Effect of a Combination of Myo-Inositol, Alpha-Lipoic Acid, and Folic Acid on Oocyte Morphology and Embryo Morphokinetics in non-PCOS Overweight/Obese Patients Undergoing IVF: A Pilot, Prospective, Randomized Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Controlled Ovarian Stimulation (COS) and Oocyte Retrieval
2.3. Polarized Light Microscopy (PLM)
2.4. Preparation of Semen Samples and Intra-Cytoplasmatic Sperm Injection (ICSI)
2.5. Fertilization Check, Embryo TLS Analysis and Embryo Transfer
2.6. Statistical Analysis
3. Results
Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergandi, L.; Basso, G.; Evangelista, F.; Canosa, S.; Dalmasso, P.; Aldieri, E.; Revelli, A.; Benedetto, C.; Ghigo, D. Inducible nitric oxide synthase and heme oxygenase 1 are expressed in human cumulus cells and may be used as biomarkers of oocyte competence. Reprod. Sci. 2014, 21, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Canosa, S.; Adriaenssens, T.; Coucke, W.; Dalmasso, P.; Revelli, A.; Benedetto, C.; Smitz, J. Zona pellucida gene mRNA expression in human oocytes is related to oocyte maturity, zona inner layer retardance and fertilization competence. Mol. Hum. Reprod. 2017, 23, 292–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revelli, A.; Canosa, S.; Bergandi, L.; Skorokhod, O.A.; Biasoni, V.; Carosso, A.; Bertagna, A.; Maule, M.; Aldieri, E.; D’Eufemia, M.D.; et al. Oocyte polarized light microscopy, assay of specific follicular fluid metabolites, and gene expression in cumulus cells as different approaches to predict fertilization efficiency after ICSI. Reprod. Biol. Endocrinol. 2017, 15, 47. [Google Scholar] [CrossRef]
- Dattilo, M.; D’Amato, G.; Caroppo, E.; Ménézo, Y. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: Mechanisms, clinical results, insights on gene-environment interactions and the role of diet. J. Assist. Reprod. Genet. 2016, 33, 1633–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciepiela, P.; Dulęba, A.J.; Kowaleczko, E.; Chełstowski, K.; Kurzawa, R. Vitamin D as a follicular marker of human oocyte quality and a serum marker of in vitro fertilization outcome. J. Assist. Reprod. Genet. 2018, 35, 1265–1276. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Girish, B.; Malhotra, N.; Mahey, R.; Perumal, V. Does double dose of recombinant human chorionic gonadotropin for final follicular maturation in in vitro fertilization cycles improve oocyte quality: A prospective randomized study. J. Hum. Reprod. Sci. 2019, 12, 310–315. [Google Scholar] [CrossRef]
- Rago, R.; Marcucci, I.; Leto, G.; Caponecchia, L.; Salacone, P.; Bonanni, P.; Fiori, C.; Sorrenti, G.; Sebastianelli, A. Effect of myo-inositol and alpha-lipoic acid on oocyte quality in polycystic ovary syndrome non-obese women undergoing in vitro fertilization: A pilot study. J. Biol. Regul. Homeost. Agents 2015, 29, 913–923. [Google Scholar]
- Orrù, B.; Circo, R.; Logoteta, P.; Petousis, S.; Carlomagno, G. Finding the best therapeutic approach for PCOS: The importance of inositol(s) bioavailability. Eur. Rev. Med. Pharmacol. Sci. 2017, 21 (Suppl. 2), 83–88. [Google Scholar]
- Caprio, F.; D’Eufemia, M.D.; Trotta, C.; Campitiello, M.R.; Ianniello, R.; Mele, D.; Colacurci, N. Myo-Inositol therapy for poor-responders during IVF: A prospective controlled observational trial. J. Ovarian Res. 2015, 8. [Google Scholar] [CrossRef] [Green Version]
- Chiu, T.T.Y.; Rogers, M.S.; Briton-Jones, C.; Haines, C. Effects of myo-inositol on the in-vitro maturation and subsequent development of mouse oocytes. Hum. Reprod. 2003, 18, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Papaleo, E.; Unfer, V.; Baillargeon, J.-P.; Fusi, F.; Occhi, F.; De Santis, L. Myo-Inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil. Steril. 2009, 91, 1750–1754. [Google Scholar] [CrossRef] [PubMed]
- De-Regil, L.M.; Peña-Rosas, J.P.; Fernández-Gaxiola, A.C.; Rayco-Solon, P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst. Rev. 2015, 12, CD007950. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. 1), 285–301. [Google Scholar] [CrossRef] [PubMed]
- Boxmeer, J.C.; Macklon, N.S.; Lindemans, J.; Beckers, N.G.M.; Eijkemans, M.J.C.; Laven, J.S.E.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. IVF outcomes are associated with biomarkers of the homocysteine pathway in monofollicular fluid. Hum. Reprod. 2009, 24, 1059–1066. [Google Scholar] [CrossRef]
- Hatami, S.; Zavareh, S.; Salehnia, M.; Lashkarbolouki, T.; Ghorbanian, M.T.; Karimi, I. Total oxidative status of mouse vitrified pre-antral follicles with pre-treatment of alpha lipoic acid. Iran Biomed. J. 2014, 18, 181–188. [Google Scholar] [CrossRef]
- Truong, T.T.; Gardner, D.K. Antioxidants increase blastocyst cryosurvival and viability post-vitrification. Hum. Reprod. 2020, 35, 12–23. [Google Scholar] [CrossRef]
- Canepa, P.; Dal Lago, A.; De Leo, C.; Gallo, M.; Rizzo, C.; Licata, E.; Anserini, P.; Rago, R.; Scaruffi, P. Combined treatment with myo-inositol, alpha-lipoic acid, folic acid and vitamins significantly improves sperm parameters of sub-fertile men: A multi-centric study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7078–7085. [Google Scholar] [CrossRef]
- Molinari, E.; Evangelista, F.; Racca, C.; Cagnazzo, C.; Revelli, A. Polarized light microscopy-detectable structures of human oocytes and embryos are related to the likelihood of conception in IVF. J. Assist. Reprod. Genet. 2012, 29, 1117–1122. [Google Scholar] [CrossRef]
- Rama Raju, G.R.; Prakash, G.J.; Krishna, K.M.; Madan, K. Meiotic spindle and zona pellucida characteristics as predictors of embryonic development: A preliminary study using PolScope imaging. Reprod. BioMed. Online 2007, 14, 166–174. [Google Scholar] [CrossRef]
- Canosa, S.; Bergandi, L.; Macrì, C.; Charrier, L.; Paschero, C.; Carosso, A.; Di Segni, N.; Silvagno, F.; Gennarelli, G.; Benedetto, C.; et al. Morphokinetic analysis of cleavage stage embryos and assessment of specific gene expression in cumulus cells independently predict human embryo development to expanded blastocyst: A preliminary study. J. Assist. Reprod. Genet. 2020, 37, 1409–1420. [Google Scholar] [CrossRef]
- Wong, C.C.; Loewke, K.E.; Bossert, N.L.; Behr, B.; De Jonge, C.J.; Baer, T.M.; Pera, R.A.R. Non-Invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat. Biotechnol. 2010, 28, 1115–1121. [Google Scholar] [CrossRef]
- Revelli, A.; Gennarelli, G.; Sestero, M.; Canosa, S.; Carosso, A.; Salvagno, F.; Pittatore, G.; Filippini, C.; Benedetto, C. A prospective randomized trial comparing corifollitropin-α late-start (day 4) versus standard administration (day 2) in expected poor, normal, and high responders undergoing controlled ovarian stimulation for IVF. J. Assist. Reprod. Genet. 2020, 37, 1163–1170. [Google Scholar] [CrossRef]
- Revelli, A.; Biasoni, V.; Gennarelli, G.; Canosa, S.; Dalmasso, P.; Benedetto, C. IVF results in patients with very low serum AMH are significantly affected by chronological age. J. Assist. Reprod. Genet. 2016, 33, 603–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holte, J.; Berglund, L.; Milton, K.; Garello, C.; Gennarelli, G.; Revelli, A.; Bergh, T. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum. Reprod. 2007, 22, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum. Reprod. 2011, 26, 1270–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ESHRE Working group on Time-lapse technology; Apter, S.; Ebner, T.; Freour, T.; Guns, Y.; Kovacic, B.; Le Clef, N.; Marques, M.; Meseguer, M.; Montjean, D.; et al. Good practice recommendations for the use of time-lapse technology. Hum. Reprod. Open 2020, 2020. [Google Scholar] [CrossRef]
- Meseguer, M.; Herrero, J.; Tejera, A.; Hilligsøe, K.M.; Ramsing, N.B.; Remohí, J. The use of morphokinetics as a predictor of embryo implantation. Hum. Reprod. 2011, 26, 2658–2671. [Google Scholar] [CrossRef] [Green Version]
- Revelli, A.; Rovei, V.; Dalmasso, P.; Gennarelli, G.; Racca, C.; Evangelista, F.; Benedetto, C. Large Randomized trial comparing transabdominal ultrasound-guided embryo transfer with a technique based on uterine length measurement before embryo transfer. Ultrasound Obstet. Gynecol. 2016, 48, 289–295. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Spiegelman, D.; Garland, M.; Hertzmark, E.; Hunter, D.J.; Colditz, G.A.; Willett, W.C.; Wand, H.; Manson, J.E. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology 2002, 13, 184–190. [Google Scholar] [CrossRef]
- Sermondade, N.; Huberlant, S.; Bourhis-Lefebvre, V.; Arbo, E.; Gallot, V.; Colombani, M.; Fréour, T. Female obesity is negatively associated with live birth rate following IVF: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 439–451. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Robker, R.L. Developmental programming of obesity and insulin resistance: Does mitochondrial dysfunction in oocytes play a role? Mol. Hum Reprod. 2015, 21, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Robker, R.L.; Akison, L.K.; Bennett, B.D.; Thrupp, P.N.; Chura, L.R.; Russell, D.L.; Lane, M.; Norman, R.J. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J. Clin. Endocrinol. Metab. 2009, 94, 1533–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talmor, A.; Dunphy, B. Female obesity and infertility. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.K.; Missmer, S.A.; Berry, K.F.; Racowsky, C.; Ginsburg, E.S. Effect of obesity on oocyte and embryo quality in women undergoing in vitro fertilization. Obstet. Gynecol. 2011, 118, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Valckx, S.D.M.; De Pauw, I.; De Neubourg, D.; Inion, I.; Berth, M.; Fransen, E.; Bols, P.E.J.; Leroy, J.L.M.R. BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatment and the consequences for oocyte and embryo quality. Hum. Reprod. 2012, 27, 3531–3539. [Google Scholar] [CrossRef] [Green Version]
- Leary, C.; Leese, H.J.; Sturmey, R.G. Human embryos from overweight and obese women display phenotypic and metabolic abnormalities. Hum. Reprod. 2015, 30, 122–132. [Google Scholar] [CrossRef]
- Shang, K.; Jia, X.; Qiao, J.; Kang, J.; Guan, Y. Endometrial abnormality in women with polycystic ovary syndrome. Reprod. Sci. 2012, 19, 674–683. [Google Scholar] [CrossRef]
- Simi, G.; Genazzani, A.R.; Obino, M.E.R.; Papini, F.; Pinelli, S.; Cela, V.; Artini, P.G. Inositol and in vitro fertilization with embryo transfer. Int. J. Endocrinol. 2017, 2017, 5469409. [Google Scholar] [CrossRef] [Green Version]
- Fruzzetti, F.; Perini, D.; Russo, M.; Bucci, F.; Gadducci, A. Comparison of two insulin sensitizers, metformin and myo-inositol, in women with Polycystic Ovary Syndrome (PCOS). Gynecol. Endocrinol. 2017, 33, 39–42. [Google Scholar] [CrossRef]
- Garg, D.; Tal, R. Inositol treatment and ART outcomes in women with PCOS. Int. J. Endocrinol. 2016, 2016, 1979654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hodgson, R.; Farquhar, C. Inositol for subfertile women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2018, 12, CD012378. [Google Scholar] [CrossRef]
- Artini, P.G.; Obino, M.E.R.; Micelli, E.; Malacarne, E.; Vacca, C.; Papini, F.; Cela, V. Effect of D-chiro-inositol and alpha-lipoic acid combination on COH outcomes in overweight/obese PCOS women. Gynecol. Endocrinol. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Montag, M.; Köster, M.; van der Ven, K.; van der Ven, H. Gamete competence assessment by polarizing optics in assisted reproduction. Hum. Reprod. Update 2011, 17, 654–666. [Google Scholar] [CrossRef] [Green Version]
- Madaschi, C.; de Souza Bonetti, T.C.; de Almeida Ferreira Braga, D.P.; Pasqualotto, F.F.; Iaconelli, A.; Borges, E. Spindle imaging: A marker for embryo development and implantation. Fertil. Steril. 2008, 90, 194–198. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Ferreira Braga, D.P.; de Cassia Savio Figueira, R.; Rodrigues, D.; Madaschi, C.; Pasqualotto, F.F.; Iaconelli, A.; Borges, E. Prognostic value of meiotic spindle imaging on fertilization rate and embryo development in in vitro-matured human oocytes. Fertil. Steril. 2008, 90, 429–433. [Google Scholar] [CrossRef]
- Montag, M.; van der Ven, H. Symposium: Innovative techniques in human embryo viability assessment. oocyte assessment and embryo viability prediction: Birefringence imaging. Reprod. BioMed. Online 2008, 17, 454–460. [Google Scholar] [CrossRef]
- Ebner, T.; Balaban, B.; Moser, M.; Shebl, O.; Urman, B.; Ata, B.; Tews, G. Automatic user-independent zona pellucida imaging at the oocyte stage allows for the prediction of preimplantation development. Fertil. Steril. 2010, 94, 913–920. [Google Scholar] [CrossRef]
- Shen, Y.; Stalf, T.; Mehnert, C.; Eichenlaub-Ritter, U.; Tinneberg, H.-R. High magnitude of light retardation by the zona pellucida is associated with conception cycles. Hum. Reprod. 2005, 20, 1596–1606. [Google Scholar] [CrossRef]
- Unfer, V.; Carlomagno, G.; Rizzo, P.; Raffone, E.; Roseff, S. Myo-Inositol rather than D-chiro-inositol is able to improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 452–457. [Google Scholar]
- Cheng, J.; Huang, L.; He, B.; Lu, F.; Wang, X.; Wu, Z.; Shi, D. Quantitative analysis of the intensity of zona pellucida birefringence of oocytes during IVF cycles. Reprod. Fertil. Dev. 2010, 22, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Madaschi, C.; Aoki, T.; de Almeida Ferreira Braga, D.P.; de Cássia Sávio Figueira, R.; Semião Francisco, L.; Iaconelli, A.; Borges, E. Zona pellucida birefringence score and meiotic spindle visualization in relation to embryo development and ICSI outcomes. Reprod. BioMed. Online 2009, 18, 681–686. [Google Scholar] [CrossRef]
- Korkmaz, C.; Cinar, O.; Akyol, M. The relationship between meiotic spindle imaging and outcome of intracytoplasmic sperm injection: A retrospective study. Gynecol. Endocrinol. 2011, 27, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Kilani, S.S.; Cooke, S.; Kan, A.K.; Chapman, M.G. Do age and extended culture affect the architecture of the zona pellucida of human oocytes and embryos? Zygote 2006, 14, 39–44. [Google Scholar] [CrossRef]
- Korkmaz, C.; Tekin, Y.B.; Sakinci, M.; Ercan, C.M. Effects of maternal ageing on ICSI outcomes and embryo development in relation to oocytes morphological characteristics of birefringent structures. Zygote 2015, 23, 550–555. [Google Scholar] [CrossRef]
- Revelli, A.; Canosa, S.; Carosso, A.; Filippini, C.; Paschero, C.; Gennarelli, G.; Delle Piane, L.; Benedetto, C. Impact of the addition of early embryo viability assessment to morphological evaluation on the accuracy of embryo selection on day 3 or day 5: A retrospective analysis. J. Ovarian Res. 2019, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Pribenszky, C.; Nilselid, A.-M.; Montag, M. Time-Lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: A meta-analysis. Reprod. BioMed. Online 2017, 35, 511–520. [Google Scholar] [CrossRef] [Green Version]
- Goodman, L.R.; Goldberg, J.; Falcone, T.; Austin, C.; Desai, N. Does the addition of time-lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertil. Steril. 2016, 105, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Kieslinger, D.C.; De Gheselle, S.; Lambalk, C.B.; De Sutter, P.; Kostelijk, E.H.; Twisk, J.W.R.; van Rijswijk, J.; Van den Abbeel, E.; Vergouw, C.G. Embryo selection using time-lapse analysis (early embryo viability assessment) in conjunction with standard morphology: A prospective two-center pilot study. Hum. Reprod. 2016, 31, 2450–2457. [Google Scholar] [CrossRef] [Green Version]
- Kirkegaard, K.; Ahlström, A.; Ingerslev, H.J.; Hardarson, T. Choosing the best embryo by time lapse versus standard morphology. Fertil. Steril. 2015, 103, 323–332. [Google Scholar] [CrossRef]
- Bartolacci, A.; Buratini, J.; Moutier, C.; Guglielmo, M.C.; Novara, P.V.; Brambillasca, F.; Renzini, M.M.; Dal Canto, M. Maternal body mass index affects embryo morphokinetics: A time-lapse study. J. Assist. Reprod. Genet. 2019, 36, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Wissing, M.L.; Bjerge, M.R.; Olesen, A.I.G.; Hoest, T.; Mikkelsen, A.L. Impact of PCOS on early embryo cleavage kinetics. Reprod. BioMed. Online 2014, 28, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provost, M.P.; Acharya, K.S.; Acharya, C.R.; Yeh, J.S.; Steward, R.G.; Eaton, J.L.; Goldfarb, J.M.; Muasher, S.J. Pregnancy outcomes decline with increasing recipient body mass index: An analysis of 22,317 fresh donor/recipient cycles from the 2008–2010 society for assisted reproductive technology clinic outcome reporting system registry. Fertil. Steril. 2016, 105, 364–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellver, J.; Martínez-Conejero, J.A.; Labarta, E.; Alamá, P.; Melo, M.A.B.; Remohí, J.; Pellicer, A.; Horcajadas, J.A. Endometrial gene expression in the window of implantation is altered in obese women especially in association with polycystic ovary syndrome. Fertil. Steril. 2011, 95, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Orrù, B.; Grandi, G.; Unfer, V. Short-Term effects of metformin and myo-inositol in women with polycystic ovarian syndrome (PCOS): A meta-analysis of randomized clinical trials. Gynecol. Endocrinol. 2019, 35, 198–206. [Google Scholar] [CrossRef]
- Di Nicuolo, F.; D’Ippolito, S.; Castellani, R.; Rossi, E.D.; Masciullo, V.; Specchia, M.; Mariani, M.; Pontecorvi, A.; Scambia, G.; Di Simone, N. Effect of alpha-lipoic acid and myoinositol on endometrial inflammasome from recurrent pregnancy loss women. Am. J. Reprod. Immunol. 2019, 82, e13153. [Google Scholar] [CrossRef]
| MI + ALA + FA (n = 20) | FA-Only (n = 20) | p | |
|---|---|---|---|
| Age (years) | 35 ± 5.5 | 37.2 ± 3.3 | ns |
| BMI (kg/m2) | 29.9 ± 2 | 29.6 ± 2.3 | ns |
| AMH (ng/mL) | 2.9 ± 2.6 | 2.8 ± 2.6 | ns |
| AFC (n) | 15.9 ± 8.9 | 14.3 ± 7.4 | ns |
| Total exogenous FSH (IU) | 3090 ± 1000 | 3053 ± 890.3 | ns |
| OSI (n) | 2.8 ± 2 | 3.3 ± 2.3 | ns |
| Peak E2 (pg/mL) | 1735 ± 689 | 2286 ± 1147 | ns |
| Endometrial thickness (mm) | 11.4 ± 2.2 | 9.9 ± 1.8 | <0.05 |
| Retrieved oocytes (n) | 7.8 ± 4 | 8.5 ± 4.3 | ns |
| Mature (MII) oocytes (n) | 6.6 ± 3.3 | 7.3 ± 4 | ns |
| Maturation rate (%) | 84.3 ± 17.2 | 87.3 ± 16.2 | ns |
| Fertilized oocytes (n) | 4.3 ± 2.5 | 4.7 ± 3.1 | ns |
| Fertilization rate (%) | 69.9 ± 25 | 64.6 ± 20.1 | ns |
| Cleaved embryos (n) | 4.2 ± 2.4 | 4.6 ± 3.2 | ns |
| Cleavage rate (%) | 98.5 ± 4.8 | 97.4 ± 8.4 | ns |
| Mean embryo score | 7.4 ± 2.3 | 6.7 ± 2.1 | <0.01 |
| Top quality embryos (n) | 1.9 ± 1.5 | 1.2 ± 1.8 | ns |
| Top quality embryos (%) | 45.2 (38/84) | 26.1 (24/92) | <0.01 |
| MI+ALA+FA (n = 155) | FA-Only (n = 169) | p | |
|---|---|---|---|
| IL-retardance (nm) | 2.1 ± 0.6 | 1.9 ± 0.5 | <0.001 |
| IL-area (µm2) | 2895 ± 574 | 2574 ± 491 | <0.0001 |
| IL-thickness (µm) | 5.3 ± 1.4 | 4.5 ± 1.2 | <0.05 |
| MS-retardance (nm) | 1.6 ± 0.5 | 1.6 ± 0.4 | ns |
| MS-area (µm2) | 85.9 ± 23.4 | 89.3 ± 21.9 | ns |
| MS-axis (µm) | 11.8 ± 1.8 | 12.3 ± 2.3 | <0.05 |
| MI+ALA+FA (n = 84) | FA-Only (n = 92) | p | |
|---|---|---|---|
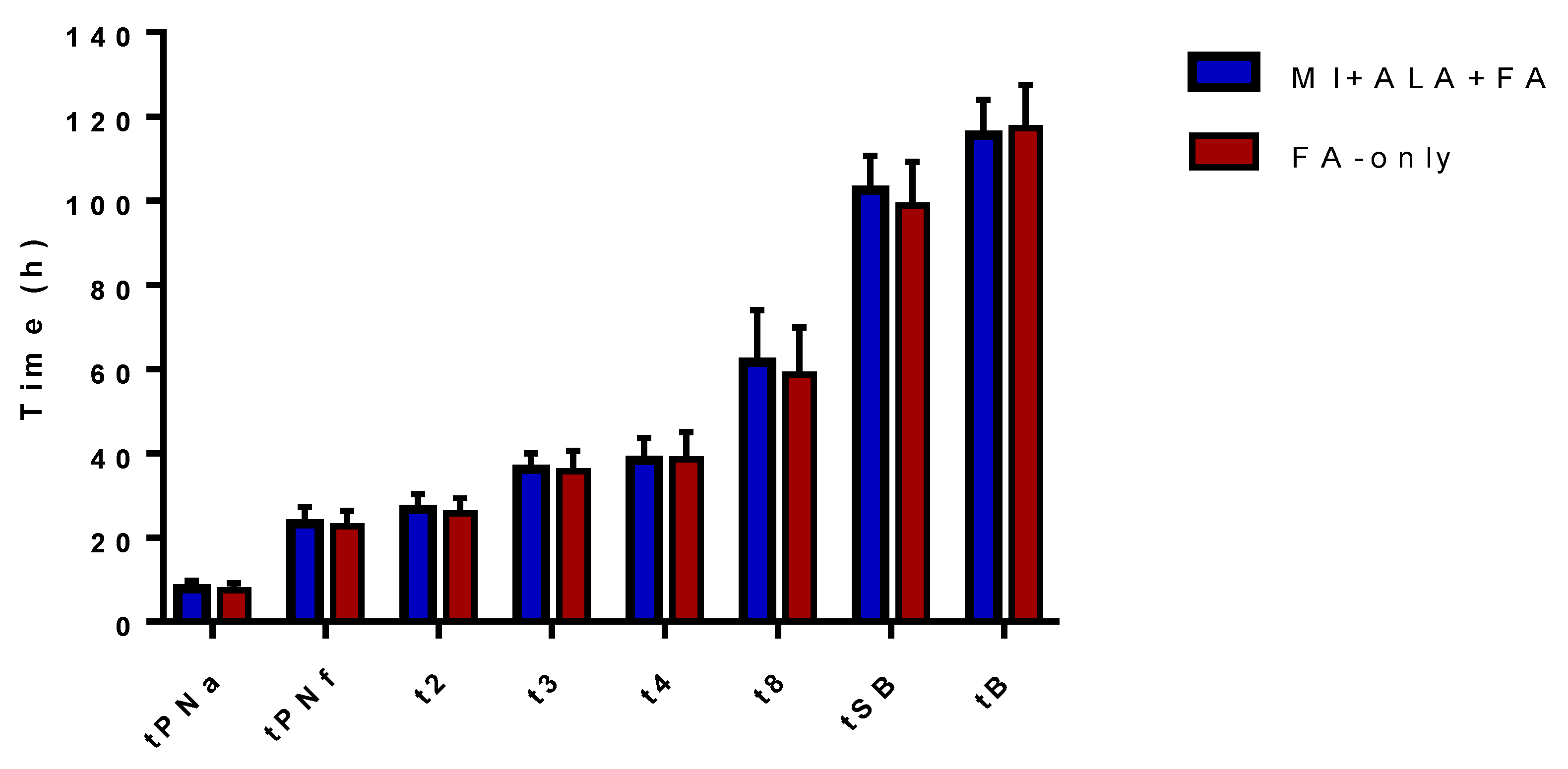
| tPNa (h) | 7.9 ± 1.8 | 7.5 ± 1.7 | ns |
| tPNf (h) | 23.3 ± 3.9 | 22.7 ± 3.6 | ns |
| t2 (h) | 26.6 ± 3.8 | 25.7 ± 3.7 | ns |
| t3 (h) | 36.2 ± 3.7 | 35.8 ± 4.8 | ns |
| t4 (h) | 38.3 ± 5.4 | 38.5 ± 6.6 | ns |
| t8 (h) | 61.6 ± 12.4 | 58.8 ± 11.1 | ns |
| tSB (h) | 102.5 ± 8.2 | 98.9 ± 10.3 | ns |
| tB (h) | 115.6 ± 8.3 | 117.2 ± 10.2 | ns |
| tPNf–tPNa (h) | 15.3 ± 3.5 | 15.2 ± 3.9 | ns |
| t2-tPNf (h) | 3.4 ± 1.6 | 3 ± 0.7 | ns |
| t3-t2 (h) | 9.6 ± 3.9 | 10.4 ± 3.6 | ns |
| t4-t3 (h) | 2.7 ± 5.1 | 2.8 ± 5.9 | ns |
| t4-t2 (h) | 12.2 ± 4.8 | 13.1 ± 5.9 | ns |
| t8-t4 (h) | 22.8 ± 13.3 | 20.5 ± 8.9 | ns |
| t2 (24.3–27.9 h) | 51.1 (43/84) | 33.6 (31/92) | <0.05 |
| t3 (35.4–40.3 h) | 47.6 (40/84) | 25 (23/92) | <0.01 |
| t4 (36.4–41.6 h) | 40.4 (34/84) | 28.2 (26/92) | ns |
| t3–t2 (≤ 11.9 h) | 75 (63/84) | 70.6 (65/92) | ns |
| t4–t3 (≤ 0.76 h) | 38 (32/84) | 47.8 (44/92) | ns |
| MI+ALA+FA (n = 20) | FA-Only (n = 20) | p | |
|---|---|---|---|
| Transferred embryos (n) | 30 | 28 | |
| Implantation rate (%) | 33.3 (10/30) | 10.7 (3/28) | <0.05 |
| Clinical pregnancy rate (CPR) (%) | 45 (9/20) | 15 (3/20) | <0.05 |
| Ongoing pregnancy rate (OPR) (%) | 35 (7/20) | 0 (0/20) | - |
| Live birth rate (cLBR) (%) | 35 (7/20) | 0 (0/20) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canosa, S.; Paschero, C.; Carosso, A.; Leoncini, S.; Mercaldo, N.; Gennarelli, G.; Benedetto, C.; Revelli, A. Effect of a Combination of Myo-Inositol, Alpha-Lipoic Acid, and Folic Acid on Oocyte Morphology and Embryo Morphokinetics in non-PCOS Overweight/Obese Patients Undergoing IVF: A Pilot, Prospective, Randomized Study. J. Clin. Med. 2020, 9, 2949. https://doi.org/10.3390/jcm9092949
Canosa S, Paschero C, Carosso A, Leoncini S, Mercaldo N, Gennarelli G, Benedetto C, Revelli A. Effect of a Combination of Myo-Inositol, Alpha-Lipoic Acid, and Folic Acid on Oocyte Morphology and Embryo Morphokinetics in non-PCOS Overweight/Obese Patients Undergoing IVF: A Pilot, Prospective, Randomized Study. Journal of Clinical Medicine. 2020; 9(9):2949. https://doi.org/10.3390/jcm9092949
Chicago/Turabian StyleCanosa, Stefano, Carlotta Paschero, Andrea Carosso, Sara Leoncini, Noemi Mercaldo, Gianluca Gennarelli, Chiara Benedetto, and Alberto Revelli. 2020. "Effect of a Combination of Myo-Inositol, Alpha-Lipoic Acid, and Folic Acid on Oocyte Morphology and Embryo Morphokinetics in non-PCOS Overweight/Obese Patients Undergoing IVF: A Pilot, Prospective, Randomized Study" Journal of Clinical Medicine 9, no. 9: 2949. https://doi.org/10.3390/jcm9092949
APA StyleCanosa, S., Paschero, C., Carosso, A., Leoncini, S., Mercaldo, N., Gennarelli, G., Benedetto, C., & Revelli, A. (2020). Effect of a Combination of Myo-Inositol, Alpha-Lipoic Acid, and Folic Acid on Oocyte Morphology and Embryo Morphokinetics in non-PCOS Overweight/Obese Patients Undergoing IVF: A Pilot, Prospective, Randomized Study. Journal of Clinical Medicine, 9(9), 2949. https://doi.org/10.3390/jcm9092949







