Organ-Sparing Surgery in Testicular Tumor: Is This the Right Approach for Lesions ≤ 20 mm?
Abstract
1. Introduction
2. Experimental Section
2.1. Study Design and Case Selection
2.2. Preoperative Examinations and Follow-Up
2.3. Surgical Approach of OSS
2.4. Outcome Measures
2.5. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Surgical Outcomes
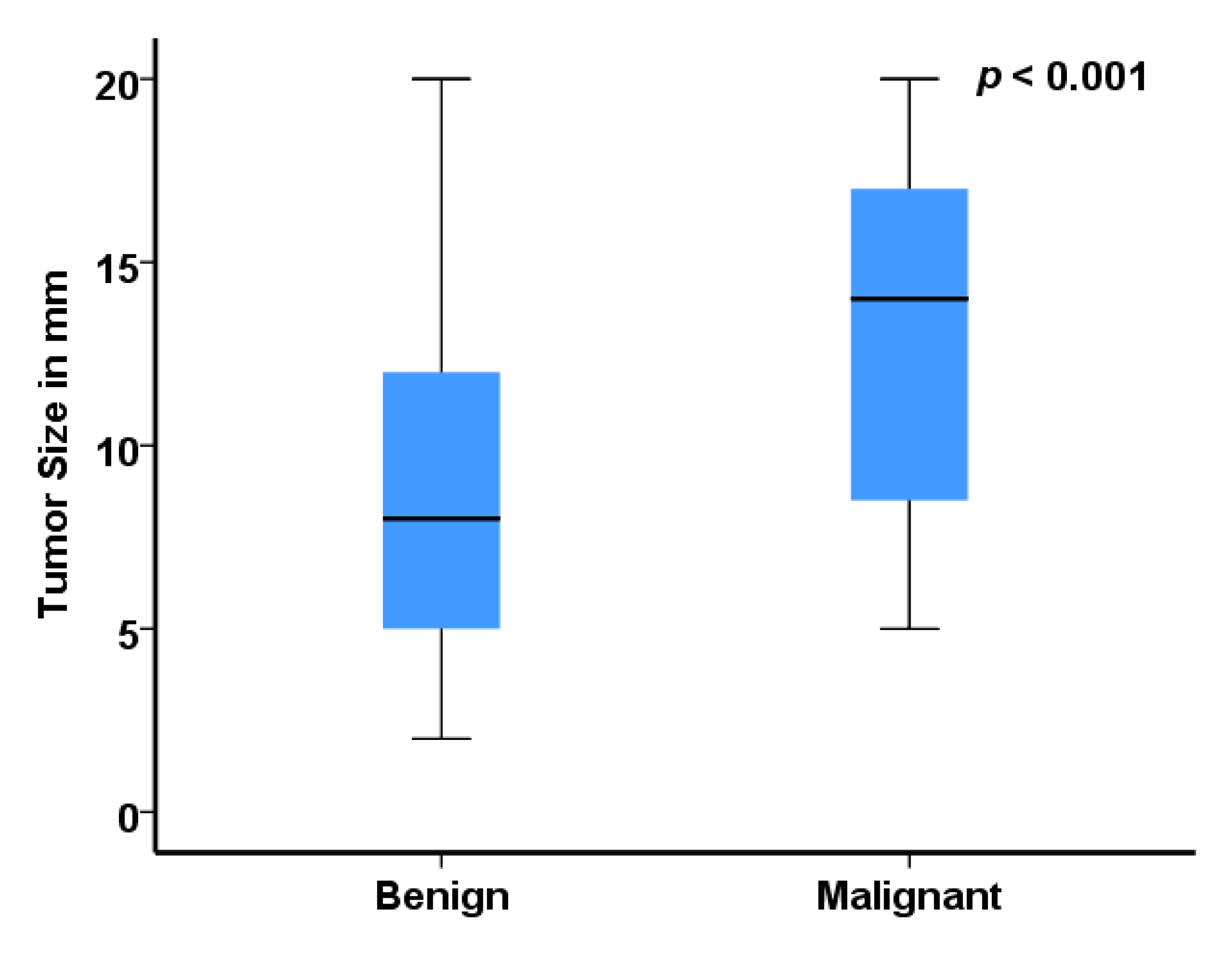
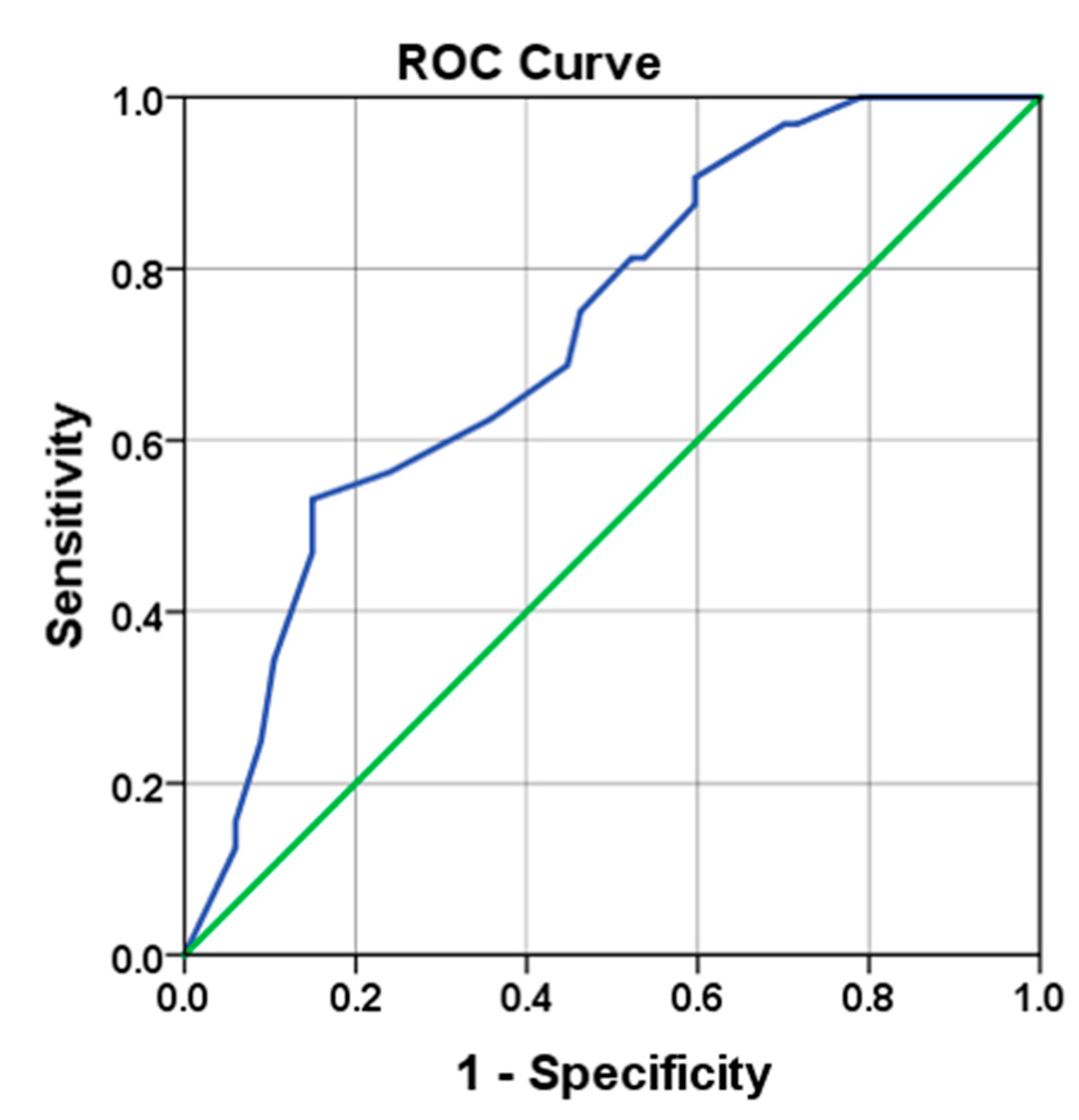
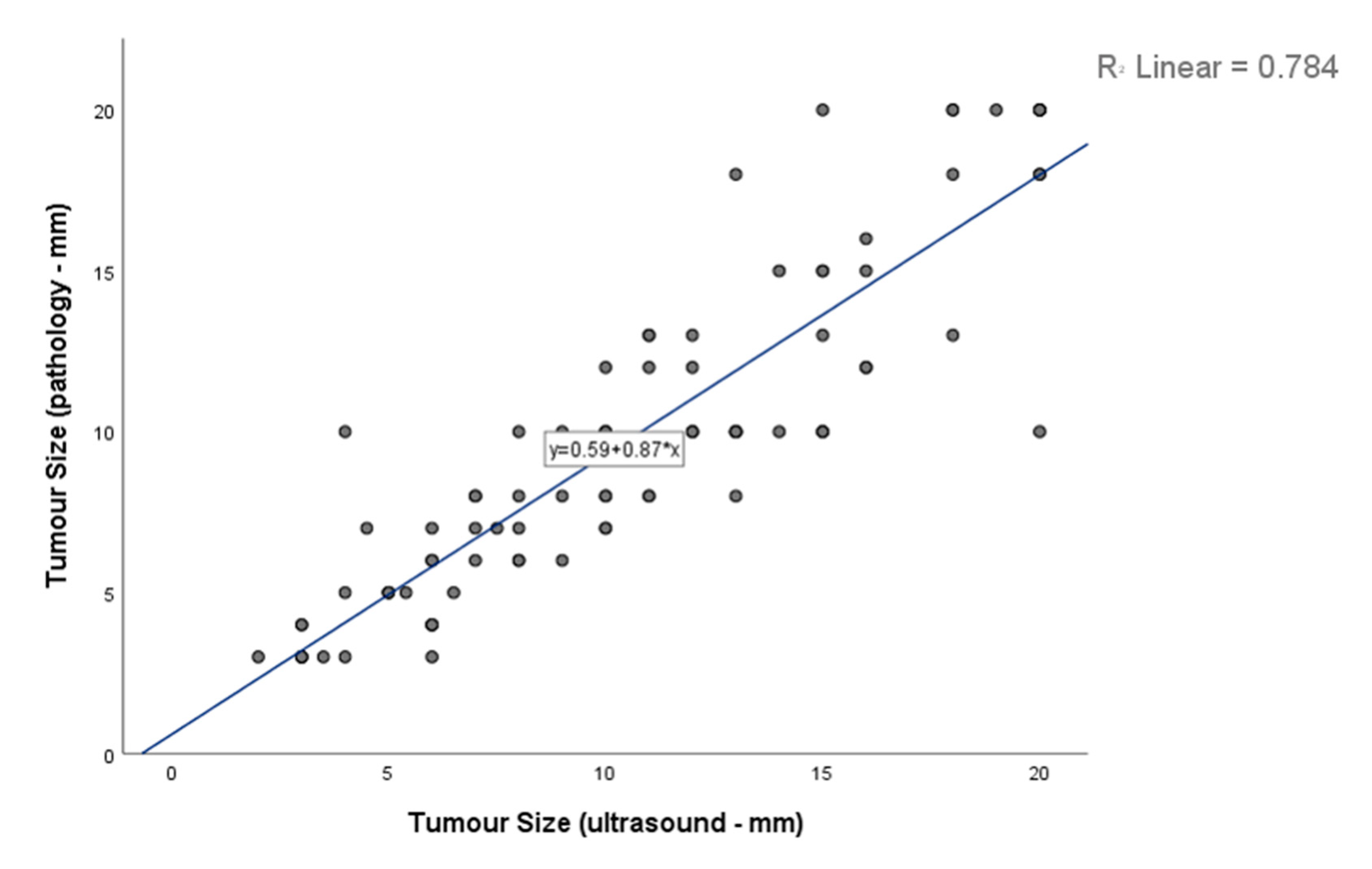
3.3. Tumor Size as Predictive Marker
3.4. Final Histopathological Findings
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFP | alpha-fetoprotein |
| AUC | area under the curve |
| CT | computed tomography |
| EAU | European Association of Urology |
| FSE | frozen section examination |
| GCNIS | germ cell neoplasia in situ |
| β-HCG | beta-human chorionic gonadotropin |
| IQR | interquartile ranges |
| IV | intravenous |
| LDH | lactate dehydrogenase |
| NPV | negative predictive values |
| OSS | organ-sparing surgery |
| PPV | positive predictive values |
| ROC | receiver operating characteristic |
| US | ultrasonography |
References
- Park, J.S.; Kim, J.; Elghiaty, A.; Ham, W.S. Recent global trends in testicular cancer incidence and mortality. Medicine (Baltimore) 2018, 97, e12390. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Roth, B. Treatment of clinical stage I germ cell tumors. Urology 2002, 59, 173–179. [Google Scholar] [CrossRef]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P.; Nicolai, N.; Oldenburg, J. Guidelines on Testicular Cancer: 2015 Update. Eur. Urol. 2015, 68, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Carmignani, L.; Gadda, F.; Gazzano, G.; Nerva, F.; Mancini, M.; Ferruti, M.; Bulfamante, G.; Bosari, S.; Coggi, G.; Rocco, F.; et al. High incidence of benign testicular neoplasms diagnosed by ultrasound. J. Urol. 2003, 170, 1783–1786. [Google Scholar] [CrossRef]
- Leonhartsberger, N.; Ramoner, R.; Aigner, F.; Stoehr, B.; Pichler, R.; Zangerl, F.; Fritzer, A.; Steiner, H. Increased incidence of Leydig cell tumours of the testis in the era of improved imaging techniques. BJU Int. 2011, 108, 1603–1607. [Google Scholar] [CrossRef]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C. European Association of Urology guidelines on Male Infertility: The 2012 update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef]
- Giannarini, G.; Dieckmann, K.P.; Albers, P.; Heidenreich, A.; Pizzocaro, G. Organ-sparing surgery for adult testicular tumours: A systematic review of the literature. Eur. Urol. 2010, 57, 780–790. [Google Scholar] [CrossRef]
- Brydøy, M.; Fosså, S.D.; Dahl, O.; Bjøro, T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol. 2007, 46, 480–489. [Google Scholar] [CrossRef]
- Fosså, S.D.; Oldenburg, J.; Dahl, A.A. Short- and long-term morbidity after treatment for testicular cancer. BJU Int. 2009, 104, 1418–1422. [Google Scholar] [CrossRef]
- Eberhard, J.; Ståhl, O.; Cwikiel, M.; Cavallin-Ståhl, E.; Giwercman, Y.; Salmonson, E.C.; Giwercman, A. Risk factors for post-treatment hypogonadism in testicular cancer patients. Eur. J. Endocrinol. 2008, 158, 561–570. [Google Scholar] [CrossRef]
- Matei, D.V.; Vartolomei, M.D.; Renne, G.; Tringali, V.M.L.; Russo, A.; Bianchi, R.; Cozzi, G.; Bottero, D.; Musi, G.; Mazzarol, G. Reliability of frozen section examination in a large cohort of testicular masses: What did we learn? Clin. Genitourin. Cancer 2017, 15, e689–e696. [Google Scholar] [CrossRef] [PubMed]
- Elert, A.; Olbert, P.; Hegele, A.; Barth, P.; Hofmann, R.; Heidenreich, A. Accuracy of frozen section examination of testicular tumors of uncertain origin. Eur. Urol. 2002, 41, 290–293. [Google Scholar] [CrossRef]
- Auer, T.; De Zordo, T.; Dejaco, C.; Gruber, L.; Pichler, R.; Jaschke, W.; Dogra, V.S.; Aigner, F. Value of Multiparametric US in the Assessment of Intratesticular Lesions. Radiology 2017, 285, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Gentile, G.; Rizzo, M.; Bianchi, L.; Falcone, M.; Dente, D.; Cilletti, M.; Franceschelli, A.; Vagnoni, V.; Garofalo, M.; Schiavina, R. Testis sparing surgery of small testicular masses: Retrospective analysis of a multicenter cohort. J. Urol. 2020, 203, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Leonhartsberger, N.; Pichler, R.; Stoehr, B.; Horninger, W.; Steiner, H. Organ-sparing surgery is the treatment of choice in benign testicular tumors. World J. Urol. 2014, 32, 1087–1091. [Google Scholar] [CrossRef]
- Leonhartsberger, N.; Pichler, R.; Stoehr, B.; Horninger, W.; Steiner, H. Organ preservation technique without ischemia in patients with testicular tumor. Urology 2014, 83, 1107–1111. [Google Scholar] [CrossRef]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P. EAU guidelines on testicular cancer: 2011 update. Eur Urol. 2011, 60, 304–319. [Google Scholar] [CrossRef]
- Steiner, H.; Leonhartsberger, N.; Stoehr, B.; Peschel, R.; Pichler, R. Postchemotherapy laparoscopic retroperitoneal lymph node dissection for low-volume, stage II, nonseminomatous germ cell tumor: First 100 patients. Eur. Urol. 2013, 63, 1013–1017. [Google Scholar] [CrossRef]
- Shilo, Y.; Zisman, A.; Raz, O.; Lang, E.; Strauss, S.; Sandbank, J.; Segal, M.; Siegel, Y.I.; Leibovici, D. Testicular sparing surgery for small masses. Urol. Oncol. 2012, 30, 188–191. [Google Scholar] [CrossRef]
- Silverio, P.C.; Schoofs, F.; Iselin, C.E.; Tille, J.-C. Fourteen-year experience with the intraoperative frozen section examination of testicular lesion in a tertiary university center. Ann. Diagn. Pathol. 2015, 19, 99–102. [Google Scholar] [CrossRef]
- Moul, J.W. Timely diagnosis of testicular cancer. Urol. Clin. N. Am. 2007, 34, 109. [Google Scholar] [CrossRef] [PubMed]
- Bosl, G.J.; Motzer, R.J. Testicular germ-cell cancer. N. Engl. J. Med. 1997, 337, 242–253. [Google Scholar] [CrossRef]
- Connolly, S.S.; D’Arcy, F.T.; Bredin, H.C.; Callaghan, J.; Corcoran, M.O. Value of frozen section analysis with suspected testicular malignancy. Urology 2006, 67, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.; Höltl, L.; Maneschg, C.; Berger, A.P.; Rogatsch, H.; Bartsch, G.; Hobisch, A. Frozen section analysis-guided organ-sparing approach in testicular tumors: Technique, feasibility, and long-term results. Urology 2003, 62, 508–513. [Google Scholar] [CrossRef]
- Gentile, G.; Brunocilla, E.; Franceschelli, A.; Schiavina, R.; Pultrone, C.; Borghesi, M.; Romagnoli, D.; Cevenini, M.; Dababneh, H.; Corcioni, B.; et al. Can testis-sparing surgery for small testicular masses be considered a valid alternative to radical orchiectomy? A prospective single-center study. Clin. Genitourin. Cancer 2013, 11, 522–526. [Google Scholar] [CrossRef]
- Bieniek, J.M.; Juvet, T.; Margolis, M.; Grober, E.D.; Lo, K.C.; Jarvi, K.A. Prevalence and management of incidental small testicular masses discovered on ultrasonographic evaluation of male infertility. J. Urol. 2018, 199, 481–486. [Google Scholar] [CrossRef]
- Cummings, J.M.; Boullier, J.A.; Sekhon, D.; Bose, K. Adult testicular torsion. J. Urol. 2002, 167, 2109–2110. [Google Scholar] [CrossRef]
- Miller, D.C.; Peron, S.E.; Keck, R.W.; Kropp, K.A. Effects of hypothermia on testicular ischemia. J. Urol. 1990, 143, 1046–1048. [Google Scholar] [CrossRef]
| All Tumors | Benign Tumors | Malignant Tumors | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Value | %, SD, IQR | Value | %, SD, IQR | Value | %, SD, IQR | ||
| Number of patients (n) | 89 | 60 | 29 | 0.581 (exact) | |||
| Unilaterally treated (n) | 79 | 88.8% | 53 | 88.3% | 26 | 89.7% | |
| Bilaterally treated (n) | 10 | 11.2% | 7 | 11.7% | 3 | 10.3% | |
| Age (years) | 0.026 | ||||||
| Available (n) | 89 | 100% | 60 | 100% | 29 | 100% | |
| Mean ± SD (years) | 38.4 | ±16.2 | 41.1 | ±16.8 | 32.9 | ±13.5 | |
| Duration of surgery (min) | 0.674 | ||||||
| Available (n) | 52 | 58.4% | 40 | 66.7% | 12 | 41.4% | |
| Mean ± SD (months) | 61.6 | ±20.2 | 62.3 | ±20.7 | 59.4 | ±19.2 | |
| Follow-up (months) | 0.017 | ||||||
| Available (n) | 89 | 100% | 60 | 100% | 29 | 100% | |
| Median (IQR) (months) | 42 | (3.5–75.5) | 32.5 | (1.3–64.8) | 59 | (29.5–100) | |
| All Tumors | Benign Tumors | Malignant Tumors | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Value | %, IQR | Value | %, IQR | Value | %, IQR | ||
| Number of treatments (n) | 99 | 67 | 32 | ||||
| Ultrasonographic size (mm) | <0.001 | ||||||
| Available (n) | 99 | 100% | 67 | 100% | 32 | 100% | |
| Median (IQR) (mm) | 10 | (6.0–15.0) | 8.0 | (5–10.0) | 14 | (8.3–17.5) | |
| Histopathological size (mm) | 0.018 | ||||||
| Available (n) | 85 | 85.9% | 56 | 56.6% | 29 | 90.6% | |
| Median (IQR) (mm) | 10 | (6.0–13.0) | 8.0 | (5.3–10.0) | 10.0 | (8.0–15.0) | |
| Palpation (n) | 0.138 | ||||||
| Available (n) | 87 | 87.9% | 60 | 89.6% | 27 | 84.4% | |
| Positive (n) | 26 | 29.9% | 15 | 25.0% | 11 | 40.7% | |
| Ischemia (n) | 0.461 (exact) | ||||||
| Available (n) | 97 | 98.0% | 66 | 98.5% | 31 | 96.9% | |
| Yes (n) | 9 | 9.3% | 5 | 7.6% | 4 | 12.9% | |
| Clinical presentation (n) | 0.001 (exact) | ||||||
| Available (n) | 82 | 82.8% | 57 | 85.1% | 25 | 78.1% | |
| Lump/swelling (n) | 23 | 28.0% | 16 | 28.1% | 7 | 28.0% | |
| Pain (n) | 11 | 13.4% | 7 | 12.3% | 4 | 16.0% | |
| Incidental (n) | 31 | 37.8% | 23 | 40.4% | 8 | 32.0% | |
| Inf/hyp/gyn 1 (n) | 11 | 13.4% | 11 | 19.3% | 0 | 0% | |
| Oncologic follow-up (n) | 6 | 7.3% | 0 | 0% | 6 | 24.0% | |
| Tumor Size (mm) | Overall (n = 99) | Benign (n = 67) | Malignant (n = 32) |
|---|---|---|---|
| ≤5 mm, n (%) | 21 | 20 (29.9) | 1 (3.1) |
| >5 mm and ≤10 mm, n (%) | 34 | 23 (34.3) | 11 (34.4) |
| >10 mm and ≤15 mm, n (%) | 26 | 17 (25.4) | 9 (28.1) |
| >15 mm and ≤20 mm, n (%) | 18 | 7 (10.4) | 11 (34.4) |
| Malignant and Benign Lesions | n | % |
|---|---|---|
| Seminomatous germ-cell tumor | 22 | 22.2 |
| Nonseminomatous: pure teratoma | 8 | 8.1 |
| Nonseminomatous: mixed germ cell 1 | 2 | 2.0 |
| Leydig cell tumor or hyperplasia | 32 | 32.3 |
| Sertoli cell tumor | 4 | 4.0 |
| Fibrotic pseudotumor | 10 | 10.1 |
| Adenomatoid tumor | 2 | 2.0 |
| Dermoid cyst | 10 | 10.1 |
| Cystic lesion | 3 | 3.0 |
| Hemangioma | 4 | 4.0 |
| Splenogonadal fusion | 1 | 1.0 |
| Leiomyoma | 1 | 1.0 |
| Total | 99 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staudacher, N.; Tulchiner, G.; Bates, K.; Ladurner, M.; Kafka, M.; Aigner, F.; Pichler, R.; Horninger, W. Organ-Sparing Surgery in Testicular Tumor: Is This the Right Approach for Lesions ≤ 20 mm? J. Clin. Med. 2020, 9, 2911. https://doi.org/10.3390/jcm9092911
Staudacher N, Tulchiner G, Bates K, Ladurner M, Kafka M, Aigner F, Pichler R, Horninger W. Organ-Sparing Surgery in Testicular Tumor: Is This the Right Approach for Lesions ≤ 20 mm? Journal of Clinical Medicine. 2020; 9(9):2911. https://doi.org/10.3390/jcm9092911
Chicago/Turabian StyleStaudacher, Nina, Gennadi Tulchiner, Katie Bates, Michael Ladurner, Mona Kafka, Friedrich Aigner, Renate Pichler, and Wolfgang Horninger. 2020. "Organ-Sparing Surgery in Testicular Tumor: Is This the Right Approach for Lesions ≤ 20 mm?" Journal of Clinical Medicine 9, no. 9: 2911. https://doi.org/10.3390/jcm9092911
APA StyleStaudacher, N., Tulchiner, G., Bates, K., Ladurner, M., Kafka, M., Aigner, F., Pichler, R., & Horninger, W. (2020). Organ-Sparing Surgery in Testicular Tumor: Is This the Right Approach for Lesions ≤ 20 mm? Journal of Clinical Medicine, 9(9), 2911. https://doi.org/10.3390/jcm9092911






