Early Biomarkers of Neurodegenerative and Neurovascular Disorders in Diabetes
Abstract
1. Introduction
2. C-Reactive Protein
2.1. MicroRNAs
2.2. Paraoxonase 1
2.3. Tau, β-Amyloid and Glycogen Synthase Kinase 3β
2.4. Phosphoinositide 3-Kinases
2.5. Amylin
2.6. Dopamine
2.7. Gamma-Glutamyl Transferase
2.8. Growth Factors
2.9. Homocysteine
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AMD | Age-related macular degeneration |
| DR | Diabetic retinopathy |
| MCI | Mild Cognitive Impairment |
| PD | Parkinson’s disease |
| T2DM | type 2 diabetes mellitus |
| Aβ-42 | amyloid beta peptide 42 |
| AGE | advanced glycation end products |
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| CRP | C-reactive protein |
| DA | dopamine |
| EGF | epidermal growth factor |
| GGT | gamma-glutamyl transpeptidase |
| GSK-3β | glycogen synthase kinase 3β |
| HCY | homocysteine |
| IL-6 | interleukin-6 |
| NGF | Nerve growth factor |
| NO | nitric oxide |
| e-NOS | endothelial nitric oxide species |
| PEDF | Pigment epithelium-derived factor |
| PI3K | Phosphoinositide 3-kinases |
| PON-1 | paraoxonase 1 |
| ROS | reactive oxygen species |
| TGF- β | transforming growth factor β1 |
| tau | tau protein |
| VEGF | vascular endothelial growth factor |
| ↑/↓1 | fluctuations or serum level disturbances |
References
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Vodolazkaia, A.; Bossuyt, X.; Fassbender, A.; Kyama, C.M.; Meuleman, C.; Peeraer, K.; Tomassetti, C.; D’Hooghe, T.M. A high sensitivity assay is more accurate than a classical assay for the measurement of plasma CRP levels in endometriosis. Reprod. Biol. Endocrinol. 2011, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Pfützner, A.; Forst, T. High-Sensitivity C-Reactive Protein as Cardiovascular Risk Marker in Patients with Diabetes Mellitus. Diabetes Technol. Ther. 2006, 8, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, S.; Liu, Y.; Bazzano, L.; He, J.; Mi, J.; Chen, W. Temporal relationship between inflammation and insulin resistance and their joint effect on hyperglycemia: The Bogalusa Heart Study. Cardiovasc. Diabetol. 2019, 18, 109–110. [Google Scholar] [CrossRef]
- Amor, S.; Peferoen, L.A.N.; Vogel, D.Y.S.; Breur, M.; Van Der Valk, P.; Baker, D.; Van Noort, J.M. Inflammation in neurodegenerative diseases—An update. Immunology 2014, 142, 151–166. [Google Scholar] [CrossRef]
- Nadrowski, P.; Chudek, J.; Skrzypek, M.; Puzianowska-Kuznicka, M.; Mossakowska, M.; Więcek, A.; Zdrojewski, T.; Grodzicki, T.; Kozakiewicz, K. Associations between cardiovascular disease risk factors and IL-6 and hsCRP levels in the elderly. Exp. Gerontol. 2016, 85, 112–117. [Google Scholar] [CrossRef]
- Gorska-Ciebiada, M.; Ciebiada, M. Association of hsCRP and vitamin D levels with mild cognitive impairment in elderly type 2 diabetic patients. Exp. Gerontol. 2020, 135, 110926. [Google Scholar] [CrossRef]
- Chen, W.-W.; Zhang, X.; Huang, W.-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Mathews, S.T.; Kothari, V.; Galdo, J.A. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [CrossRef]
- Rotermund, C.; Machetanz, G.; Fitzgerald, J.C. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Front. Endocrinol. 2018, 9, 400. [Google Scholar] [CrossRef]
- Luan, Y.-Y.; Yao, Y.-M. The Clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immunol. 2018, 9, 1302. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xiao, Y.; Wu, J.; Gan, L.; Huang, Y.; Wang, J. C-Reactive Protein and Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Meneses, M.J.; Silvestre, R.; Sousa-Lima, I.; Macedo, M.; Lima, S. Paraoxonase-1 as a Regulator of Glucose and Lipid Homeostasis: Impact on the Onset and Progression of Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4049. [Google Scholar] [CrossRef] [PubMed]
- Shunmoogam, N.; Naidoo, P.; Chilton, R. Paraoxonase (PON)-1: A brief overview on genetics, structure, polymorphisms and clinical relevance. Vasc. Health Risk Manag. 2018, 14, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, P.; Ozdemir, B.; Mert, M.; Okuturlar, Y. Relation of Paraoxonase 1 Activity with Biochemical Variables, Brachial Artery Intima-Media Thickness in Patients with Diabetes with or without Obesity. Obes. Facts 2018, 11, 56–66. [Google Scholar] [CrossRef]
- Bigagli, E.; Lodovici, M. Circulating Oxidative Stress Biomarkers in Clinical Studies on Type 2 Diabetes and Its Complications. Oxid. Med. Cell. Longev. 2019, 2019, 5953685. [Google Scholar] [CrossRef]
- Meaney, E.; Sierra-Vargas, P.; Meaney, A.; Guzmán-Grenfell, M.; Ramírez-Sánchez, I.; Hicks, J.J.; Olivares-Corichi, I.; Ceballos, G. Does Metformin Increase Paraoxonase Activity in Patients with the Metabolic Syndrome? Additional Data from the MEFISTO Study. Clin. Transl. Sci. 2012, 5, 265–268. [Google Scholar] [CrossRef]
- Menini, T.; Gugliucci, A. Paraoxonase 1 in neurological disorders. Redox Rep. 2014, 19, 49–58. [Google Scholar] [CrossRef]
- Gratuze, M.; Joly-Amado, A.; Buee, L.; Vieau, D.; Blum, D. Tau, Diabetes and Insulin. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2019; Volume 1184, pp. 259–287. [Google Scholar]
- Huang, H.-C.; Jiang, Z.-F. Accumulated Amyloid-β Peptide and Hyperphosphorylated Tau Protein: Relationship and Links in Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 16, 15–27. [Google Scholar] [CrossRef]
- Kubis-Kubiak, A.; Dyba, A.; Piwowar, A. The Interplay between Diabetes and Alzheimer’s Disease—In the Hunt for Biomarkers. Int. J. Mol. Sci. 2020, 21, 2744. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, N.Q.; Yan, F.; Jin, H.; Zhou, S.-Y.; Shi, J.-S.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mudher, A. Alzheimer’s Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front. Mol. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef] [PubMed]
- Maffei, A.; Lembo, G.; Carnevale, D. PI3Kinases in Diabetes Mellitus and Its Related Complications. Int. J. Mol. Sci. 2018, 19, 4098. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Na, L.; Li, Y.; Chen, L. Roles of the PI3K/AKT/mTOR signaling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pillay, K.; Govender, P. Amylin Uncovered: A Review on the Polypeptide Responsible for Type II Diabetes. BioMed. Res. Int. 2013, 2013, 826706. [Google Scholar] [CrossRef] [PubMed]
- Lutz, T.A.; Meyer, U. Amylin at the interface between metabolic and neurodegenerative disorders. Front. Mol. Neurosci. 2015, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Grizzanti, J.; Corrigan, R.; Servizi, S.; Casadesus, G. Amylin Signaling in Diabetes and Alzheimer’s Disease: Therapy or Pathology? J. Neurol. Neuromed. 2019, 4, 12–16. [Google Scholar] [CrossRef]
- Msc, I.M.; Valenti-Azcarate, R.; Amat-Villegas, I.; Riverol, M.; Marcilla, I.; Andrea, C.E.; Sánchez-Arias, J.A.; Carmona-Abellan, M.; Marti, G.; Erro, M.; et al. Amylin as a potential link between type 2 diabetes and alzheimer disease. Ann. Neurol. 2019, 86, 539–551. [Google Scholar] [CrossRef]
- Qiu, W.; Zhu, H. Amylin and its analogs: A friend or foe for the treatment of Alzheimer’s disease? Front. Aging Neurosci. 2014, 6, 186. [Google Scholar] [CrossRef]
- Bharadwaj, P.; Wijesekara, N.; Liyanapathirana, M.; Newsholme, P.; Ittner, L.M.; Fraser, P.; Verdile, G. The Link between Type 2 Diabetes and Neurodegeneration: Roles for Amyloid-β, Amylin, and Tau Proteins. J. Alzheimer’s Dis. 2017, 59, 421–432. [Google Scholar] [CrossRef]
- Ko, J.H.; Strafella, A.P. Dopaminergic neurotransmission in the human brain: New lessons from perturbation and imaging. Neuroscientist 2012, 18, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, J. Degeneration of Dopaminergic Neurons Due to Metabolic Alterations and Parkinson’s Disease. Front. Aging Neurosci. 2016, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Jaspan, J.B.; Kastin, A.J. Effect of Diabetes Mellitus on the Permeability of the Blood–Brain Barrier to Insulin. Peptides 1997, 18, 1577–1584. [Google Scholar] [CrossRef]
- Lee, S.E.; Han, K.; Baek, J.Y.; Ko, K.S.; Lee, K.-U.; Koh, E.H. Taskforce Team for Diabetes Fact Sheet of the Korean Diabetes Association Association Between Diabetic Retinopathy and Parkinson Disease: The Korean National Health Insurance Service Database. J. Clin. Endocrinol. Metab. 2018, 103, 3231–3238. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Lee, J.-Y.; Kim, T.W.; Yoon, E.J.; Oh, S.; Kim, Y.K.; Kim, J.-M.; Woo, S.J.; Kim, K.W.; Jeon, B. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology 2018, 91, e1003–e1012. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Mo, E.Y.; Shin, S.J.; Moon, S.D.; Han, J.H.; Kim, E.S. Serum gamma-glutamyltransferase is not associated with subclinical atherosclerosis in patients with type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Björk, M.P.; Johansson, B. Gamma-Glutamyltransferase (GGT) as a biomarker of cognitive decline at the end of life: Contrasting age and time to death trajectories. Int. Psychogeriatr. 2017, 30, 981–990. [Google Scholar] [CrossRef]
- Hong, S.-H.; Han, K.; Park, S.; Kim, S.M.; Kim, N.H.; Choi, K.M.; Baik, S.H.; Park, Y.-G.; Yoo, H.J. Gamma-Glutamyl Transferase Variability and Risk of Dementia in Diabetes Mellitus: A Nationwide Population-Based Study. J. Clin. Endocrinol. Metab. 2020, 105, e119–e129. [Google Scholar] [CrossRef]
- Divya, R.; Ashok, V. Evaluation of serum gamma glutamyl transferase levels as a marker of oxidative stress in type 2 diabetes patients with and without retinopathy. MedPulse Int. J. Physiol. 2019, 9, 30–34. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, J.; Zhou, X.; Zhang, R.; Xie, T.; Zou, Z.; Liao, L.; Dong, J. Thiazolidinediones versus metformin on improving abnormal liver enzymes in patients with type 2 diabetes mellitus: A meta-analysis. Oncotarget 2018, 9, 12389–12399. [Google Scholar] [CrossRef][Green Version]
- Bennett, S.; Grant, M.M.; Aldred, S. Oxidative Stress in Vascular Dementia and Alzheimer’s Disease: A Common Pathology. J. Alzheimer’s Dis. 2009, 17, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.; Lang, A. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.C. The Association between Serum GGT Concentration and Diabetic Peripheral Polyneuropathy in Type 2 Diabetic Patients. Korean Diabetes J. 2010, 34, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Romero, C.; Sadidi, M.; Feldman, E.L. Mechanisms of disease: The oxidative stress theory of diabetic neuropathy. Rev. Endocr. Metab. Disord. 2008, 9, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-W.; Li, M.; Hou, W.-S.; Li, K.; Zhou, J.-R.; Tang, Z.-Y. Association between Gamma-Glutamyltransferase Level and Risk of Stroke: A Systematic Review and Meta-analysis of Prospective Studies. J. Stroke Cereb. Dis. 2015, 24, 2816–2823. [Google Scholar] [CrossRef]
- Yao, T.; Li, J.; Long, Q.; Li, G.; Ding, Y.; Cui, Q.; Liu, Z. Association between Serum Gamma-glutamyl transferase and Intracranial Arterial Calcification in Acute Ischemic Stroke Subjects. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Shi, G.-J.; Zhou, J.-Y.; Zhang, W.-J.; Gao, C.-Y.; Jiang, Y.-P.; Zi, Z.-G.; Zhao, H.-H.; Yang, Y.; Yu, J.-Q.; Shi, G.-R. Involvement of growth factors in diabetes mellitus and its complications: A general review. Biomed. Pharmacother. 2018, 101, 510–527. [Google Scholar] [CrossRef]
- Falkowski, B.; Rogowicz-Frontczak, A.; Szczepanek-Parulska, E.; Krygier, A.; Wrotkowska, E.; Uruska, A.; Araszkiewicz, A.; Ruchała, M.; Zozulińska-Ziółkiewicz, D. Novel Biochemical Markers of Neurovascular Complications in Type 1 Diabetes Patients. J. Clin. Med. 2020, 9, 198. [Google Scholar] [CrossRef]
- Zorena, K.; Raczyńska, D.; Raczynska, K. Biomarkers in Diabetic Retinopathy and the Therapeutic Implications. Mediat. Inflamm. 2013, 2013, 193604. [Google Scholar] [CrossRef]
- Zhang, Q.; Fang, W.; Ma, L.; Wang, Z.-D.; Yang, Y.-M.; Lu, Y.-Q. VEGF levels in plasma in relation to metabolic control, inflammation, and microvascular complications in type-2 diabetes. Medicine 2018, 97, e0415. [Google Scholar] [CrossRef]
- Adamska, A.; Pilacinski, S.; Zozulinska-Ziolkiewicz, R.; Gandecka, A.; Grzelka, A.; Konwerska, A.; Malinska, A.; Nowicki, M.; Araszkiewicz, A. An increased skin microvessel density is associated with neurovascular complications in type 1 diabetes mellitus. Diabetes Vasc. Dis. Res. 2019, 16, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.; Periasamy, R.; Gangaraju, R. Adult Stem Cell Therapeutics in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4876. [Google Scholar] [CrossRef] [PubMed]
- Izuta, H.; Matsunaga, N.; Shimazawa, M.; Sugiyama, T.; Ikeda, T.; Hara, H. Proliferative diabetic retinopathy and relations among antioxidant activity, oxidative stress, and VEGF in the vitreous body. Mol. Vis. 2010, 16, 130–136. [Google Scholar] [PubMed]
- Pusparajah, P.; Lee, L.-H.; Kadir, K.A. Molecular Markers of Diabetic Retinopathy: Potential Screening Tool of the Future? Front. Physiol. 2016, 7, 200. [Google Scholar] [CrossRef]
- Falero-Perez, J.; Park, S.; Sorenson, C.M.; Sheibani, N. PEDF expression affects retinal endothelial cell proangiogenic properties through alterations in cell adhesive mechanisms. Am. J. Physiol. Physiol. 2017, 313, C405–C420. [Google Scholar] [CrossRef]
- Haque, S.; Morris, J.C. Transforming growth factor-β: A therapeutic target for cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef]
- Li, Q.; Pang, L.; Yang, W.; Liu, X.; Su, G.; Dong, Y. Long Non-Coding RNA of Myocardial Infarction Associated Transcript (LncRNA-MIAT) Promotes Diabetic Retinopathy by Upregulating Transforming Growth Factor-β1 (TGF-β1) Signaling. Med. Sci. Monit. 2018, 24, 9497–9503. [Google Scholar] [CrossRef]
- Thomas, P.K. Growth Factors and Diabetic Neuropathy. Diabet. Med. 1994, 11, 732–739. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.L.; Bianchi, P.; Manni, L. Nerve growth factor: From the early discoveries to the potential clinical use. J. Transl. Med. 2012, 10, 239. [Google Scholar] [CrossRef]
- El-Abyary, M.M.; El-Fattah, D.A.; Selem, H.A.; El-Mosalamy, F.A.; Elazizi, N.M.A. Assessment of nerve growth factor and nerve conduction velocity in diabetic patients with neuropathy. Egypt. J. Neurol. Psychiatry Neurosurg. 2009, 46, 101–109. [Google Scholar]
- Decroli, E.; Manaf, A.; Syahbuddin, S.; Syafrita, Y.; Dillasamola, D. The Correlation between Malondialdehyde and Nerve Growth Factor Serum Level with Diabetic Peripheral Neuropathy Score. Open Access Maced. J. Med. Sci. 2019, 7, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.-J.; Li, Z.-Z.; Wang, L.-M.; Sun, W.; Yu, J.-C.; Wang, B. Association of lower serum Brain-derived neurotrophic factor levels with larger infarct volumes in acute ischemic stroke. J. Neuroimmunol. 2017, 307, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mirowska-Guzel, D.; Gromadzka, G.; Mendel, T.A.; Janus-Laszuk, B.; Dzierka, J.; Sarzyńska-Długosz, I.; Członkowski, A.; Członkowska, A. Impact of BDNF -196 G>A and BDNF -270 C>T Polymorphisms on Stroke Rehabilitation Outcome: Sex and Age Differences. Top. Stroke Rehabil. 2014, 21, S33–S41. [Google Scholar] [CrossRef] [PubMed]
- Mirowska-Guzel, D.; Gromadzka, G.; Seniów, J.; Leśniak, M.; Bilik, M.; Waldowski, K.; Gruchala, K.; Członkowski, A.; Członkowska, A. Association between BDNF-196 G>A and BDNF-270 C>T polymorphisms, BDNF concentration, and rTMS-supported long-term rehabilitation outcome after ischemic stroke. Neurorehabilitation 2013, 32, 573–582. [Google Scholar] [CrossRef]
- Eyileten, C.; Mirowska-Guzel, D.; Milanowski, L.; Zaremba, M.; Rosiak, M.; Cudna, A.; Kapłon-Cieślicka, A.; Opolski, G.; Filipiak, K.J.; Małek, Ł.; et al. Serum Brain-Derived Neurotrophic Factor is Related to Platelet Reactivity and Metformin Treatment in Adult Patients with Type 2 Diabetes Mellitus. Can. J. Diabetes 2019, 43, 19–26. [Google Scholar] [CrossRef]
- Eyileten, C.; Zaremba, M.; Janicki, P.K.; Rosiak, M.; Cudna, A.; Kapłon-Cieślicka, A.; Opolski, G.; Filipiak, K.J.; Kosior, D.A.; Mirowska-Guzel, D.; et al. Serum Brain-Derived Neurotrophic Factor is Related to Platelet Reactivity but not to Genetic Polymorphisms within BDNF Encoding Gene in Patients with Type 2 Diabetes. Med. Sci. Monit. 2016, 22, 69–76. [Google Scholar] [CrossRef]
- Pearse, R.N.; Swendeman, S.L.; Li, Y.; Rafii, D.; Hempstead, B.L. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood 2005, 105, 4429–4436. [Google Scholar] [CrossRef]
- Ahmad, S.; Akhtar, N.; Wasim, S.; Irfan, S. Implication of serum homocyst eine level in type-2 diabetes: A pilot study. J. Evol. Med. Dent. Sci. 2015, 4, 7021–7025. [Google Scholar] [CrossRef]
- Obeid, R.; Herrmann, W. Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 2006, 580, 2994–3005. [Google Scholar] [CrossRef]
- Lehotský, J.; Tothová, B.; Kovalská, M.; Dobrota, D.; Beňová, A.; Kalenská, D.; Kaplan, P. Role of Homocysteine in the Ischemic Stroke and Development of Ischemic Tolerance. Front. Mol. Neurosci. 2016, 10, 538. [Google Scholar] [CrossRef]
- Tawfik, A.; Mohamed, R.; Elsherbiny, N.M.; DeAngelis, M.M.; Bartoli, M.; Al-Shabrawey, M. Homocysteine: A Potential Biomarker for Diabetic Retinopathy. J. Clin. Med. 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Tiwari, M.; Tiwari, R.K. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic Clin. Pharmacol. Toxicol. 2015, 117, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, S.; Li, L.; Li, Q.; Ren, K.; Sun, X.; Li, J. Metformin treatment and homocysteine: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2016, 8, 798. [Google Scholar] [CrossRef]
- Dai, Z.; Jiao, Y.; Fan, Q.; Qi, A.; Xiao, L.; Li, J. Homocysteine, interleukin-1β, and fasting blood glucose levels as prognostic markers for diabetes mellitus complicated with cerebral infarction and correlated with carotid intima-media thickness. Exp. Ther. Med. 2020, 19, 1167–1174. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sera, Y.; Nakamura, H.; Ueki, Y.; Miyake, S. Correlation between common carotid arterial wall thickness and ischemic stroke in patients with type 2 diabetes mellitus. Metabolism 2002, 51, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, O.; Turkcuoglu, P.; Güler, M.; Celiker, U.; Ustundag, B.; Yilmaz, T.; Metin, K. Plasma and Vitreous Homocysteine Concentrations in Patients with Proliferative Diabetic Retinopathy. Retina 2008, 28, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, H.; Guan, Z.; Hu, M.; Zhang, T.; Ge, S. Elevated serum homocysteine level in the development of diabetic peripheral neuropathy. Genet. Mol. Res. 2015, 14, 15365–15375. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yan, X.; Guo, J.; Xu, Q.; Tang, B.; Sun, Q. Recent Advances in Biomarkers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 305. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Anwar, F.; Zamzami, M.; Choudhry, H.; Shaik, M.; Tamargo, I.; Kamal, M.A. miRNAs as Circulating Biomarkers for Alzheimer’s Disease and Parkinson’s Disease. Med. Chem. 2016, 12, 217–225. [Google Scholar] [CrossRef]
- Eyileten, C.; Wicik, Z.; De Rosa, S.; Mirowska-Guzel, D.; Soplinska, A.; Indolfi, C.; Jastrzebska-Kurkowska, I.; Członkowska, A.; Postula, M. MicroRNAs as Diagnostic and Prognostic Biomarkers in Ischemic Stroke—A Comprehensive Review and Bioinformatic Analysis. Cells 2018, 7, 249. [Google Scholar] [CrossRef] [PubMed]
- Pordzik, J.; Jakubik, D.; Jarosz-Popek, J.; Wicik, Z.; Eyileten, C.; De Rosa, S.; Indolfi, C.; Siller-Matula, J.M.; Czajka, P.; Postula, M. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: Bioinformatic analysis and review. Cardiovasc. Diabetol. 2019, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, J.; Wicik, Z.; De Rosa, S.; Eyileten, C.; Jakubik, D.; Spaccarotella, C.; Mongiardo, A.; Postula, M.; Indolfi, C.; Jolanda, S.; et al. MicroRNAs fingerprint of bicuspid aortic valve. J. Mol. Cell. Cardiol. 2019, 134, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Pordzik, J.; Pisarz, K.; De Rosa, S.; Jones, A.D.; Eyileten, C.; Indolfi, C.; Małek, Ł.; Postula, M. The Potential Role of Platelet-Related microRNAs in the Development of Cardiovascular Events in High-Risk Populations, Including Diabetic Patients: A Review. Front. Endocrinol. 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Rome, S. Are extracellular microRNAs involved in type 2 diabetes and related pathologies? Clin. Biochem. 2013, 46, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wan, S.; Yang, T.; Niu, D.; Zhang, A.; Yang, C.; Cai, J.; Wu, J.; Song, J.; Zhang, C.-Y.; et al. Increased serum microRNAs are closely associated with the presence of microvascular complications in type 2 diabetes mellitus. Sci. Rep. 2016, 6, 20032. [Google Scholar] [CrossRef]
- Prado, M.S.G.; De Jesus, M.L.; De Goes, T.C.; Mendonça, L.S.O.; Kaneto, C.M. Downregulation of circulating miR-320a and target gene prediction in patients with diabetic retinopathy. BMC Res. Notes 2020, 13, 155–157. [Google Scholar] [CrossRef]
- Lee, C.S.; Larson, E.B.; Gibbons, L.E.; Lee, A.Y.; McCurry, S.M.; Bowen, J.D.; McCormick, W.C.; Crane, P.K. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimer’s Dement. 2018, 15, 34–41. [Google Scholar] [CrossRef]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef]
- Angelucci, F.; Cechova, K.; Valis, M.; Kuca, K.; Zhang, B.; Hort, J. MicroRNAs in Alzheimer’s Disease: Diagnostic Markers or Therapeutic Agents? Front. Pharmacol. 2019, 10, 665. [Google Scholar] [CrossRef]
- Siedlecki-Wullich, D.; Català-Solsona, J.; Fábregas, C.; Hernandez, I.; Clarimon, J.; Lleo, A.; Boada, M.; Saura, C.A.; Rodríguez, J.; Miñano-Molina, A.J. Altered microRNAs related to synaptic function as potential plasma biomarkers for Alzheimer’s disease. Alzheimer’s Res. Ther. 2019, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Børsheim, E.; Carvalho, E. The Role of MicroRNAs in Diabetic Complications—Special Emphasis on Wound Healing. Genes 2014, 5, 926–956. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-P.; Yang, S.-L.; Zhao, S.; Zheng, C.-H.; Li, H.-H.; Zhang, Y.; Huang, R.-X.; Li, M.-Z.; Gao, Y.; Zhang, S.-J.; et al. Biomarkers for Early Diagnostic of Mild Cognitive Impairment in Type-2 Diabetes Patients: A Multicentre, Retrospective, Nested Case-Control Study. EBioMedicine 2016, 5, 105–113. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Q.; Lv, Y.; Sun, L.; Gang, X.; Wang, G. Biomarkers for cognitive decline in patients with diabetes mellitus: Evidence from clinical studies. Oncotarget 2017, 9, 7710–7726. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, X.; Liu, S.; Li, M. Changes in Cerebrospinal Fluid Tau and β-Amyloid Levels in Diabetic and Prediabetic Patients: A Meta-Analysis. Front. Aging Neurosci. 2018, 10, 271. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Szydłowska, A.; Skupień, A.; Mikiciuk-Olasik, E.; Huttunen, K.M. Metformin–A future therapy for neurodegenerative diseases. Pharm. Res. 2017, 34, 2614–2627. [Google Scholar] [CrossRef]
- Katila, N.; Bhurtel, S.; Shadfar, S.; Srivastav, S.; Neupane, S.; Ojha, U.; Jeong, G.S.; Choi, D.Y. Metformin lowers alpha-synuclein phosphorylation and upregulates neurotrophic factor in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 2017, 125, 396–407. [Google Scholar] [CrossRef]
- Vicchi, F.L.; Luque, G.M.; Brie, B.; Nogueira, J.P.; Tornadu, I.G.; Becu-Villalobos, D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol. Res. 2016, 109, 74–80. [Google Scholar] [CrossRef]
- Fiory, F.; Perruolo, G.; Cimmino, I.; Cabaro, S.; Pignalosa, F.C.; Miele, C.; Beguinot, F.; Formisano, P.; Oriente, F. The Relevance of Insulin Action in the Dopaminergic System. Front. Mol. Neurosci. 2019, 13, 868. [Google Scholar] [CrossRef]
- Pagano, G.; Polychronis, S.; Wilson, H.; Giordano, B.; Ferrara, N.; Niccolini, F.; Politis, M. Diabetes mellitus and Parkinson disease. Neurology 2018, 90, e1654–e1662. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Kim, R.; Jung, Y.J.; Han, K.; Shin, C.M.; Lee, J.-Y. Serum gamma-glutamyltransferase activity and Parkinson’s disease risk in men and women. Sci. Rep. 2020, 10, 1258. [Google Scholar] [CrossRef] [PubMed]
- Aarti, V.; Nirankar, N.; Sukhraj, K.; Avtar, D.; Bhoj, S.; Rohit, B.; Jaswinder, S. Association between serum Gamma-Glutamyl Transferase (GGT) level and acute stroke. Int. J. Curr. Res. Biol. Med. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Merimee, T. The Interface Between Diabetic Retinopathy, Diabetes Management, and Insulin-Like Growth Factors. J. Clin. Endocrinol. Metab. 1997, 82, 2806–2808. [Google Scholar] [CrossRef]
- Drela, E.; Kulwas, A.; Ruszkowska, B.; Rość, D. The diversity of angiogenesis in diabetic vascular complications. Folia Med. Copernic. 2013, 1, 53–57. [Google Scholar]
- Nano, J.; Muka, T.; Ligthart, S.; Hofman, A.; Murad, S.D.; Janssen, H.L.A.; Franco, O.H.; Dehghan, A. Gamma-glutamyltransferase levels, prediabetes and type 2 diabetes: A Mendelian randomization study. Int. J. Epidemiol. 2017, 46, 1400–1409. [Google Scholar] [CrossRef]
- Kim, H.C.; Cho, Y.J.; Ahn, C.; Park, K.S.; Kim, J.C.; Nam, J.S.; Im, Y.S.; Lee, J.E.; Lee, S.C.; Lee, H.K. Nerve growth factor and expression of its receptors in patients with diabetic neuropathy. Diabet. Med. 2009, 26, 1228–1234. [Google Scholar] [CrossRef]
- Deguchi, T.; Hashiguchi, T.; Horinouchi, S.; Uto, T.; Oku, H.; Kimura, K.; Makisumi, K.; Arimura, K. Serum VEGF increases in diabetic polyneuropathy, particularly in the neurologically active symptomatic stage. Diabet Med. 2009, 26, 247–252. [Google Scholar] [CrossRef]
- Eyileten, C.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D.; Małek, Ł.; Postula, M. Antidiabetic Effect of Brain-Derived Neurotrophic Factor and Its Association with Inflammation in Type 2 Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 2823671. [Google Scholar] [CrossRef]
- Vanhecke, E.; Adriaenssens, E.; Verbeke, S.; Meignan, S.; Germain, E.; Berteaux, N.; Nurcombe, V.; Le Bourhis, X.; Hondermarck, H. Brain-derived neurotrophic factor and neurotrophin-4/5 are expressed in breast cancer and can be targeted to inhibit tumor cell survival. Clin. Cancer Res. 2011, 17, 1741–1752. [Google Scholar] [CrossRef]
- Chahine, L.M.; Stern, M.B.; Chen-Plotkin, A. Blood-based biomarkers for Parkinson’s disease. Park. Relat. Disord. 2014, 20, S99–S103. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A. Cerebrospinal fluid biochemical studies in patients with Parkinson’s disease: Toward a potential search for biomarkers for this disease. Front. Cell Neurosci. 2014, 8, 369. [Google Scholar] [CrossRef] [PubMed]
- Fiala, M.; Veerhuis, R. Biomarkers of inflammation and amyloid-β phagocytosis in patients at risk of Alzheimer disease. Exp. Gerontol. 2010, 45, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Qi, W.; Fang, S.; Jiang, P.; Yang, C.; Mo, Y.; Dong, C.; Li, Y.; Zhong, J.; Cai, W.; et al. Pigment Epithelium-Derived Factor Plays a Role in Alzheimer’s Disease by Negatively Regulating Aβ42. Neurotherapeutics 2018, 15, 728–741. [Google Scholar] [CrossRef]
- Herrmann, W.; Obeid, R. Homocysteine: A biomarker in neurodegenerative diseases. Clin. Chem. Lab. Med. 2011, 49, 435–441. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, Y.; Zhang, S.; Tie, T.; Cheng, Y.; Su, X.; Man, Z.; Hou, J.; Sun, L.; Tian, M.; et al. The association between homocysteine and ischemic stroke subtypes in Chinese. Medicine 2020, 99, e19467. [Google Scholar] [CrossRef]
- Ashjazadeh, N.; Fathi, M.; Shariat, A. Evaluation of Homocysteine Level as a Risk Factor among Patients with Ischemic Stroke and Its Subtypes. Iran. J. Med. Sci. 2013, 38, 233–239. [Google Scholar]
- Tai, M.-L.S.; Toh, T.H.; Hussain, H.; Ong, K.G. Serum Homocysteine and Intracranial Aneurysms. New Insight Cereb. Dis. Updated Compr. Rev. 2019, 3, 1–4. [Google Scholar]
- Ren, J.-R.; Ren, S.-H.; Ning, B.; Wu, J.; Cao, Y.; Ding, X.-M.; Zhen, Z.-G.; Hao, X.-D.; Wang, S. Hyperhomocysteinemia as a Risk Factor for Saccular Intracranial Aneurysm: A Cohort Study in a Chinese Han Population. J. Stroke Cereb. Dis. 2017, 26, 2720–2726. [Google Scholar] [CrossRef]
- Damanik, J.; Mayza, A.; Rachman, A.; Sauriasari, R.; Kristanti, M.; Agustina, P.S.; Angianto, A.R.; Prawiroharjo, P.; Yunir, E. Association between serum homocysteine level and cognitive function in middle-aged type 2 diabetes mellitus patients. PLoS ONE 2019, 14, e0224611. [Google Scholar] [CrossRef]
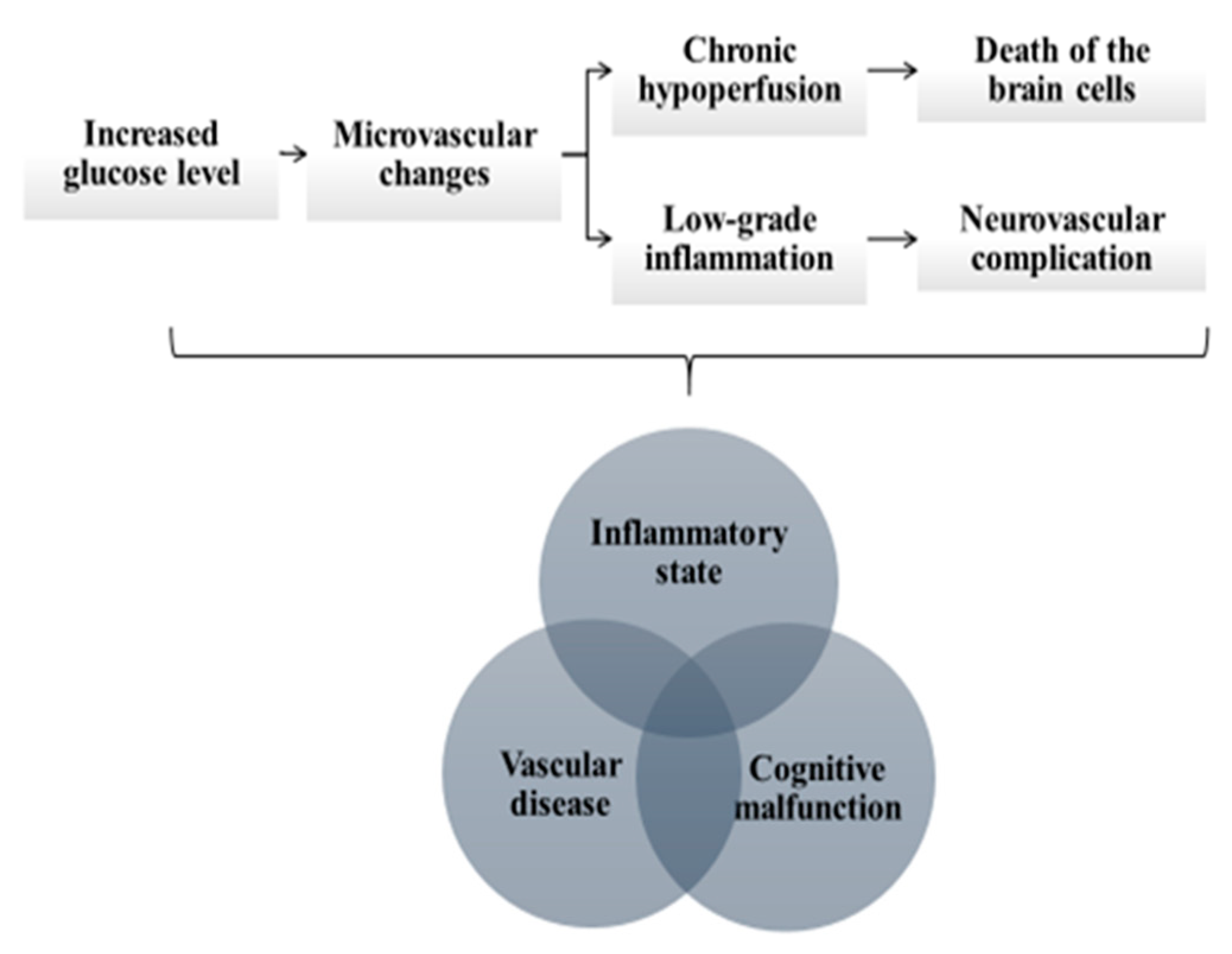
| Neurodegenerative Diseases | Neurovascular Disorders |
|---|---|
| Alzheimer’s disease | Diabetic retinopathy |
| Parkinson’s disease | Diabetic neuropathy |
| Mild cognitive impairment | Stroke |
| Dementia |
| Biomarker | Role/Effect in Human Body | Link with T2DM | Link with Neurological Complications | Example of Neurological Complications | Direction of Change | Ref |
|---|---|---|---|---|---|---|
| CRP | acute phase protein, produced in response to inflammation and interleukin IL-6 | increased glucose concentration →inflammation →microvascular changes | microvascular changes →chronic brain hypoperfusion → degeneration of the brain cells | AD, MCI, PD | ↑ | [6,7,8,9,10,11] |
| microvascular changes →choroidal endothelial cell dysfunction | AMD | ↑ | ||||
| PON-1 | antiatherogenic, antioxidant and anti-inflammatory properties (inhibition of lipid oxidation, breakdown of lipid peroxides) | increased glucose concentration → enzymatic glycation and oxidative stress | oxidative stress → endothelial damage → atherosclerosis in the brain arteries | stroke | ↓ | [14,15,16,17,18,19] |
| oxidative stress → dysregulated acetylcholine metabolism and organo-phosphates detoxification | AD, PD | ↓ | ||||
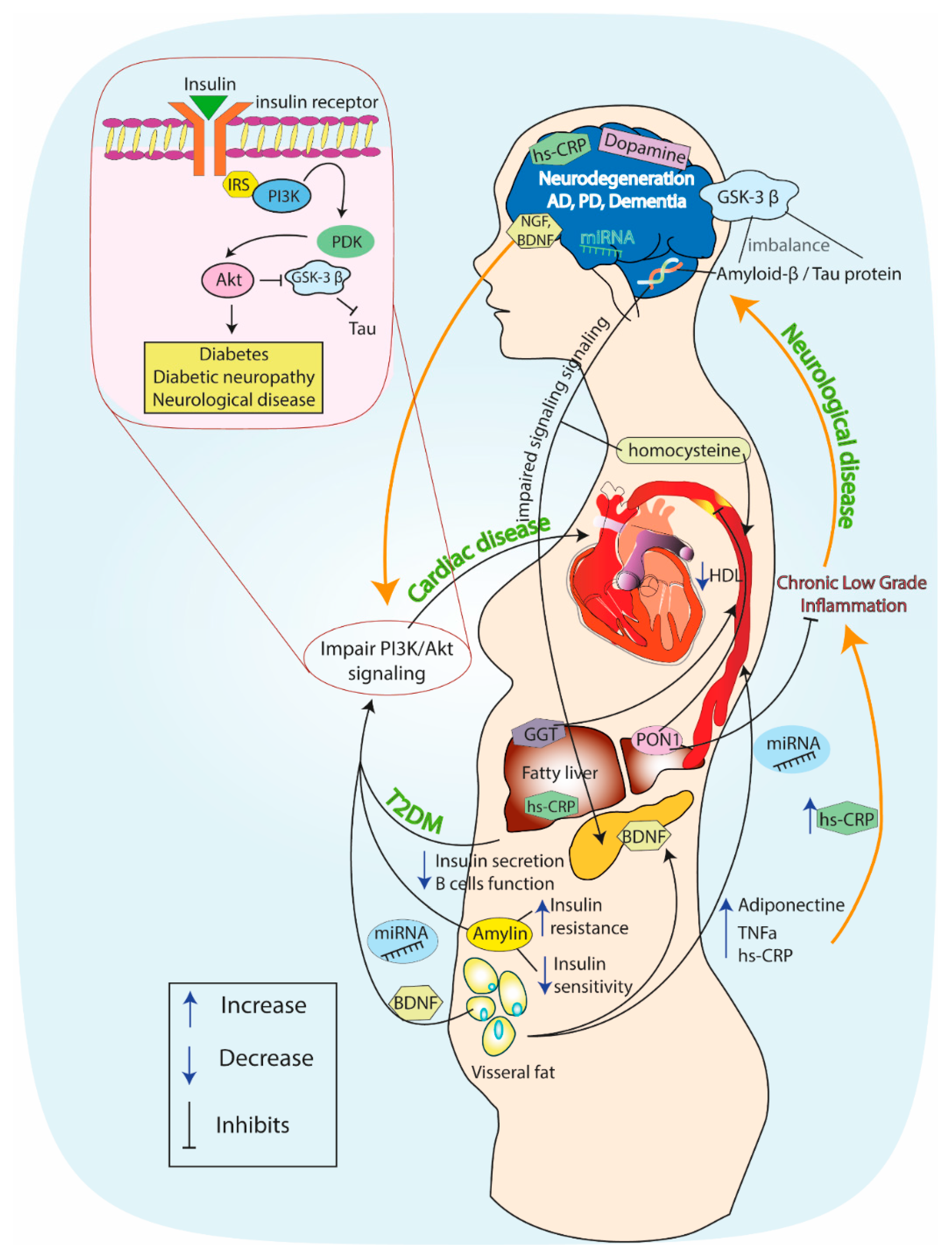
| GSK-3β, tau protein, amyloidβ | morphogenesis, cell division and intracellular transport | impaired insulin signaling → increased GSK-3β activity →Increased AGE formation | increased AGE formation → tau hyperphosphorylation, Aβ accumulation → amyloid plaque aggregation on nerve cells → synaptic loss | AD, PD | ↑ | [20,21,22,23,24] |
| PI3K | glucose homeostasis, antiatherogenic and vasodilatory effect, e-NOS activation and NO production | impairment of insulin/PI3K/Akt signaling → alteration of mTOR signaling | alteration of mTOR signaling → tau hyper-phosphorylation, Aβ accumulation → neurotoxicity | AD, PD | ↓ | [25,26] |
| key role in nerve survival | impairment of insulin signaling → decreased PI3K activity in peripheral nerves | decreased PI3K activity → decreased retrograde transport of neurotrophins and nerve growth factor | diabetic neuropathy | ↓ | ||
| promotion of the survival of dopamine-producing neurons | impairment of insulin signaling →decreased PI3K activity in CNS | decreased PI3K activity → dopamine neuron degeneration | PD | ↓ | ||
| Amylin | glucose homeostasis, decrease of secretion of gastric acid and glucagon | amylin aggregates → cytotoxic effect on pancreatic β-cells | amylin aggregates →mixed plaque formation (amylin, β-amyloid) | AD, MCI | ↑ | [27,28,29,30,31,32] |
| DA | glucose homeostasis motor control executive functions | upregulated insulin activity through insulin receptors → modulation of dopaminergic neurons in midbrain | modulation of dopaminergic neurons →boost of DA elimination from the synapse; degeneration of dopaminergic neurons | PD | ↓ | [33,34,35,36,37] |
| neuro-transmitter in retina | chronically disturbed glucose homeostasis | disturbed glucose homeostasis → decline of DA production → neuronal damage | DR | ↓ | ||
| GGT | glutathione metabolism | oxidative stress →insulin resistance | upregulated oxidative stress and lipid accumulation in the retina → retinopathy | DR | ↑ | [38,39,40,41,42,43,44,45,46,47,48] |
| cellular antioxidant | increased glucose concentration → oxidative stress | oxidative stress → neuronal damage decline of cognitive function, plaque progression | AD, dementia | ↑/↓1 | ||
| glutathione metabolism, cellular antioxidant | increased glucose concentration → oxidative stress, neuro-inflammation | toxic abnormal protein aggregation and mitochondrial dysfunction in substantia nigra → oxidative stress, neuro-inflammation → neuronal cell death | PD | ↑ | ||
| cellular antioxidant | increased glucose concentration → oxidative stress | oxidative stress → progression of neuronal damage in diabetic neuropathy | diabetic neuropathy | ↑/↓1 | ||
| cellular antioxidant, independent risk factor of stroke | increased glucose concentration → oxidative stress | oxidative stress → GGT presence in calcified intracranial atherosclerotic plaques | stroke | ↑ | ||
| GF | [49,50,51] | |||||
| EGF | proliferative and wound healing processes | increased glucose concentration → inflammation →microvascular complications | microvascular complications → retinal ischemia | DR | ↑ | [50] |
| VEGF | endothelial cell proliferation and migration, collagen production, macrophage chemotaxis | increased glucose concentration → inflammation →microvascular complications | ↑ | [49,51,52,53,54,55] | ||
| PEDF | angiogenesis inhibition | increased glucose concentration →oxidative and inflammatory conditions | oxidative and inflammatory conditions → retinal microvascular endothelial cell dysfunction | DR | ↓ | [51,56,57] |
| TGF-β | cell growth and differentiation, angiogenesis | increased glucose concentration →oxidative and inflammatory conditions | oxidative and inflammatory conditions →thickening of basal lamina of retinal vessels | DR | ↑ | [51,58,59] |
| NGF | neuronal development, growth and survival of neurons in the nervous system | glucotoxicity, insulin deficiency →ischemia and oxidative stress | ischemia and oxidative stress → decreased nerve conduction velocity | peripheral neuropathy | ↓ | [60,61,62,63] |
| BDNF | supporting the survival of existing neurons, neurogenesis instigation | dysregulation of glucose level → impairment of insulin signaling →altered MAPK and PI3K activity | altered MAPK and PI3K activity → potential role in stroke recovery (plasticity promotion) | ischemic stroke | ↓ | [64,65,66,67,68,69] |
| Homo-cysteine | insulin homeostasis, suppression of endothelial NO production, increase of ROS | increased glucose concentration → oxidative stress | oxidative stress → endothelial dysfunction→ platelet activation, atherothrombotic incidents, carotid intima–media thickness | stroke | ↑ | [70,71,72,73,74,75,76,77,78,79,80] |
| activation of inflammatory and oxidative stress mechanisms | increased glucose concentration → oxidative stress; increased levels of VEGF → micro-angiopathy | micro-angiopathy → direct effect on blood–retina barrier, apoptosis in retinal ganglion cells | DR | ↑ | ||
| independent risk factor | increased glucose concentration→inflammation →micro and macrovascular complications | inflammation → micro and macrovascular complications → nerve injury | diabetic neuropathy | ↑ | ||
| role in brain damage, cognitive and memory decline, activation of oxidative stress mechanisms | increased glucose concentration → oxidative stress → ROS production | deficiency of cofactors related to homocysteine metabolism → HCY neurotoxicity → ROS production → Aβ accumulation in the brain →apoptosis and neuronal death | AD, PD, dementia | ↑ | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasecka, A.; Siwik, D.; Gajewska, M.; Jaguszewski, M.J.; Mazurek, T.; Filipiak, K.J.; Postuła, M.; Eyileten, C. Early Biomarkers of Neurodegenerative and Neurovascular Disorders in Diabetes. J. Clin. Med. 2020, 9, 2807. https://doi.org/10.3390/jcm9092807
Gasecka A, Siwik D, Gajewska M, Jaguszewski MJ, Mazurek T, Filipiak KJ, Postuła M, Eyileten C. Early Biomarkers of Neurodegenerative and Neurovascular Disorders in Diabetes. Journal of Clinical Medicine. 2020; 9(9):2807. https://doi.org/10.3390/jcm9092807
Chicago/Turabian StyleGasecka, Aleksandra, Dominika Siwik, Magdalena Gajewska, Miłosz J. Jaguszewski, Tomasz Mazurek, Krzysztof J. Filipiak, Marek Postuła, and Ceren Eyileten. 2020. "Early Biomarkers of Neurodegenerative and Neurovascular Disorders in Diabetes" Journal of Clinical Medicine 9, no. 9: 2807. https://doi.org/10.3390/jcm9092807
APA StyleGasecka, A., Siwik, D., Gajewska, M., Jaguszewski, M. J., Mazurek, T., Filipiak, K. J., Postuła, M., & Eyileten, C. (2020). Early Biomarkers of Neurodegenerative and Neurovascular Disorders in Diabetes. Journal of Clinical Medicine, 9(9), 2807. https://doi.org/10.3390/jcm9092807







