A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Measurements
2.3. DRI Development
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 3. Prevention or delay of type 2 diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S29–S33. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus statement by the American association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm—2019 Executive summary. Endocr. Pract. 2019, 25, 69–100. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, R.T. From programs to policy and back again: The push and pull of realizing type 2 diabetes prevention on a national scale. Diabetes Care 2017, 40, 1298–1301. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association; National Institute of Diabetes, Digestive and Kidney Diseases. The prevention or delay of type 2 diabetes. Diabetes Care 2002, 25, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; Kahn, R. A step backward–or is it forward? Diabetes Care 2008, 31, 1093–1096. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Daniel, S.; Soleymani, T.; Garvey, W.T. A complications-based clinical staging of obesity to guide treatment modality and intensity. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 377–388. [Google Scholar] [CrossRef]
- Torgerson, J.S.; Hauptman, J.; Boldrin, M.N.; Sjostrom, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004, 27, 155–161. [Google Scholar] [CrossRef]
- Garvey, W.T.; Ryan, D.H.; Henry, R.; Bohannon, N.J.; Toplak, H.; Schwiers, M.; Troupin, B.; Day, W.W. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended-release. Diabetes Care 2013. [Google Scholar] [CrossRef]
- Scott, R.A.; Scott, L.J.; Mägi, R.; Marullo, L.; Gaulton, K.J.; Kaakinen, M.; Pervjakova, N.; Pers, T.H.; Johnson, A.D.; Eicher, J.D. An expanded genome-wide association study of type 2 diabetes in Europeans. Diabetes 2017, 66, 2888–2902. [Google Scholar] [CrossRef]
- Mahajan, A.; Taliun, D.; Thurner, M.; Robertson, N.R.; Torres, J.M.; Rayner, N.W.; Payne, A.J.; Steinthorsdottir, V.; Scott, R.A.; Grarup, N. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018, 50, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Sahlqvist, A.-S.; Lotta, L.; Brosnan, J.M.; Vollenweider, P.; Giabbanelli, P.; Nunez, D.J.; Waterworth, D.; Scott, R.A.; Langenberg, C. A systematic review of biomarkers and risk of incident type 2 diabetes: An overview of epidemiological, prediction and aetiological research literature. PLoS ONE 2016, 11, e0163721. [Google Scholar] [CrossRef]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef] [PubMed]
- Matyus, S.P.; Braun, P.J.; Wolak-Dinsmore, J.; Jeyarajah, E.J.; Shalaurova, I.; Xu, Y.; Warner, S.M.; Clement, T.S.; Connelly, M.A.; Fischer, T.J. NMR measurement of LDL particle number using the Vantera Clinical Analyzer. Clin. Biochem. 2014, 47, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Shalaurova, I.; Connelly, M.A.; Garvey, W.T.; Otvos, J.D. Lipoprotein insulin resistance index: A lipoprotein particle-derived measure of insulin resistance. Metab. Syndr. Relat. Disord. 2014, 12, 422–429. [Google Scholar] [CrossRef]
- Mackey, R.H.; Mora, S.; Bertoni, A.G.; Wassel, C.L.; Carnethon, M.R.; Sibley, C.T.; Goff, D.C., Jr. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care 2015. [Google Scholar] [CrossRef]
- Harada, P.H.N.; Demler, O.V.; Dugani, S.B.; Akinkuolie, A.O.; Moorthy, M.V.; Ridker, P.M.; Cook, N.R.; Pradhan, A.D.; Mora, S. Lipoprotein insulin resistance score and risk of incident diabetes during extended follow-up of 20 years: The Women’s Health Study. J. Clin. Lipidol. 2017, 11, 1257–1267.e2. [Google Scholar] [CrossRef]
- Dugani, S.B.; Akinkuolie, A.O.; Paynter, N.; Glynn, R.J.; Ridker, P.M.; Mora, S. Association of lipoproteins, insulin resistance, and rosuvastatin with incident type 2 diabetes mellitus: Secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016, 1, 136–145. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Connelly, M.A.; Shalaurova, I.; Gruppen, E.G.; Kieneker, L.M.; Dullaart, R.P.F.; Bakker, S.J.L. Lipoprotein insulin resistance index, a high-throughput measure of insulin resistance, is associated with incident type II diabetes mellitus in the Prevention of Renal and Vascular End-Stage Disease study. J. Clin. Lipidol. 2019, 13, 129–137.e1. [Google Scholar] [CrossRef]
- Ellsworth, D.L.; Costantino, N.S.; Blackburn, H.L.; Engler, R.J.; Kashani, M.; Vernalis, M.N. Lifestyle modification interventions differing in intensity and dietary stringency improve insulin resistance through changes in lipoprotein profiles. Obes. Sci. Pract. 2016, 2, 282–292. [Google Scholar] [CrossRef]
- Fernandez-Castillejo, S.; Valls, R.M.; Castaner, O.; Rubio, L.; Catalan, U.; Pedret, A.; Macia, A.; Sampson, M.L.; Covas, M.I.; Fito, M.; et al. Polyphenol rich olive oils improve lipoprotein particle atherogenic ratios and subclasses profile: A randomized, crossover, controlled trial. Mol. Nutr. Food Res. 2016, 60, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Tuccinardi, D.; Farr, O.M.; Upadhyay, J.; Oussaada, S.M.; Mathew, H.; Paschou, S.A.; Perakakis, N.; Koniaris, A.; Kelesidis, T.; Mantzoros, C.S. Lorcaserin treatment decreases body weight and reduces cardiometabolic risk factors in obese adults: A six-month, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes. Metab. 2019, 21, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Yoon, M.S. The emerging role of branched-chain amino acids in insulin resistance and metabolism. Nutrients 2016, 8, 405. [Google Scholar] [CrossRef]
- Holecek, M. Branched-chain amino acids in health and disease: Metabolism, alterations in blood plasma, and as supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef]
- Flores-Guerrero, J.L.; Oste, M.C.J.; Kieneker, L.M.; Gruppen, E.G.; Wolak-Dinsmore, J.; Otvos, J.D.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Plasma branched-chain amino acids and risk of incident type 2 diabetes: Results from the PREVEND prospective cohort study. J. Clin. Med. 2018, 7, 513. [Google Scholar] [CrossRef]
- Zhou, M.; Shao, J.; Wu, C.Y.; Shu, L.; Dong, W.; Liu, Y.; Chen, M.; Wynn, R.M.; Wang, J.; Wang, J. Targeting BCAA catabolism to treat obesity-associated insulin resistance. Diabetes 2019, 68, 1730–1746. [Google Scholar] [CrossRef]
- Wolak-Dinsmore, J.; Gruppen, E.G.; Shalaurova, I.; Matyus, S.P.; Grant, R.P.; Gegen, R.; Bakker, S.J.L.; Otvos, J.D.; Connelly, M.A.; Dullaart, R.P.F. A novel NMR-based assay to measure circulating concentrations of branched-chain amino acids: Elevation in subjects with type 2 diabetes mellitus and association with carotid intima media thickness. Clin. Biochem. 2018, 54, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lambers Heerspink, H.J.; Brantsma, A.H.; de Zeeuw, D.; Bakker, S.J.; de Jong, P.E.; Gansevoort, R.T.; Group, P.S. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am. J. Epidemiol. 2008, 168, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; Postmus, D.; Gansevoort, R.T.; de Jong, P.E.; Gans, R.O.; Struck, J.; Hillege, H.L.; Stolk, R.P.; Navis, G.; et al. Plasma procalcitonin and risk of type 2 diabetes in the general population. Diabetologia 2011, 54, 2463–2465. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.; Hillege, H.L.; Stolk, R.P.; Navis, G.; Bakker, S.J.; Dullaart, R.P. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: The PREVEND study. J. Clin. Endocrinol. Metab. 2013, 98, E1352–E1359. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, J.P.; Bakker, S.J.; Sparks, C.E.; Dullaart, R.P. Apolipoprotein A-II influences apolipoprotein E-linked cardiovascular disease risk in women with high levels of HDL cholesterol and C-reactive protein. PLoS ONE 2012, 7, e39110. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. 2019. Available online: http://www.r-project.org/ (accessed on 1 June 2020).
- RStudio Team. RStudio: Integrated Development Environment for R. 2019. Available online: http://www.rstudio.com/ (accessed on 1 June 2020).
- Selvin, S. Statistical Analysis of Epidemiologic Data; Oxford University Press: Oxford, UK, 2004. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Koehler, A.B.; Murphree, E.S. A comparison of the Akaike and Schwarz criteria for selecting model order. Appl. Stat. 1988, 37, 187. [Google Scholar] [CrossRef]
- McNemar, Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika 1947, 12, 153–157. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B.; D’Agostino, R.B.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; Meigs, J.B.; Sullivan, L.; Fox, C.S.; Nathan, D.M.; D’Agostino, R.B. Prediction of incident diabetes mellitus in middle-aged adults. Arch. Intern. Med. 2007, 167, 1068. [Google Scholar] [CrossRef]
- Lyssenko, V.; Jonsson, A.; Almgren, P.; Pulizzi, N.; Isomaa, B.; Tuomi, T.; Berglund, G.; Altshuler, D.; Nilsson, P.; Groop, L. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N. Engl. J. Med. 2008, 359, 2220–2232. [Google Scholar] [CrossRef]
- Hirschel, J.; Vogel, M.; Baber, R.; Garten, A.; Beuchel, C.; Dietz, Y.; Dittrich, J.; Körner, A.; Kiess, W.; Ceglarek, U. Relation of whole blood amino acid and acylcarnitine metabolome to age, sex, bmi, puberty, and metabolic markers in children and adolescents. Metabolites 2020, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.; Park, H.; Park, K. Estimation of dietary amino acid intake and independent correlates of skeletal muscle mass index among Korean adults. Nutrients 2020, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Merz, B.; Frommherz, L.; Rist, M.; Kulling, S.; Bub, A.; Watzl, B. Dietary pattern and plasma BCAA-variations in healthy men and women—Results from the KarMeN study. Nutrients 2018, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Lotta, L.A.; Scott, R.A.; Sharp, S.J.; Burgess, S.; Luan, J.; Tillin, T.; Schmidt, A.F.; Imamura, F.; Stewart, I.D.; Perry, J.R.B.; et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: A mendelian randomisation analysis. PLoS Med. 2016, 13, e1002179. [Google Scholar] [CrossRef] [PubMed]
- Glynn, E.L.; Piner, L.W.; Huffman, K.M.; Slentz, C.A.; Elliot-Penry, L.; AbouAssi, H.; White, P.J.; Bain, J.R.; Muehlbauer, M.J.; Ilkayeva, O.R.; et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 2015, 58, 2324–2335. [Google Scholar] [CrossRef]
- Taheri, S.; Zaghloul, H.; Chagoury, O.; Elhadad, S.; Ahmed, S.H.; el Khatib, N.; Amona, R.A.; el Nahas, K.; Suleiman, N.; Alnaama, A.; et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): An open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 477–489. [Google Scholar] [CrossRef]
- Collins, G.S.; Mallett, S.; Omar, O.; Yu, L.M. Developing risk prediction models for type 2 diabetes: A systematic review of methodology and reporting. BMC Med. 2011, 9. [Google Scholar] [CrossRef]
- Kengne, A.P.; Beulens, J.W.J.; Peelen, L.M.; Moons, K.G.M.; van der Schouw, Y.T.; Schulze, M.B.; Spijkerman, A.M.W.; Griffin, S.J.; Grobbee, D.E.; Palla, L.; et al. Non-invasive risk scores for prediction of type 2 diabetes (EPIC-InterAct): A validation of existing models. Lancet Diabetes Endocrinol. 2014, 2, 19–29. [Google Scholar] [CrossRef]
- Carrillo-Larco, R.M.; Aparcana-Granda, D.J.; Mejia, J.R.; Barengo, N.C.; Bernabe-Ortiz, A. Risk scores for type 2 diabetes mellitus in Latin America: A systematic review of population-based studies. Diabet. Med. 2019, 36, 1573–1584. [Google Scholar] [CrossRef]
- Udler, M.S.; McCarthy, M.I.; Florez, J.C.; Mahajan, A. Genetic risk scores for diabetes diagnosis and precision medicine. Endocr. Rev. 2019, 40, 1500–1520. [Google Scholar] [CrossRef] [PubMed]
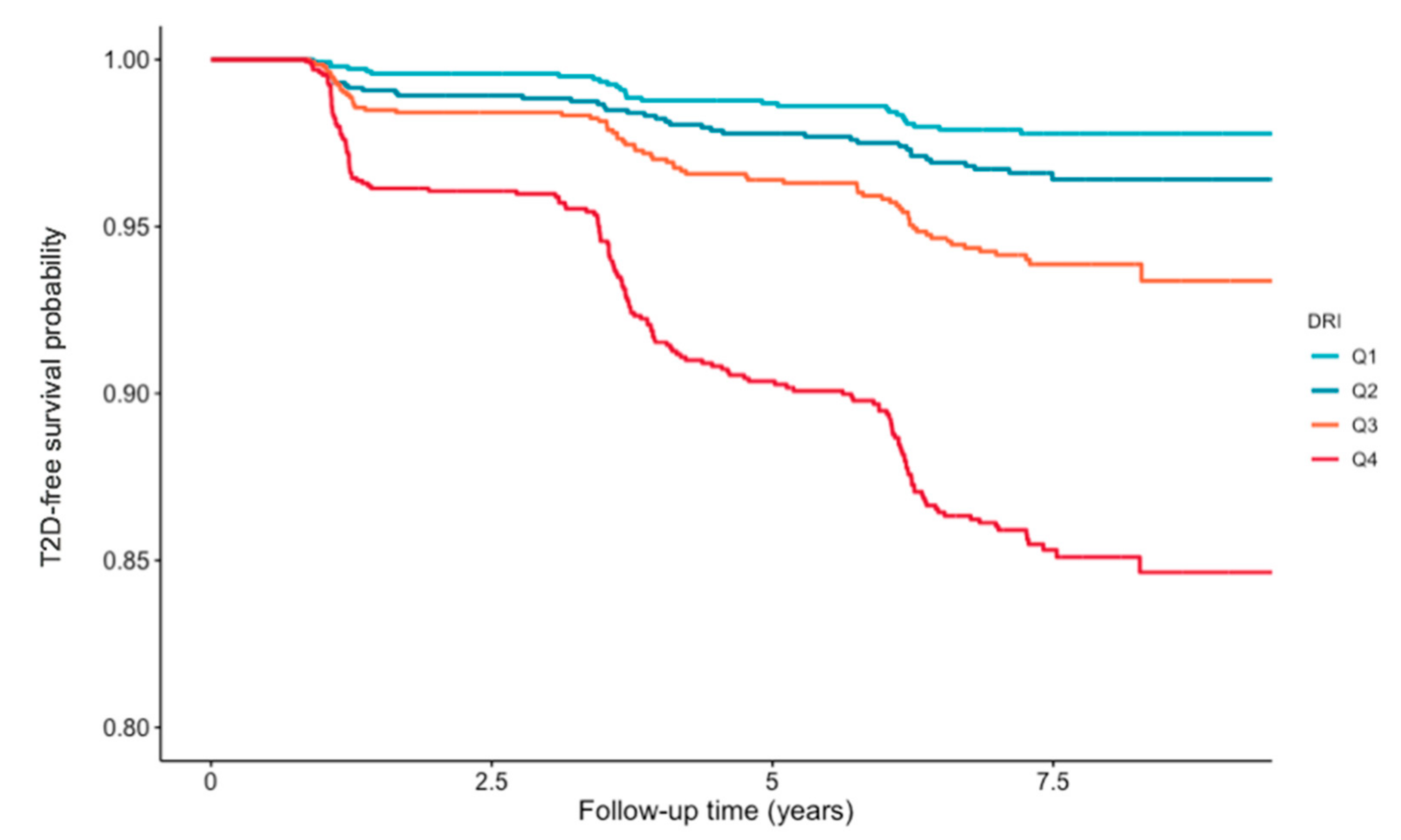
| Quartiles of DRI | ||||||
|---|---|---|---|---|---|---|
| Variables | All Participants | Q1 | Q2 | Q3 | Q4 | p-Value |
| Participants, n | 6134 | 1455 | 1596 | 1489 | 1594 | |
| DRI score | 33 (19–48) | 11 (5–15) | 25 (22–29) | 39 (36–43) | 58 (52–64) | <0.001 |
| Sex, men, % | 49.3 | 16.4 | 42.7 | 56.7 | 79.2 | <0.001 |
| Age, years | 53.2 ± 12.0 | 50.6 ± 11.7 | 53.0 ± 12.5 | 54.5 ± 11.9 | 54.5 ± 11.3 | <0.001 |
| BMI, kg/m2 | 26.5 ± 4.2 | 24.2 ± 3.4 | 25.7 ± 3.8 | 27.2 ± 4.2 | 28.7 ± 4.0 | <0.001 |
| SBP, mm Hg | 125.8 ± 18.6 | 118.4 ±17.8 | 123.1 ±18.1 | 127.5 ± 17.9 | 132.7 ± 17.5 | <0.001 |
| DBP, mm Hg | 73.2 ± 9.0 | 69.5 ± 8.9 | 71.9 ± 8.7 | 74.3 ± 8.5 | 76.9 ± 8.4 | <0.001 |
| Parental history of T2DM, yes, % | 14.4 | 11.9 | 13.7 | 15.1 | 16.7 | <0.001 |
| Smoking status | <0.001 | |||||
| Never, % | 28.5 | 34.4 | 30.3 | 25.9 | 23.9 | |
| Former, % | 42.1 | 37.2 | 41.4 | 44.3 | 45.3 | |
| Current, % | 28.1 | 26.9 | 27.3 | 28.3 | 29.9 | |
| Alcohol consumption | <0.001 | |||||
| <1 drinks/week, % | 24.1 | 23.7 | 24.0 | 23.8 | 21.8 | |
| 1–7 drinks/week, % | 48.6 | 50.0 | 51.9 | 46.5 | 46.1 | |
| >7 drinks/week, % | 26.3 | 21.7 | 23.4 | 28.0 | 31.4 | |
| Antihypertensive drugs, % | 18.2 | 10.3 | 15.9 | 20.1 | 25.8 | <0.001 |
| Lipid-lowering drugs, % | 7.2 | 3.4 | 5.8 | 8.1 | 11.0 | <0.001 |
| TC, mmol/L | 5.4 ± 1.0 | 5.2 ± 1.0 | 5.3 ± 1.0 | 5.5 ± 1.0 | 5.7 ± 1.0 | <0.001 |
| HDL-C, mmol/L | 1.2 ± 0.3 | 1.5 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.3 | 1.0 ± 0.2 | <0.001 |
| LDL-C, mmol/L | 2.9 ± 0.7 | 2.7 ± 0.7 | 2.9 ± 0.7 | 3.0 ± 0.7 | 3.0 ± 0.8 | <0.001 |
| TG, mmol/L | 1.1 (0.8–1.6) | 0.8 (0.6–0.9) | 0.9 (0.7–1.2) | 1.2 (1.0–1.5) | 1.9 (1.4–2.4) | <0.001 |
| Glucose, mmol/L | 4.8 ± 0.6 | 4.6 ± 0.5 | 4.8 ± 0.6 | 4.9 ± 0.6 | 5.0 ± 0.7 | <0.001 |
| Total BCAA, μM | 377.0 ± 72.6 | 301.7 ± 35.1 | 354.9 ± 38.8 | 393.8 ± 44.4 | 455.0 ± 61.5 | <0.001 |
| Valine, μM | 207.1 ± 37.2 | 171.0 ± 21.4 | 197.4 ± 24.1 | 216.0 ± 26.6 | 241.4 ± 33.7 | <0.001 |
| Leucine, μM | 127.2 ± 27.7 | 99.1 ±13.9 | 118.8 ± 15.1 | 131.7 ± 17.2 | 156.5 ± 24.6 | <0.001 |
| LP-IR score | 40 (21–61) | 15 (8–23) | 29 (20–39) | 47 (38–57) | 73 (62–85) | <0.001 |
| Large VLDL-P, nmol/L | 3.3 (1.6–6.6) | 1.4 (0.75–2.2) | 2.3 (1.4–3.7) | 4.1 (2.7–6.4) | 9.2 (6.1–13.8) | <0.001 |
| VLDL size, nm | 49.8 ± 9.1 | 45.7 ± 8.3 | 46.9 ± 7.5 | 49.6 ± 7.6 | 56.5 ± 8.7 | <0.001 |
| Small LDL-P, nmol/L | 336 (189–534) | 166 (1–274) | 274 (166–289) | 378 (251–522) | 624 (434–848) | <0.001 |
| LDL size, nm | 20.9 ± 1.6 | 21.2 ± 1.8 | 21.1 ± 1.9 | 21.0 ± 1.1 | 20.5 ± 1.1 | <0.001 |
| Large HDL-P, μmol/L | 5.1 ± 2.8 | 7.6 ± 4.7 | 5.8 ± 2.4 | 4.3 ± 2.3 | 2.8 ± 1.6 | <0.001 |
| HDL size, nm | 9.1 ± 0.6 | 9.7 ± 0.5 | 9.3 ± 0.5 | 9.0 ± 0.5 | 8.7± 0.4 | <0.001 |
| Q1 | Q2 | Q3 | Q4 | DRI Per 1 SD Increment | |||||
|---|---|---|---|---|---|---|---|---|---|
| DRI < 19 | DRI 19–33 | DRI 33–48 | DRI > 48 | ||||||
| Participants, n | 1455 | 1596 | 1489 | 1594 | 6134 | ||||
| Events, n | 15 | 39 | 71 | 181 | 306 | ||||
| HR (95 % CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Crude Model | (ref) | 2.48 (1.37; 4.50) | 0.002 | 4.89 (2.80; 8.53) | <0.001 | 12.05 (7.12; 20.41) | <0.001 | 2.34 (2.09; 2.62) | <0.001 |
| Model 1 | (ref) | 2.42 (1.33; 4.40) | 0.003 | 4.69 (2.66; 8.26) | <0.001 | 12.07 (6.97; 20.89) | <0.001 | 2.46 (2.17; 2.80) | <0.001 |
| Model 2 | (ref) | 1.84 (1.01; 3.36) | 0.04 | 2.83 (1.59; 5.04) | <0.001 | 6.01 (3.42; 10.58) | <0.001 | 2.02 (1.76; 2.31) | <0.001 |
| Model 3 | (ref) | 1.71 (0.93; 3.14) | 0.08 | 2.22 (1.23; 4.03) | 0.008 | 3.20 (1.73; 5.95) | <0.001 | 1.50 (1.25; 1.79) | 0.001 |
| Q1 | Q2 | Q3 | Q4 | ||||
|---|---|---|---|---|---|---|---|
| ♀ DRI < 13 | ♀ DRI 13–23 | ♀ DRI 23–36 | ♀ DRI > 36 | ||||
| ♂ DRI < 30 | ♂ DRI 30–43 | ♂ DRI 43–56 | ♂ DRI > 56 | ||||
| Participants, n | 1628 | 1494 | 1534 | 1478 | |||
| Males, % | 48.6 | 50.7 | 49.4 | 48.8 | |||
| Events, n | 28 | 42 | 70 | 166 | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | ||
| Crude Model | (ref) | 1.63 (1.00; 2.65) | 0.05 | 2.87 (1.84; 4.47) | <0.001 | 7.27 (4.84; 10.92) | <0.001 |
| Model 1 | (ref) | 1.55 (0.95; 2.52) | 0.08 | 2.62 (1.68; 4.09) | <0.001 | 6.74 (4.49; 10.13) | <0.001 |
| Model 2 | (ref) | 1.34 (0.81; 2.20) | 0.25 | 1.75 (1.10; 2.78) | 0.018 | 3.75 (2.44; 5.76) | <0.001 |
| Model 3 | (ref) | 1.30 (0.77; 2.20) | 0.32 | 1.35 (0.81; 2.24) | 0.25 | 1.80 (1.07; 3.02) | 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Guerrero, J.L.; Gruppen, E.G.; Connelly, M.A.; Shalaurova, I.; Otvos, J.D.; Garcia, E.; Bakker, S.J.L.; Dullaart, R.P.F. A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort. J. Clin. Med. 2020, 9, 2781. https://doi.org/10.3390/jcm9092781
Flores-Guerrero JL, Gruppen EG, Connelly MA, Shalaurova I, Otvos JD, Garcia E, Bakker SJL, Dullaart RPF. A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort. Journal of Clinical Medicine. 2020; 9(9):2781. https://doi.org/10.3390/jcm9092781
Chicago/Turabian StyleFlores-Guerrero, Jose L., Eke. G. Gruppen, Margery A. Connelly, Irina Shalaurova, James D. Otvos, Erwin Garcia, Stephan J. L. Bakker, and Robin P. F. Dullaart. 2020. "A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort" Journal of Clinical Medicine 9, no. 9: 2781. https://doi.org/10.3390/jcm9092781
APA StyleFlores-Guerrero, J. L., Gruppen, E. G., Connelly, M. A., Shalaurova, I., Otvos, J. D., Garcia, E., Bakker, S. J. L., & Dullaart, R. P. F. (2020). A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort. Journal of Clinical Medicine, 9(9), 2781. https://doi.org/10.3390/jcm9092781







