.-Y., Sohn, I., & Jhun, B. W. (2020). Outcomes of Inhaled Amikacin and Clofazimine-Containing Regimens for Treatment of Refractory Mycobacterium avium Complex Pulmonary Disease. Journal of Clinical Medicine, 9(9), 2968. https://doi.org/10.3390/jcm9092968





