Efficacy of Plasma-Polymerized Allylamine Coating of Zirconia after Five Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Characterization
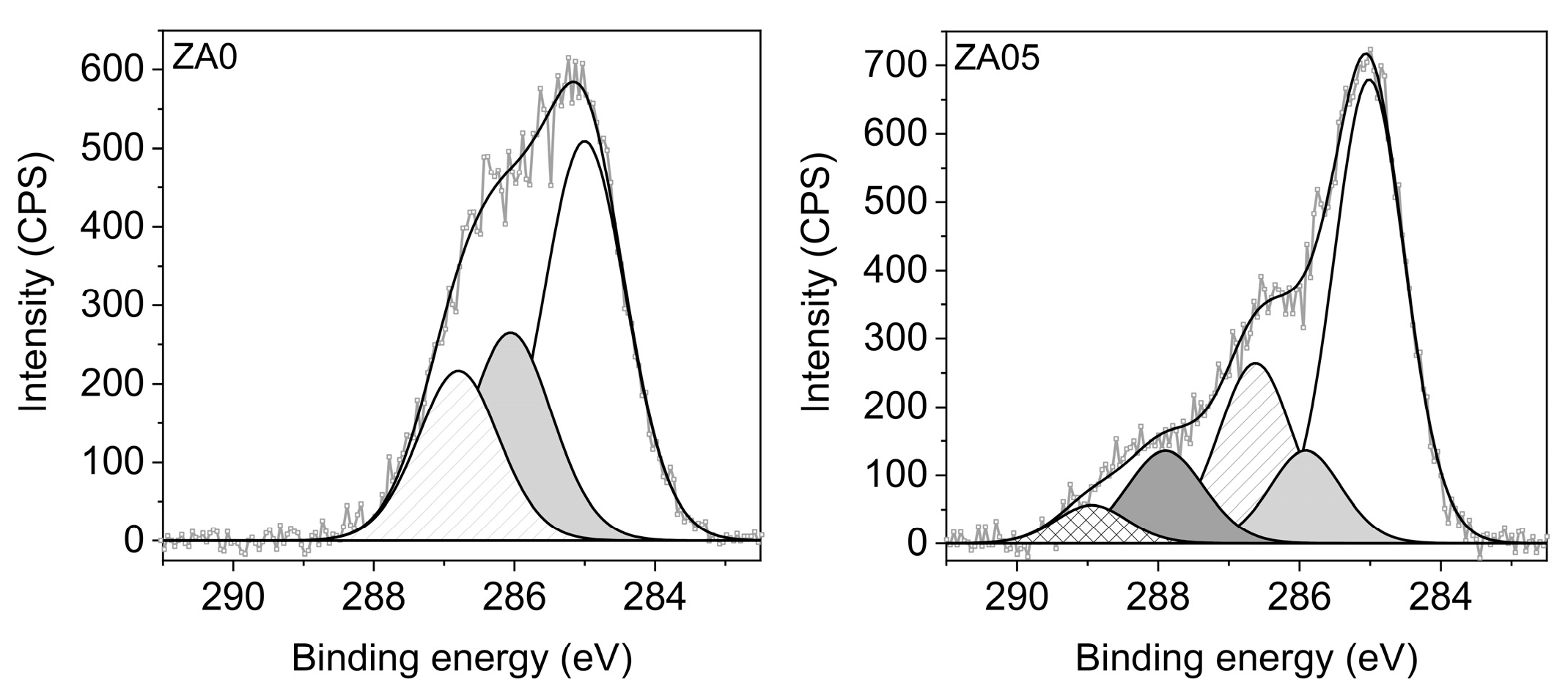
2.1.1. Elemental Composition (XPS)
2.1.2. SEM Imaging
2.2. Cell Behavior
2.2.1. Cell Cultivation
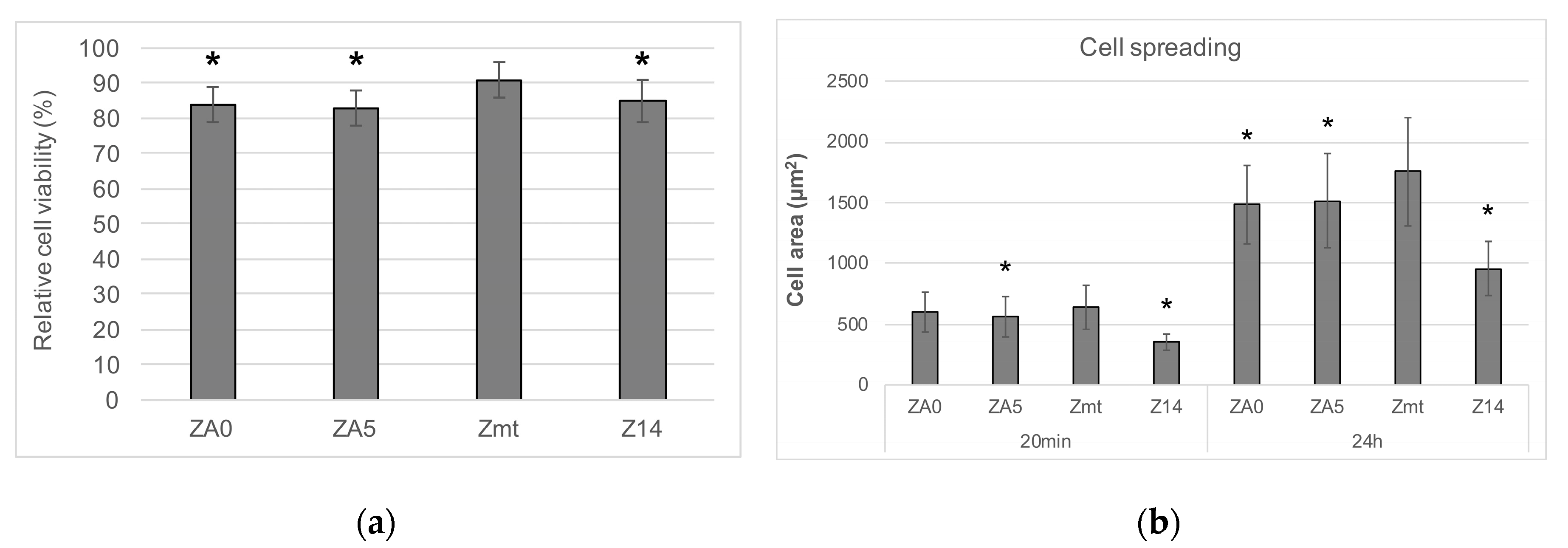
2.2.2. Cell Viability
2.2.3. Cell Spreading
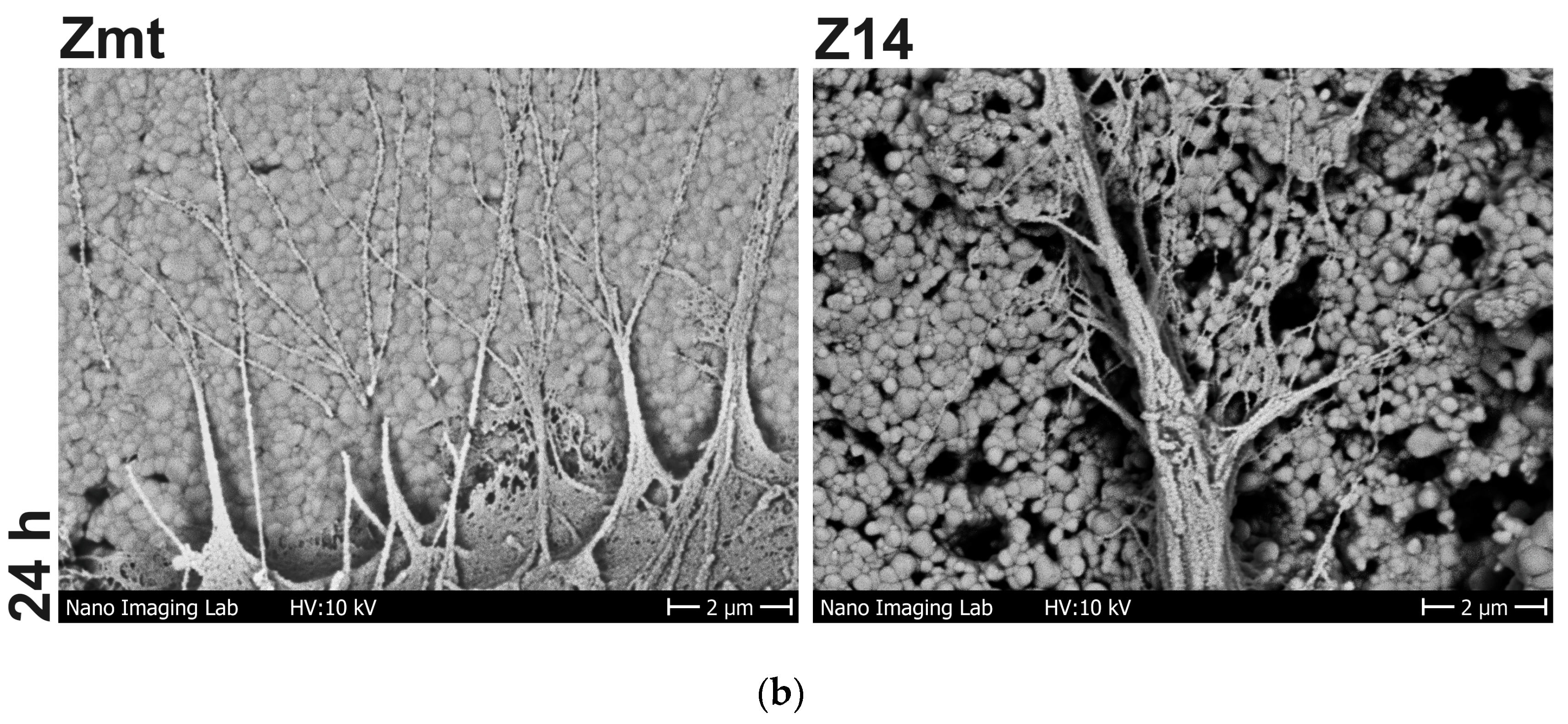
2.2.4. Cell Morphology
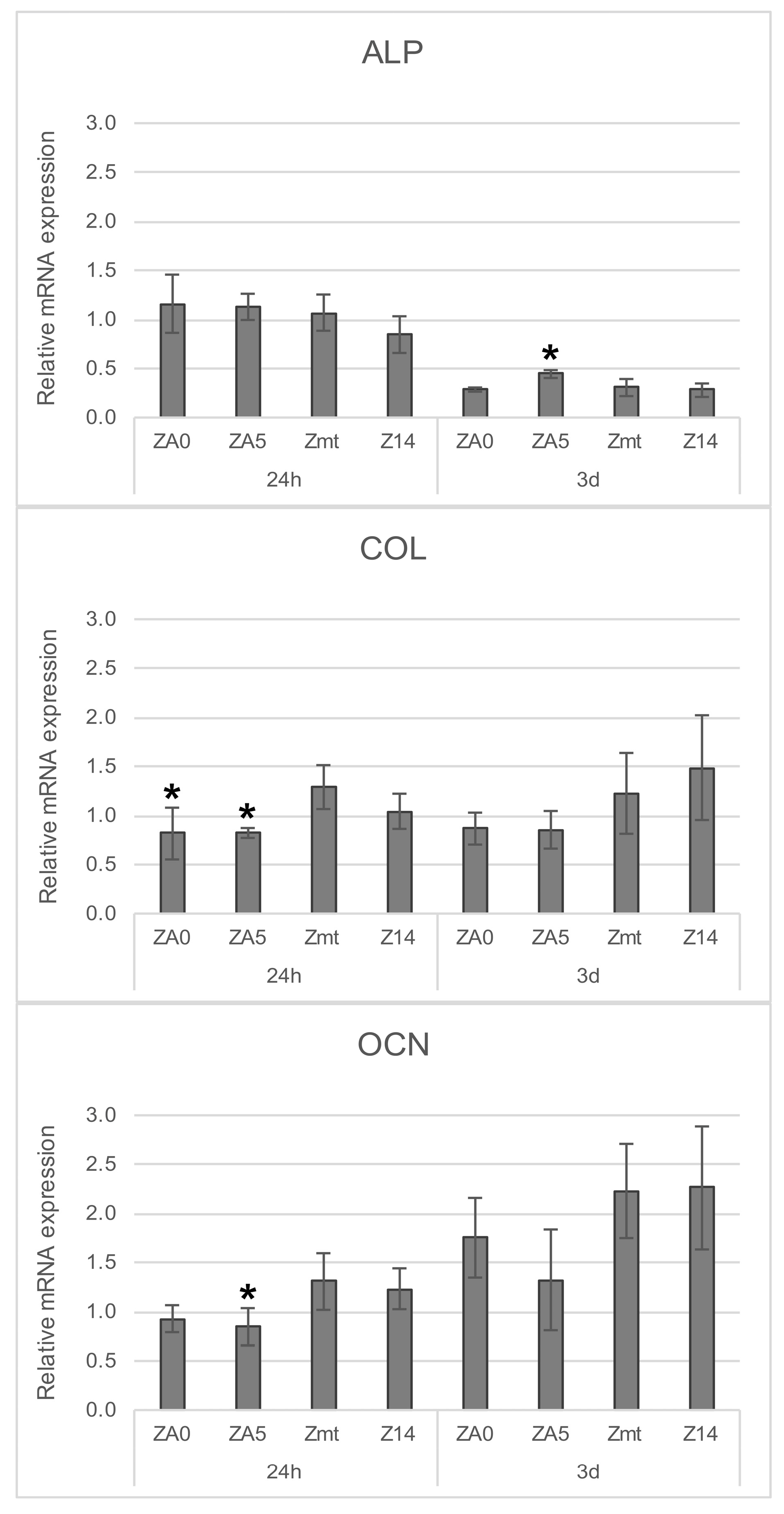
2.2.5. Gene Expression
2.3. Statistical Analysis
3. Results
3.1. Specimen Characterization
3.2. Cell Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin. Oral Implant. Res. 2018, 29, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Cadosch, D.; Gautschi, O.P.; Chan, E.; Filgueira, L.; Simmen, H.-P. Titanium induced production of chemokines CCL17/TARC and CCL22/MDC in human osteoclasts and osteoblasts. J. Biomed. Mater. Res. Part A 2009, 9999, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Cadosch, D.; Al-Mushaiqri, M.S.; Gautschi, O.P.; Meagher, J.; Simmen, H.-P.; Filgueira, L. Biocorrosion and uptake of titanium by human osteoclasts. J. Biomed. Mater. Res. Part A 2010, 95, 1004–1010. [Google Scholar] [CrossRef]
- Bormann, K.H.; Gellrich, N.-C.; Kniha, H.; Schild, S.; Weingart, D.; Gahlert, M. A prospective clinical study to evaluate the performance of zirconium dioxide dental implants in single-tooth edentulous area: 3-year follow-up. BMC Oral Health 2018, 18, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmer, M.; Spies, B.C.; Kohal, R.-J.; Hämmerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-year results of a prospective cohort investigation. Clin. Oral Implant. Res. 2020, 31, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Adánez, M.H.; Nishihara, H.; Att, W. A systematic review and meta-analysis on the clinical outcome of zirconia implant–restoration complex. J. Prosthodont. Res. 2018, 62, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Binon, P.P. Implants and components: Entering the new millennium. Int. J. Oral Maxillofac. Implant. 2000, 15, 76–94. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Fischer, J.; Schott, A.; Martin, S. Surface micro-structuring of zirconia dental implants. Clin. Oral Implant. Res. 2015, 27, 162–166. [Google Scholar] [CrossRef]
- Pieralli, S.; Kohal, R.-J.; Hernandez, E.L.; Doerken, S.; Spies, B.C. Osseointegration of zirconia dental implants in animal investigations: A systematic review and meta-analysis. Dent. Mater. 2017, 34, 171–182. [Google Scholar] [CrossRef]
- Nishihara, H.; Adanez, M.H.; Att, W. Current status of zirconia implants in dentistry: Preclinical tests. J. Prosthodont. Res. 2019, 63, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kniha, K.; Schlegel, K.; Kniha, H.; Modabber, A.; Hölzle, F. Evaluation of peri-implant bone levels and soft tissue dimensions around zirconia implants—A three-year follow-up study. Int. J. Oral Maxillofac. Surg. 2018, 47, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Morra, M. Biomolecular modification of implant surfaces. Expert Rev. Med Devices 2007, 4, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 85, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Faucheux, N.; Tzoneva, R.; Nagel, M.D.; Groth, T. The dependence of fibrillar adhesions in human fibroblasts on substratum chemistry. Biomaterials 2006, 27, 234–245. [Google Scholar] [CrossRef]
- Aziz, G.; De Geyter, N.; Morent, R. Incorporation of Primary Amines via Plasma Technology on Biomaterials. In Advances in Bioengineering; Serra, P.A., Ed.; InTech: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Feng, Q.; Bachhuka, A.; Vasilev, K. Surface Modification by Allylamine Plasma Polymerization Promotes Osteogenic Differentiation of Human Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2014, 6, 9733–9741. [Google Scholar] [CrossRef]
- Gallino, E.; Massey, S.; Tatoulian, M.; Mantovani, D. Plasma polymerized allylamine films deposited on 316L stainless steel for cardiovascular stent coatings. Surf. Coatings Technol. 2010, 205, 2461–2468. [Google Scholar] [CrossRef]
- Crespin, M.; Moreau, N.; Masereel, B.; Feron, O.; Gallez, B.; Vander Borght, T.; Michiels, C.; Lucas, S. Surface properties and cell adhesion onto allylamine-plasma and amine-plasma coated glass coverslips. J. Mater. Sci. Mater. Electron. 2011, 22, 671–682. [Google Scholar] [CrossRef]
- Testrich, H.; Rebl, H.; Finke, B.; Hempel, F.; Nebe, B.; Meichsner, J. Aging effects of plasma polymerized ethylenediamine (PPEDA) thin films on cell-adhesive implant coatings. Mater. Sci. Eng. C 2013, 33, 3875–3880. [Google Scholar] [CrossRef]
- Manakhov, A.; Landová, M.; Medalová, J.; Michlíček, M.; Polčák, J.; Nečas, D.; Zajíčková, L. Cyclopropylamine plasma polymers for increased cell adhesion and growth. Plasma Process. Polym. 2016, 14, 1600123. [Google Scholar] [CrossRef]
- Chan, K.V.; Asadian, M.; Onyshchenko, I.; Declercq, H.; Morent, R.; De Geyter, N. Biocompatibility of Cyclopropylamine-Based Plasma Polymers Deposited at Sub-Atmospheric Pressure on Poly (ε-caprolactone) Nanofiber Meshes. Nanomaterials 2019, 9, 1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buddhadasa, M.; Lerouge, S.; Girard-Lauriault, P.-L. Plasma polymer films to regulate fibrinogen adsorption: Effect of pressure and competition with human serum albumin. Plasma Process. Polym. 2018, 15, 1800040. [Google Scholar] [CrossRef]
- Schweikl, H.; Müller, R.; Englert, C.; Hiller, K.-A.; Kujat, R.; Nerlich, M.; Schmalz, G.; Müller, R. Proliferation of osteoblasts and fibroblasts on model surfaces of varying roughness and surface chemistry. J. Mater. Sci. Mater. Electron. 2007, 18, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Nebe, J.B.; Finke, B.; Lüthen, F.; Bergemann, C.; Schröder, K.; Rychly, J.; Liefeith, K.; Ohl, A. Improved initial osteoblast functions on amino-functionalized titanium surfaces. Biomol. Eng. 2007, 24, 447–454. [Google Scholar] [CrossRef]
- Finke, B.; Luethen, F.; Schroeder, K.; Mueller, P.D.; Bergemann, C.; Frant, M.; Ohl, A.; Nebe, J.B. The effect of positively charged plasma polymerization on initial osteoblastic focal adhesion on titanium surfaces. Biomaterials 2007, 28, 4521–4534. [Google Scholar] [CrossRef]
- Rebl, H.; Finke, B.; Lange, R.; Weltmann, K.-D.; Nebe, J.B. Impact of plasma chemistry versus titanium surface topography on osteoblast orientation. Acta Biomater. 2012, 8, 3840–3851. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Finke, B.; Mueller, P.; Gruening, M.; Nebe, J.B. Enhanced calcium ion mobilization in osteoblasts on amino group containing plasma polymer nanolayer. Cell Biosci. 2018, 8, 22. [Google Scholar] [CrossRef]
- Hoene, A.; Walschus, U.; Patrzyk, M.; Finke, B.; Lucke, S.; Nebe, B.; Schroeder, K.; Ohl, A.; Schlosser, M. In vivo investigation of the inflammatory response against allylamine plasma polymer coated titanium implants in a rat model. Acta Biomater. 2010, 6, 676–683. [Google Scholar] [CrossRef]
- Finke, B.; Rebl, H.; Hempel, F.; Schäfer, J.; Liefeith, K.; Weltmann, K.-D.; Nebe, J.B. Aging of Plasma-Polymerized Allylamine Nanofilms and the Maintenance of Their Cell Adhesion Capacity. Langmuir 2014, 30, 13914–13924. [Google Scholar] [CrossRef]
- Rebl, H.; Finke, B.; Schmidt, J.; Mohamad, H.S.; Ihrke, R.; Helm, C.A.; Nebe, J.B. Accelerated cell-surface interlocking on plasma polymer-modified porous ceramics. Mater. Sci. Eng. C 2016, 69, 1116–1124. [Google Scholar] [CrossRef]
- Nebe, J.B.; Rebl, H.; Schlosser, M.; Staehlke, S.; Gruening, M.; Weltmann, K.-D.; Walschus, U.; Finke, B. Plasma Polymerized Allylamine—The Unique Cell-Attractive Nanolayer for Dental Implant Materials. Polymers 2019, 11, 1004. [Google Scholar] [CrossRef] [Green Version]
- Moerke, C.; Mueller, P.; Nebe, J.B. Sensing of micropillars by osteoblasts involves complex intracellular signaling. J. Mater. Sci. Mater. Med. 2017, 28, 171. [Google Scholar] [CrossRef] [Green Version]
- Rohr, N.; Zeller, B.; Matthisson, L.; Fischer, J. Surface structuring of zirconia to increase fibroblast viability. Dent. Mater. 2020, 36, 779–786. [Google Scholar] [CrossRef]
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages: MG-63 cells: Receptors, cell cycle, signaling. Cell Boil. Int. 2018, 43, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Hong, G.; Matsui, H.; Endo, K.; Wan, Q.; Sasaki, K. Initial osteoblast adhesion and subsequent differentiation on zirconia surfaces are regulated by integrins and heparin-sensitive molecule. Int. J. Nanomed. 2018, 13, 7657–7667. [Google Scholar] [CrossRef] [Green Version]
- Rohr, N.; Nebe, J.B.; Schmidli, F.; Müller, P.; Weber, M.; Fischer, H.; Fischer, J. Influence of bioactive glass-coating of zirconia implant surfaces on human osteoblast behavior in vitro. Dent. Mater. 2019, 35, 862–870. [Google Scholar] [CrossRef]
- Bergemann, C.; Duske, K.; Nebe, J.B.; Schöne, A.; Bulnheim, U.; Seitz, H.; Fischer, J. Microstructured zirconia surfaces modulate osteogenic marker genes in human primary osteoblasts. J. Mater. Sci. Mater. Med. 2015, 26, 26. [Google Scholar] [CrossRef] [Green Version]
- Hempel, U.; Hefti, T.; Dieter, P.; Schlottig, F. Response of human bone marrow stromal cells, MG-63, and SaOS-2 to titanium-based dental implant surfaces with different topography and surface energy. Clin. Oral Implant. Res. 2011, 24, 174–182. [Google Scholar] [CrossRef]
- Lüthen, F.; Lange, R.; Becker, P.; Rychly, J.; Beck, U.; Nebe, J.B. The influence of surface roughness of titanium on β1- and β3-integrin adhesion and the organization of fibronectin in human osteoblastic cells. Biomaterials 2005, 26, 2423–2440. [Google Scholar] [CrossRef]
- Hidalgo-Bastida, L.A.; Cartmell, S. Mesenchymal Stem Cells, Osteoblasts and Extracellular Matrix Proteins: Enhancing Cell Adhesion and Differentiation for Bone Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 405–412. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; De Souza, J.C.M.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Billiard, J.; Moran, R.A.; Whitley, M.Z.; Chatterjee-Kishore, M.; Gillis, K.; Brown, E.L.; Komm, B.; Bodine, P. Transcriptional profiling of human osteoblast differentiation. J. Cell. Biochem. 2003, 89, 389–400. [Google Scholar] [CrossRef]


| Group | Surface Pretreatment |
|---|---|
| ZA0 | heat treated for 1 h at 1250 °C, plasma-polymerized allylamine coating September 2018 |
| ZA5 | heat treated for 1 h at 1250 °C, plasma-polymerized allylamine coating August 2013 |
| Zmt | heat treated for 1 h at 1250 °C |
| Z14 | sandblasted Al2O3 105 µm, etched 1 h hydrofluoric acid 38–40%, heat treated for 1 h at 1250 °C |
| C (at.%) | N (at.%) | O (at.%) | N/C (%) | O/C (%) | NH2/C (%) | |
|---|---|---|---|---|---|---|
| ZA0 | 74.5 ± 2.1 | 22.9 ± 2.3 | 2.6 ± 0.3 | 30.7 ± 3.8 | 3.5 ± 0.3 | 3.4 ± 0.1 |
| ZA5 | 73.9 ± 0.8 | 13.9 ± 0.8 | 11.4 ± 0.3 | 18.8 ± 1.3 | 15.4 ± 0.1 | 0.3 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohr, N.; Fricke, K.; Bergemann, C.; Nebe, J.B.; Fischer, J. Efficacy of Plasma-Polymerized Allylamine Coating of Zirconia after Five Years. J. Clin. Med. 2020, 9, 2776. https://doi.org/10.3390/jcm9092776
Rohr N, Fricke K, Bergemann C, Nebe JB, Fischer J. Efficacy of Plasma-Polymerized Allylamine Coating of Zirconia after Five Years. Journal of Clinical Medicine. 2020; 9(9):2776. https://doi.org/10.3390/jcm9092776
Chicago/Turabian StyleRohr, Nadja, Katja Fricke, Claudia Bergemann, J Barbara Nebe, and Jens Fischer. 2020. "Efficacy of Plasma-Polymerized Allylamine Coating of Zirconia after Five Years" Journal of Clinical Medicine 9, no. 9: 2776. https://doi.org/10.3390/jcm9092776
APA StyleRohr, N., Fricke, K., Bergemann, C., Nebe, J. B., & Fischer, J. (2020). Efficacy of Plasma-Polymerized Allylamine Coating of Zirconia after Five Years. Journal of Clinical Medicine, 9(9), 2776. https://doi.org/10.3390/jcm9092776







