Analysis of Risk Factors and Long-Term Outcomes in Kidney Transplant Patients with Identified Lymphoceles
Abstract
:1. Introduction
2. Experimental Section
2.1. Patient Selection and Data Acquisition
2.2. Definitions
2.3. Rejection Episodes
2.4. Immunosuppression
2.5. Evaluation of De Novo Donor-Specific Antibodies
2.6. Surgical Technique and Postoperative Treatment
2.7. Data Analysis
3. Results
3.1. Patient characteristics
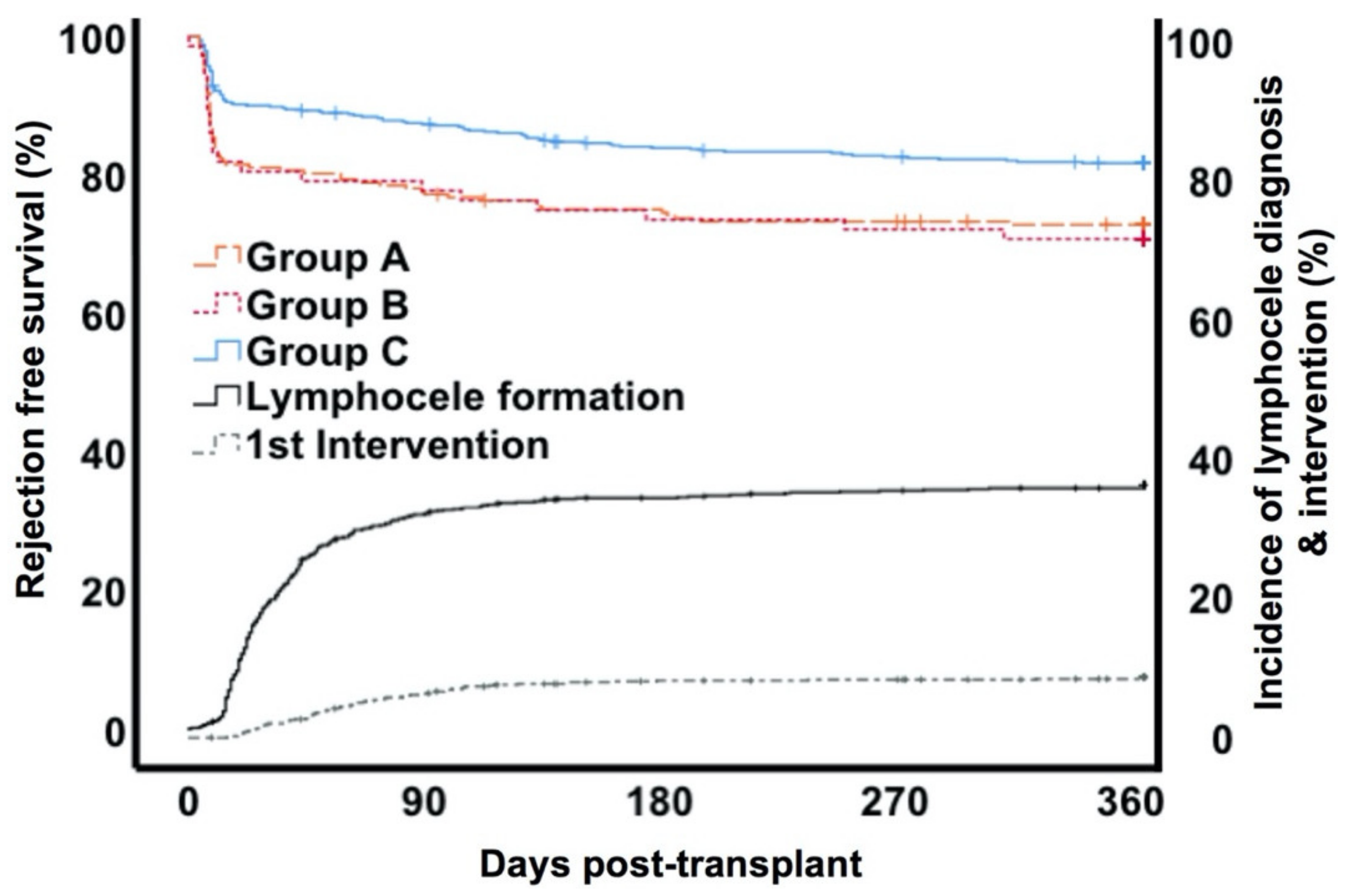
3.2. Course and Treatment Of Lymphoceles
3.3. Rejection and Graft Function
3.4. Risk Factors for Lymphocele Formation and Treatment
3.5. Donor Specific Antibody Formation
3.6. Patient and Graft Survival
4. Discussion
Considerations for Clinical Practice
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Goel, M.; Flechner, S.M.; Zhou, L.; Mastroianni, B.; Savas, K.; Derweesh, I.; Patel, P.; Modlin, C.; Goldfarb, D.; Novick, A.C. The influence of various maintenance immunosuppressive drugs on lymphocele formation and treatment after kidney transplantation. J. Urol. 2004, 171, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Ranghino, A.; Segoloni, G.P.; Lasaponara, F.; Biancone, L. Lymphatic disorders after renal transplantation: New insights for an old complication. Clin. Kidney J. 2015, 8, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caglar, M.; Ergun, E.L. Lymphatic leakage in a renal transplant: The role of lymphoscintigraphy. Clin. Nucl. Med. 2006, 31, 486–489. [Google Scholar] [PubMed]
- Minetti, E.E. Lymphocele after renal transplantation, a medical complication. J. Nephrol. 2011, 24, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Pacovsky, J.; Hyšpler, R.; Navratil, P.; Tichá, A.; Brodak, M. The estimation of post-transplant lymphocele origin using creatine kinase activity. Ups. J. Med. Sci. 2010, 115, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebadzadeh, M.R.; Tavakkoli, M. Lymphocele after kidney transplantation: Where are we standing now? Urol. J. 2008, 5, 144–148. [Google Scholar]
- De Lima, M.L.; Cotrim, C.A.C.; Moro, J.C.; Miyaoka, R.; D’Ancona, C.A.L. Laparoscopic treatment of lymphoceles after renal transplantation. Int. Braz. J. Urol. 2012, 38, 215–221. [Google Scholar] [CrossRef]
- Khauli, R.B.; Stoff, J.S.; Lovewell, T.; Ghavamian, R.; Baker, S. Post-transplant lymphoceles: A critical look into the risk factors, pathophysiology and management. J. Urol. 1993, 150, 22–26. [Google Scholar] [CrossRef]
- Risaliti, A.; Corno, V.; Donini, A.; Cautero, N.; Baccarani, U.; Pasqualucci, A.; Terrosu, G.; Cedolini, C.; Bresadola, F. Laparoscopic treatment of symptomatic lymphoceles after kidney transplantation. Surg. Endosc. 2000, 14, 293–295. [Google Scholar] [CrossRef]
- Ulrich, F.; Niedzwiecki, S.; Fikatas, P.; Nebrig, M.; Schmidt, S.C.; Kohler, S.; Weiss, S.; Schumacher, G.; Pascher, A.; Reinke, P.; et al. Symptomatic lymphoceles after kidney transplantation - multivariate analysis of risk factors and outcome after laparoscopic fenestration. Clin. Transplant. 2009, 24, 273–280. [Google Scholar] [CrossRef]
- Zagdoun, E.; Ficheux, M.; Lobbedez, T.; Chatelet, V.; Thuillier-Lecouf, A.; Bensadoun, H.; Ryckelynck, J.-P.; De Ligny, B.H. Complicated lymphoceles after kidney transplantation. Transpl. Proc. 2010, 42, 4322–4325. [Google Scholar] [CrossRef] [PubMed]
- Sansalone, C.V.; Aseni, P.; Minetti, E.; Di Benedetto, F.; Rossetti, O.; Manoochehri, F.; Vertemati, M.; Giacomoni, A.; Civati, G.; Forti, D. Is lymphocele in renal transplantation an avoidable complication? Am. J. Surg. 2000, 179, 182–185. [Google Scholar] [CrossRef]
- Dean, P.G.; Lund, W.J.; Larson, T.S.; Prieto, M.; Nyberg, S.L.; Ishitani, M.B.; Kremers, W.K.; Stegall, M.D. Wound-healing complications after kidney transplantation: A prospective, randomized comparison of sirolimus and tacrolimus. Transplantation 2004, 77, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Giessing, M.; Budde, K. Sirolimus and lymphocele formation after kidney transplantation: An immunosuppressive medication as co-factor for a surgical problem? Nephrol. Dial. Transplant. 2003, 18, 448–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langer, R.M.; Kahan, B.D. Incidence, therapy, and consequences of lymphocele after sirolimus-cyclosporine-prednisone immunosuppression in renal transplant recipients. Transplantation 2002, 74, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Veeramani, M.; Mishra, S.; Kurien, A.; Ganpule, A.; Sabnis, R. Does rejection have a role in lymphocele formation post renal transplantation? A single centre experience. Indian J. Urol. 2010, 26, 193–195. [Google Scholar] [CrossRef]
- Rashid, A.; Posen, G.; Couture, R.; McKay, D.; Wellington, J. Accumulation of lymph around the transplanted kidney (lymphocele) mimicking renal allograft rejection. J. Urol. 1974, 111, 145–147. [Google Scholar] [CrossRef]
- Kerjaschki, D. The crucial role of macrophages in lymphangiogenesis. J. Clin. Investig. 2005, 115, 2316–2319. [Google Scholar] [CrossRef] [Green Version]
- Kerjaschki, D. Lymphatic neoangiogenesis in renal transplants: A driving force of chronic rejection? J. Nephrol. 2006, 19, 403–406. [Google Scholar]
- Kerjaschki, D.; Huttary, N.; Raab, I.; Regele, H.; Bojarski-Nagy, K.; Bartel, G.; Kröber, S.M.; Greinix, H.; Rosenmaier, A.; Karlhofer, F.; et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat. Med. 2006, 12, 230–234. [Google Scholar] [CrossRef]
- Kerjaschki, D. Lymphatic Neoangiogenesis in Human Kidney Transplants Is Associated with Immunologically Active Lymphocytic Infiltrates. J. Am. Soc. Nephrol. 2004, 15, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuht, S.; Gwinner, W.; Franz, I.; Schwarz, A.; Jonigk, D.; Kreipe, H.; Kerjaschki, D.; Haller, H.; Mengel, M. Lymphatic neoangiogenesis in human renal allografts: Results from sequential protocol biopsies. Am. J. Transplant. 2007, 7, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, I.; Yamaguchi, Y.; Yamamoto, H.; Hosoya, T.; Horita, S.; Tanabe, K.; Fuchinoue, S.; Teraoka, S.; Toma, H. A pathological analysis of lymphatic vessels in early renal allograft. Transplant. Proc. 2006, 38, 3300–3303. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.E.; Gralla, J.; Cagle, L.; Goldberg, R.; Chan, L.; Wiseman, A.C. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation 2011, 91, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Devos, J.M.; Gaber, A.O.; Teeter, L.D.; Graviss, E.A.; Patel, S.J.; Land, G.A.; Moore, L.W.; Knight, R.J. Intermediate-term graft loss after renal transplantation is associated with both donor-specific antibody and acute rejection. Transplantation 2014, 97, 534–540. [Google Scholar] [CrossRef]
- Loupy, A.; Hill, G.S.; Jordan, S.C. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat. Rev. Nephrol. 2012, 8, 348–357. [Google Scholar] [CrossRef]
- Loupy, A.; Lefaucheur, C.; Vernerey, D.; Prugger, C.; Van Huyen, J.-P.D.; Mooney, N.; Suberbielle, C.; Fremeaux-Bacchi, V.; Méjean, A.; Desgrandchamps, F.; et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N. Engl. J. Med. 2013, 369, 1215–1226. [Google Scholar] [CrossRef] [Green Version]
- Loupy, A.; Suberbielle-Boissel, C.; Hill, G.S.; Lefaucheur, C.; Anglicheau, D.; Zuber, J.; Martinez, F.; Thervet, E.; Mejean, A.; Charron, D.; et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am. J. Transplant. 2009, 9, 2561–2570. [Google Scholar] [CrossRef]
- Schröter, K.; Trzebiatowski, G.L.; Fritsche, L. Tbase—Aninternet-BASED transplantation Database. Workshop on Concurrency, Specification and Programming; CS&P: Berlin, Germany, 1998; Volume 110, p. 214. [Google Scholar]
- OPTN. A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI). Available online: http://optn.transplant.hrsa.gov/ContentDocuments/Guide_to_Calculating_Interpreting_KDPI.pdf (accessed on 13 March 2018).
- OPTN. KDRI to KDPI Mapping Table. Available online: http://optn.transplant.hrsa.gov/ContentDocuments/KDRI_to_KDPI_Mapping_Table.pdf (accessed on 22 March 2016).
- Massie, A.B.; Leanza, J.; Fahmy, L.M.; Chow, E.K.H.; Desai, N.M.; Luo, X.; King, E.A.; Bowring, M.G.; Segev, D.L. A risk index for living donor kidney transplantation. Am. J. Transplant. 2016, 16, 2077–2084. [Google Scholar] [CrossRef] [Green Version]
- Haas, M.; Sis, B.; Racusen, L.C.; Solez, K.; Glotz, D.; Colvin, R.B.; De Castro, M.C.R.; David, D.S.R.; David-Neto, E.; Bagnasco, S.M.; et al. Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am. J. Transplant. 2014, 14, 272–283. [Google Scholar] [CrossRef]
- Lachmann, N.; Terasaki, P.I.; Budde, K.; Liefeldt, L.; Kahl, A.; Reinke, P.; Pratschke, J.; Rudolph, B.; Schmidt, D.; Salama, A.; et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation 2009, 87, 1505–1513. [Google Scholar] [CrossRef]
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Tamura, M.K.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735. [Google Scholar] [CrossRef] [Green Version]
- Braun, W.E.; Banowsky, L.H.; Straffon, R.A.; Nakamoto, S.; Kiser, W.S.; Popowniak, K.L.; Hewitt, C.B.; Stewart, B.H.; Zelch, J.V.; Magalhaes, R.L.; et al. Lymphocytes associated with renal transplantation. Report of 15 cases and review of the literature. Am. J. Med. 1974, 57, 714–729. [Google Scholar] [CrossRef]
- Lucewicz, A.; Wong, G.; Lam, V.W.; Hawthorne, W.J.; Allen, R.; Craig, J.C.; Pleass, H.C. Management of primary symptomatic lymphocele after kidney transplantation: A systematic review. Transplantation 2011, 92, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Lipay, M.A.S.; Noronha, I.D.L.; Júnior, A.V.; Júnior, J.E.R.; Campagnari, J.C.; Srougi, M. Lymphocele: A possible relationship with acute cellular rejection in kidney transplantation. Sao Paulo Med. J. 1999, 117, 238–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ristimaki, A.; Narko, K.; Enholm, B.; Joukov, V.; Alitalo, K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J. Biol. Chem. 1998, 273, 8413–8418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gago, M.; Cornell, L.D.; Kremers, W.K.; Stegall, M.; Cosio, F.G. Kidney allograft inflammation and fibrosis, causes and consequences. Am. J. Transplant. 2012, 12, 1199–1207. [Google Scholar] [CrossRef]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on ischemia-reperfusion injury in kidney transplantation: Pathogenesis and treatment. World J. Transplant. 2015, 5, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.P.; Beitz, G.; Eng, M.; Gibbons, N.; Hickey, D.; Little, D. Long-term outcome of cadaveric renal transplant after treatment of symptomatic lymphocele. J. Urol. 2006, 176, 1069–1072. [Google Scholar] [CrossRef]
- Institute for Applied Quality Promotion and Research in Health Care GmbH. Federal evaluation for the year 2012. NTX - Kidney Transplantation Quality Indicators. Basic evaluation. 2013. Available online: http://www.sqg.de/downloads/Bundesauswertungen/2012/bu_Gesamt_NTX_2012.pdf (accessed on 23 August 2020).
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Wilk, A.R.; Robinson, A.; Wainright, J.L.; Haynes, C.R.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2018 annual data report: Kidney. Am. J. Transplant. 2020, 20, 20–130. [Google Scholar] [CrossRef]
- Rodriguez, F.O.; Boissier, R.; Budde, K.; Figueiredo, A.; Taylor, C.F.; Hevia, V.; García, E.L.; Regele, H.; Zakri, R.H.; Olsburgh, J.; et al. European association of urology guidelines on renal transplantation: Update 2018. Eur. Urol. Focus 2018, 4, 208–215. [Google Scholar] [CrossRef] [PubMed]




| Group A | Group B | Group C | p-Value | |
|---|---|---|---|---|
| n (number), (%) | 233 (26.8%) | 72 (8.3%) | 562 (64.8%) | |
| Mean follow up, years (SD) | 6.2 (2.8) | 6.5 (2.8) | 5.4 (2.8) | 0.006 |
| Mean recipient age, years (SD) | 52 (14) | 54 (15) | 50 (15) | 0.016 |
| Mean donor age, years (SD) | 53 (15) | 58 (13) | 53 (15) | 0.027 |
| Male, n | 149 (64%) | 40 (56%) | 335 (60%) | 0.339 |
| Re-transplantation, n | 21 (9%) | 12 (17%) | 60 (11%) | 0.189 |
| Median time on dialysis, months (IQR) | 53 (21–87) | 61 (22–85) | 47 (12–84) | 0.112 |
| Dialysis modality, n | 0.09 | |||
| Hemodialysis: | 201 (86.3%) | 61 (84.7%) | 436 (77.6%) | |
| Peritoneal dialysis: | 14 (6%) | 7 (9.7%) | 47 (8.4%) | |
| Preemptive: | 10 (4.3%) | 2 (2.8%) | 44 (7.8%) | |
| Unknown: | 8 (3.4%) | 2 (2.8%) | 35 (6.2%) | |
| Mean HLA-A, B, DR mismatches (SD) | 2.8 (1.7) | 2.9 (1.5) | 2.8 (1.7) | 0.906 |
| Median cold ischemia time (cadaveric donation), hours (IQR) | 11 (8.1–15.2) | 11.1 (7.5–15.5) | 11.2 (8–14.4) | 0.961 |
| Median cold ischemia time (living donation), hours (IQR) | 2.5 (2.2–3) | 2.4 (2.1–3.2) | 2.5 (2.2–3.1) | 0.99 |
| Body mass index, kg/m2 (SD) | 25.8 (4.5) | 25.6 (4.3) | 25.8 (4.6) | 0.939 |
| Pre-Tx Diabetes (all types) | 22 (9.4%) | 8 (11.1%) | 42 (7.5%) | 0.391 |
| Living donation, n | 60 (26%) | 14 (19%) | 210 (37%) | <0.001 |
| Donor creatinine, mg/dL (IQR) | 0.86 (0.70–1.04) | 0.87 (0.71–1.11) | 0.80 (0.69–0.99) | 0.103 |
| KDPI (SD)(deceased donor kidneys, n = 581) | 65 (29) | 77 (23) | 63 (30) | 0.004 |
| Living kidney donor profile indices (LKDPI) (living donor kidneys, n = 284) | 20 (24) | 20 (29) | 23 (24) | 0.572 |
| Delayed graft function, n | 98 (42.1%) | 38 (52.7%) | 182 (32.4%) | <0.001 |
| Preformed donor specific antibodies, n | 6 (0.7%) | 1 (0.1%) | 27 (3.1%) | 0.173 |
| Rejection episode (T-cell mediated rejection + antibody mediated rejection + Borderline) | 72 (30.9%) | 25 (34.7%) | 122 (21.7%) | 0.004 |
| T-cell mediated rejection | 48 (20.6%) | 18 (25%) | 92 (16.4%) | 0.111 |
| Primary immunosuppression at transplantation | ||||
| - Tacrolimus | 99 (43%) | 22 (31%) | 314 (56%) | <0.001 |
| - Cyclosporine | 127 (55%) | 47 (65%) | 233 (42%) | <0.001 |
| - mammalian target of rapamycin (mTOR) inhibitor | 4 (2%) | 1 (1%) | 5 (1%) | 0.499 |
| - mycophenolic acid | 224 (96%) | 71 (99%) | 542 (96%) | 0.699 |
| - others | 19 (8%) | 3 (4%) | 37 (7%) | 0.523 |
| - Basiliximab induction | all patients | |||
| Group A | Group B | |||||||
|---|---|---|---|---|---|---|---|---|
| Monovariate | Multivariate | Monovariate | Multivariate * | |||||
| Variable | OR | p-Value | OR | p-Value | OR | p-Value | OR | p-Value |
| Mean recipient age per year | 1.01 | 0.008 | 1.02 | 0.039 | ||||
| Mean donor age per year | 1.01 | 0.131 | 1.02 | 0.008 | 1.02 | 0.023 | ||
| Male sex | 0.91 | 0.498 | 1.25 | 0.377 | ||||
| Re-transplantation | 1.02 | 0.948 | 1.76 | 0.093 | ||||
| Median time on dialysis per month | 1.00 | 0.079 | 1.00 | 0.568 | ||||
| Mean HLA-mismatches per mismatch | 1.01 | 0.835 | 1.03 | 0.657 | ||||
| Median cold ischemia time per hour | 1.03 | 0.005 | 1.04 | 0.035 | ||||
| Donor creatinine per mg/dL | 1.15 | 0.189 | 1.15 | 0.373 | ||||
| Living donation | 0.54 | <0.001 | 0.55 | <0.001 | 0.47 | 0.014 | ||
| BMI per kg/m2 | 1.00 | 0.779 | 0.99 | 0.755 | ||||
| Diabetes | 1.35 | 0.23 | 1.43 | 0.37 | ||||
| Rejection episode | 1.68 | 0.001 | 1.61 | 0.003 | 1.65 | 0.056 | ||
| DGF | 1.68 | 0.001 | 2.06 | 0.004 | 1.9 | 0.011 | ||
| Monovariate | Multivariate * | |||
|---|---|---|---|---|
| n | HR | p-Value | HR | p-Value |
| Mean recipient age per year | 1.03 | <0.001 | ||
| Mean donor age per year | 1.04 | <0.001 | 1.04 | <0.001 |
| Male sex | 1.29 | 0.180 | ||
| Re-transplantation | 1.59 | 0.079 | 1.74 | 0.046 |
| Median time on dialysis per month | 1.00 | 0.230 | ||
| Mean HLA-mismatches per mismatch | 1.24 | <0.001 | ||
| Median cold ischemia time per hour | 1.03 | 0.056 | ||
| Living donation | 0.47 | 0.002 | ||
| Diabetes | 2.34 | 0.001 | ||
| BMI per kg/m2 | 1.06 | 0.006 | ||
| Rejection episode | 2.5 | <0.001 | 1.94 | 0.001 |
| DGF | 2.52 | <0.001 | 1.73 | 0.008 |
| Lymphocele treatment | 0.91 | 0.788 | n.a. | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehner, L.J.; Hohberger, A.; Marschke, L.; Lachmann, N.; Peters, R.; Friedersdorff, F.; Khadzhynov, D.; Halleck, F.; Budde, K.; Staeck, O.; et al. Analysis of Risk Factors and Long-Term Outcomes in Kidney Transplant Patients with Identified Lymphoceles. J. Clin. Med. 2020, 9, 2841. https://doi.org/10.3390/jcm9092841
Lehner LJ, Hohberger A, Marschke L, Lachmann N, Peters R, Friedersdorff F, Khadzhynov D, Halleck F, Budde K, Staeck O, et al. Analysis of Risk Factors and Long-Term Outcomes in Kidney Transplant Patients with Identified Lymphoceles. Journal of Clinical Medicine. 2020; 9(9):2841. https://doi.org/10.3390/jcm9092841
Chicago/Turabian StyleLehner, Lukas J., Arnim Hohberger, Lisanne Marschke, Nils Lachmann, Robert Peters, Frank Friedersdorff, Dmytro Khadzhynov, Fabian Halleck, Klemens Budde, Oliver Staeck, and et al. 2020. "Analysis of Risk Factors and Long-Term Outcomes in Kidney Transplant Patients with Identified Lymphoceles" Journal of Clinical Medicine 9, no. 9: 2841. https://doi.org/10.3390/jcm9092841
APA StyleLehner, L. J., Hohberger, A., Marschke, L., Lachmann, N., Peters, R., Friedersdorff, F., Khadzhynov, D., Halleck, F., Budde, K., Staeck, O., & Duerr, M. (2020). Analysis of Risk Factors and Long-Term Outcomes in Kidney Transplant Patients with Identified Lymphoceles. Journal of Clinical Medicine, 9(9), 2841. https://doi.org/10.3390/jcm9092841





